Effects of Sodium–Glucose Cotransporter 2 Inhibitors in Diabetic and Non-Diabetic Patients with Advanced Chronic Kidney Disease in Peritoneal Dialysis on Residual Kidney Function: In Real-World Data
Abstract
1. Introduction
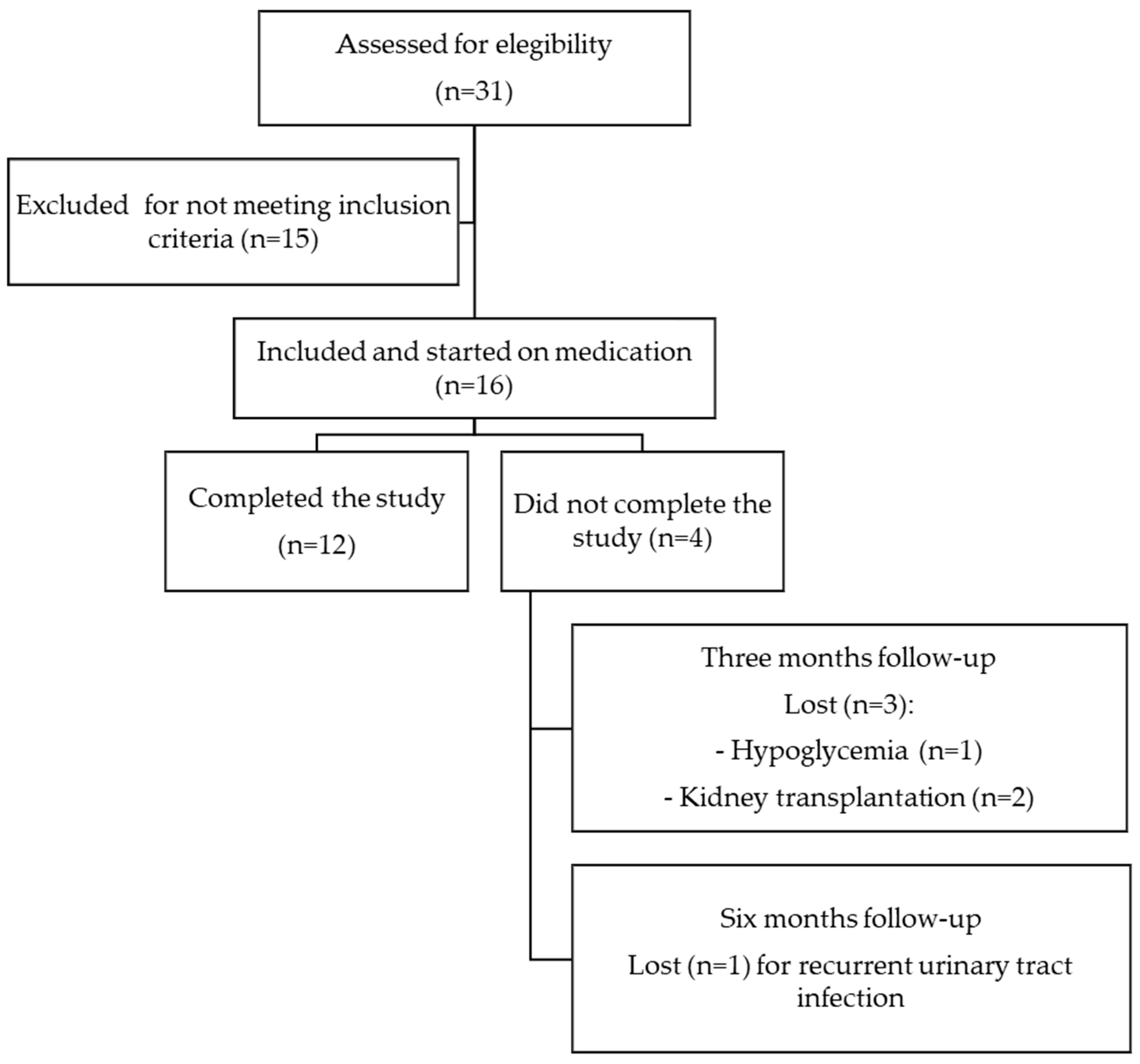
2. Materials and Methods
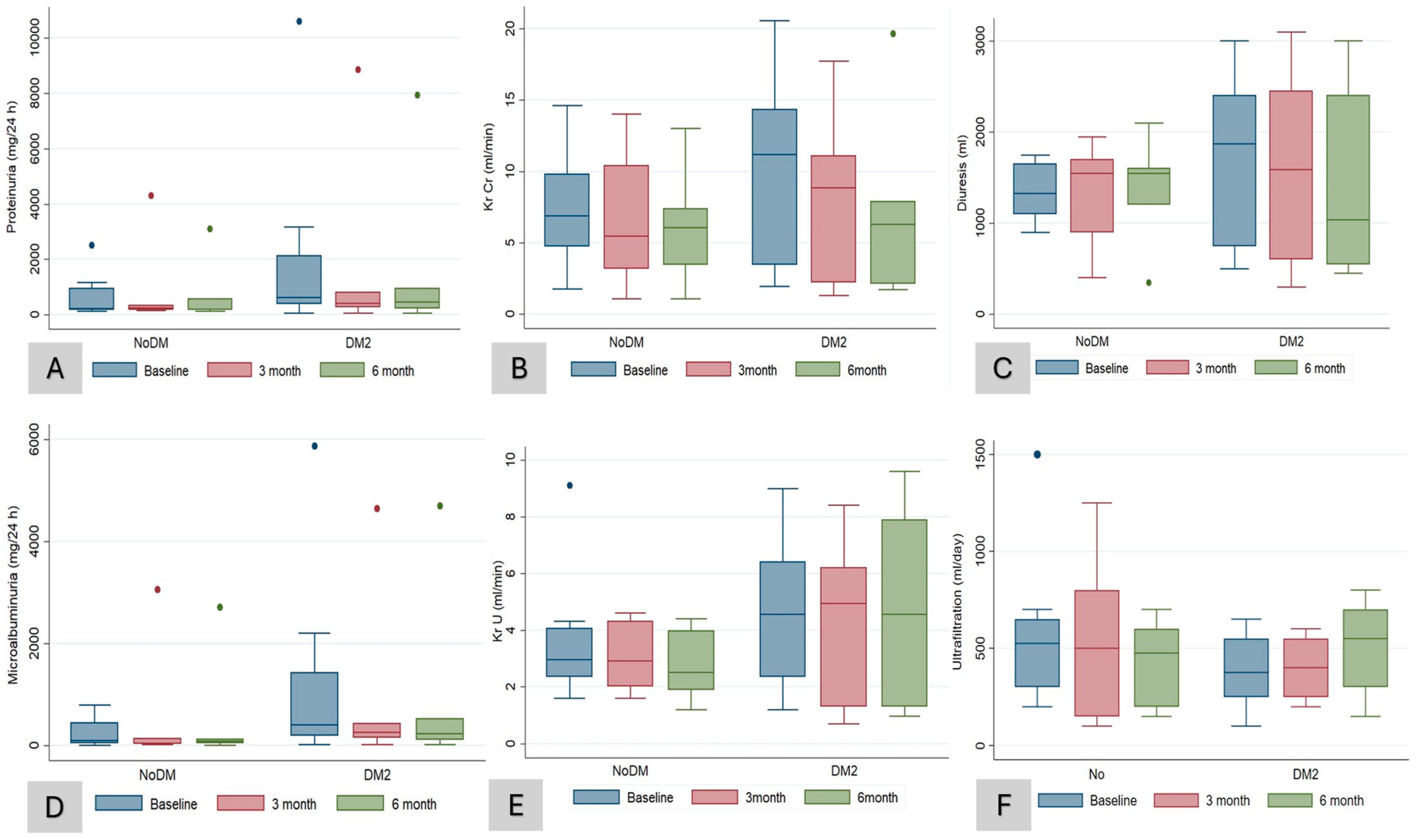
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Manzi, G.; Pierucci, N.; Laviola, D.; Piro, A.; D’Amato, A.; Filomena, D.; Matteucci, A.; Severino, P.; Miraldi, F.; et al. SGLT2i effect on atrial fibrillation: A network meta-analysis of randomized controlled trials. J. Cardiovasc. Electrophysiol. 2024; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Yau, K.; Dharia, A.; Alrowiyti, I.; Cherney, D.Z.I. Prescribing SGLT2 Inhibitors in Patients With CKD: Expanding Indications and Practical Considerations. Kidney Int. Rep. 2022, 7, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Barreto, J.; Borges, C.; Rodrigues, T.B.; Jesus, D.C.; Campos-Staffico, A.M.; Nadruz, W.; da Costa, J.L.; de Oliveira, R.B.; Sposito, A.C. Pharmacokinetic Properties of Dapagliflozin in Hemodialysis and Peritoneal Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2023, 18, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.; Oshima, M.; Mahaffey, K.W.; Agarwal, R.; Cannon, C.P.; Capuano, G.; Charytan, D.M.; De Zeeuw, D.; Edwards, R.; Greene, T.; et al. Effects of Canagliflozin in Patients with Baseline eGFR < 30 mL/min per 1.73 m2. Clin. J. Am. Soc. Nephrol. 2020, 15, 1705–1714. [Google Scholar] [CrossRef]
- Heerspink, H.L.; Wheeler, D.C.; Jong, N.; Correa-Rotter, R.; Rossing, P.; Gansevoort, R.; Mcmurray, J.; Langkilde, A.M.; Toto, R.; Chertow, G. #3382 Reasons for dialysis initiation and safety of dapagliflozin among dialysis participants: New insights from dapa-ckd. Nephrol. Dial. Transplant. 2023, 38. [Google Scholar] [CrossRef]
- Cao, H.; Rao, X.; Jia, J.; Yan, T.; Li, D. Effects of sodium-glucose co-transporter-2 inhibitors on kidney, cardiovascular, and safety outcomes in patients with advanced chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2022, 60, 325–335. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Balzer, M.S.; Rong, S.; Nordlohne, J.; Zemtsovski, J.D.; Schmidt, S.; Stapel, B.; Bartosova, M.; von Vietinghoff, S.; Haller, H.; Schmitt, C.P.; et al. SGLT2 Inhibition by Intraperitoneal Dapagliflozin Mitigates Peritoneal Fibrosis and Ultrafiltration Failure in a Mouse Model of Chronic Peritoneal Exposure to High-Glucose Dialysate. Biomolecules 2020, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fan, J.; Zheng, C.; Yin, P.; Wu, H.; Li, X.; Luo, N.; Yu, X.; Chen, C. SGLT-2 inhibitors reduce glucose absorption from peritoneal dialysis solution by suppressing the activity of SGLT-2. Biomed. Pharmacother. 2019, 109, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Marrón, B.; Remón, C.; Pérez-Fontán, M.; Quirós, P.; Ortíz, A. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int. 2008, 73, S42–S51. [Google Scholar] [CrossRef] [PubMed]
- Borkum, M.; Jamal, A.; Singh, R.S.; Levin, A. The rationale for the need to study sodium-glucose co-transport 2 inhibitor usage in peritoneal dialysis patients. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2023, 43, 139–144. [Google Scholar] [CrossRef]
- Alhwiesh, A.K.; Sarah Al-Wa, I.; Abdul-Rahman, S.; Ahmad Nasreldin, M.; Moaz Mohammed, A.; Al-Oudah, S.; Al-Thwainy, R. The use of SGLT2 Inhibitors in Peritoneal Dialysis Patients: A Shade of Light on Dapagliflozin. Arch Nephrol. Urol. 2022, 5, 1–8. [Google Scholar]
- Lai, J.-W.; Lin, H.-J.; Chou, C.-Y. SGLT-2 inhibitors may increase ultrafiltration in incident peritoneal dialysis patients: A case report. BMC Nephrol. 2023, 24, 1–4. [Google Scholar] [CrossRef]
- Hamdan, Z.; Abdel-Hafez, Y.; Enaya, A.; Sarsour, A.; Kharraz, L.; Nazzal, Z. Dapagliflozin in peritoneal dialysis patients: A pilot study evaluating peritoneal membrane function. BMC Nephrol. 2024, 25, 1–8. [Google Scholar] [CrossRef]
- Bargman, J.M.; Thorpe, K.E.; Churchill, D.N. Relative Contribution of Residual Renal Function and Peritoneal Clearance to Adequacy of Dialysis: A Reanalysis of the CANUSA Study. J. Am. Soc. Nephrol. 2001, 12, 2158–2162. [Google Scholar] [CrossRef]
- Li, P.K.T.; Chow, K.M.; Wong, T.Y.H.; Leung, C.B.; Szeto, C.C. Effects of an Angiotensin-Converting Enzyme Inhibitor on Residual Renal Function in Patients Receiving Peritoneal Dialysis: A Randomized, Controlled Study. Ann. Intern. Med. 2003, 139, 105. [Google Scholar] [CrossRef]
- Suzuki, H.; Kanno, Y.; Sugahara, S.; Okada, H.; Nakamoto, H. Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. Am. J. Kidney Dis. 2004, 43, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, L.; Beretta, A.; Jovane, C.; Peiti, S.; Genovesi, S. A Role for SGLT-2 Inhibitors in Treating Non-diabetic Chronic Kidney Disease. Drugs 2021, 81, 1491–1511. [Google Scholar] [CrossRef] [PubMed]
- Hodrea, J.; Balogh, D.B.; Hosszu, A.; Lenart, L.; Besztercei, B.; Koszegi, S.; Sparding, N.; Genovese, F.; Wagner, L.J.; Szabo, A.J.; et al. Reduced O-GlcNAcylation and tubular hypoxia contribute to the antifibrotic effect of SGLT2 inhibitor dapagliflozin in the diabetic kidney. Am. J. Physiol. Ren. Physiol. 2020, 318, F1017-29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nakano, D.; Guan, Y.; Hitomi, H.; Uemura, A.; Masaki, T.; Kobara, H.; Sugaya, T.; Nishiyama, A. A sodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor–dependent pathway after renal injury in mice. Kidney Int. 2018, 94, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhao, Y.; Wang, Q.; Hillebrands, J.L.; Born, J.V.D.; Ji, L.; An, T.; Qin, G. Dapagliflozin Attenuates Renal Tubulointerstitial Fibrosis Associated With Type 1 Diabetes by Regulating STAT1/TGFβ1 Signaling. Front. Endocrinol. 2019, 3, 441. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, G.; Carletti, R.; Ippolito, S.; Colzani, M.; Barzaghi, F.; Stella, A.; Zerbini, G.; Perseghin, G.; di Gioia, C.R. Renal Anti-Fibrotic Effect of Sodium Glucose Cotransporter 2 Inhibition in Angiotensin II-Dependent Hypertension. Am. J. Nephrol. 2020, 51, 119–129. [Google Scholar] [CrossRef] [PubMed]
- De La Flor, J.C.; Villa, D.; Cruzado, L.; Apaza, J.; Valga, F.; Zamora, R.; Marschall, A.; Cieza, M.; Deira, J.; Rodeles, M. Efficacy and Safety of the Use of SGLT2 Inhibitors in Patients on Incremental Hemodialysis: Maximizing Residual Renal Function, Is There a Role for SGLT2 Inhibitors? Biomedicines 2023, 11, 1908. [Google Scholar] [CrossRef] [PubMed]
- Ethier, I.; Cho, Y.; Hawley, C.; Pascoe, E.M.; Viecelli, A.K.; Campbell, S.B.; van Eps, C.; Isbel, N.M.; Cooper, B.A.; Harris, D.C.; et al. Rate of decline in residual kidney function pre and post peritoneal dialysis initiation: A post hoc analysis of the IDEAL study. PLoS ONE 2020, 15, e0242254. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.-S.; Hwu, C.-M.; Liu, J.-S.; Wu, Y.-L.; Chong, K.; Hsu, C.-C. Sodium–Glucose Cotransporter-2 Inhibitors and the Risk for Dialysis and Cardiovascular Disease in Patients With Stage 5 Chronic Kidney Disease. Ann. Intern. Med. 2024, 177, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Medcalf, J.F.; Harris, K.P.G.; Walls, J. Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 2001, 59, 1128–1133. [Google Scholar] [CrossRef]
- Thomas, M.C.; Cherney, D.Z.I. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia 2018, 61, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Zaccardi, F.; Webb, D.R.; Htike, Z.Z.; Youssef, D.; Khunti, K.; Davies, M.J. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: Systematic review and network meta-analysis. Diabetes Obes. Metab. 2016, 18, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Fu, Q.; Zhou, L.; Fan, Y.; Liu, F.; Fan, Y.; Zhang, X.; Lin, W.; Wu, X. Effect of SGLT-2 inhibitor, empagliflozin, on blood pressure reduction in Chinese elderly hypertension patients with type 2 diabetes and its possible mechanisms. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Krämer, B.K.; Hauske, S.J.; Chilton, R.; Mann, J.F.; Gullestad, L.; Fitchett, D.; Mattheus, M.; Steubl, D.; Wanner, C. Changes in cardiac and vascular haemodynamics as potential mediators of improvements in cardiovascular and kidney outcomes with empagliflozin in type 2 diabetes. J. Diabetes Its Complicat. 2023, 37, 108588. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, A.; Burnier, M.; Muller, M.; Ghajarzadeh-Wurzner, A.; Maillard, M.; Loncle, N.; Milani, B.; Dufour, N.; Bonny, O.; Pruijm, M. Acute and Chronic Effects of SGLT2 Inhibitor Empagliflozin on Renal Oxygenation and Blood Pressure Control in Nondiabetic Normotensive Subjects: A Randomized, Placebo-Controlled Trial. J. Am. Hearth Assoc. 2020, 9, e016173. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, A.; Pruijm, M.; Muller, M.-E.; Ghajarzadeh-Wurzner, A.; Maillard, M.; Dufour, N.; Bonny, O.; Wuerzner, G.; Burnier, M. Twenty-Four Hour Blood Pressure Response to Empagliflozin and Its Determinants in Normotensive Non-diabetic Subjects. Front. Cardiovasc. Med. 2022, 9, 854230. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, N. SGLT2 inhibitors in peritoneal dialysis: A promising frontier toward improved patient outcomes. Ren. Replace. Ther. 2024, 10, 5. [Google Scholar] [CrossRef]
- Cherney, D.Z.; Cooper, M.E.; Tikkanen, I.; Pfarr, E.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Lund, S.S. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018, 93, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Osonoi, T.; Shirabe, S.; Saito, M.; Hosoya, M.; Watahiki, N.; Douguchi, S.; Ofuchi, K.; Katoh, M. Dapagliflozin Improves Erythropoiesis and Iron Metabolism in Type 2 Diabetic Patients with Renal Anemia. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 1799–1808. [Google Scholar] [CrossRef]
- Koshino, A.; Schechter, M.; Chertow, G.M.; Vart, P.; Jongs, N.; Toto, R.D.; Rossing, P.; Correa-Rotter, R.; McMurray, J.J.; Górriz, J.L.; et al. Dapagliflozin and Anemia in Patients with Chronic Kidney Disease. NEJM Evid. 2023, 2, EVIDoa2300049. [Google Scholar] [CrossRef]
- Fuchs Andersen, C.; Omar, M.; Glenthøj, A.; El Fassi, D.; Møller, H.J.; Lindholm Kurtzhals, J.A.; Styrishave, B.; Kistorp, C.; Tuxen, C.; Poulsen, M.K.; et al. Effects of empagliflozin on erythropoiesis in heart failure: Data from the Empire HF trial. Eur. J. Heart Fail. 2023, 25, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Cases, A.; Cigarrán, S.; Górriz, J.L.; Nuñez, J. Efecto de los inhibidores del cotransportador sodio-glucosa tipo 2 sobre la anemia: Posibles implicaciones clínicas. Nefrología 2024, 44, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Goto, S. Possible Mechanism of Hematocrit Elevation by Sodium Glucose Cotransporter 2 Inhibitors and Associated Beneficial Renal and Cardiovascular Effects. Circulation 2019, 139, 1985–1987. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation 2022, 146, 1383–1405. [Google Scholar] [CrossRef]
- Mayne, K.J.; Staplin, N.; Keane, D.F.; Wanner, C.; Brenner, S.; Cejka, V.; Stegbauer, J.; Judge, P.K.; Preiss, D.; Emberson, J.; et al. Effects of Empagliflozin on Fluid Overload, Weight and Blood Pressure in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2024, 35, 202–215. [Google Scholar] [CrossRef]
- Tang, J.; Ye, L.; Yan, Q.; Zhang, X.; Wang, L. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Water and Sodium Metabolism. Front. Pharmacol. 2022, 13, 800490. [Google Scholar] [CrossRef] [PubMed]
- Albakr, R.B.; Sridhar, V.S.; Cherney, D.Z. Novel Therapies in Diabetic Kidney Disease and Risk of Hyperkalemia: A Review of the Evidence From Clinical Trials. Am. J. Kidney Dis. 2023, 82, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Gabai, P.; Fouque, D. SGLT2 inhibitors: New kids on the block to control hyperkalemia. Nephrol. Dial. Transplant. 2023, 38, 1345–1348. [Google Scholar] [CrossRef]
- Neuen, B.L.; Oshima, M.; Agarwal, R.; Arnott, C.; Cherney, D.Z.; Edwards, R.; Langkilde, A.M.; Mahaffey, K.W.; McGuire, D.K.; Neal, B.; et al. Sodium-Glucose Cotransporter 2 Inhibitors and Risk of Hyperkalemia in People With Type 2 Diabetes: A Meta-Analysis of Individual Participant Data From Randomized, Controlled Trials. Circulation 2022, 145, 1460–1470. [Google Scholar] [CrossRef]
- Rau, M.; Thiele, K.; Hartmann, N.-U.K.; Möllmann, J.; Wied, S.; Hohl, M.; Marx, N.; Lehrke, M. Effects of empagliflozin on markers of calcium and phosphate homeostasis in patients with type 2 diabetes—Data from a randomized, placebo-controlled study. Bone Rep. 2022, 16, 101175. [Google Scholar] [CrossRef]
- Goicoechea, M.; de Vinuesa, S.G.; Verdalles, U.; Ruiz-Caro, C.; Ampuero, J.; Rincón, A.; Arroyo, D.; Luño, J. Effect of Allopurinol in Chronic Kidney Disease Progression and Cardiovascular Risk. Clin. J. Am. Soc. Nephrol. 2010, 5, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Shiau, C.-H.; Tsau, L.-Y.; Kao, C.-C.; Peng, Y.-C.; Bai, C.-H.; Wu, J.; Hou, W.-H. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors in patients with chronic kidney disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2023, 56, 1359–1381. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Barreto, E.F.; Kellum, J.A.; Awdishu, L.; Murray, P.T.; Ostermann, M.; Bihorac, A.; Mehta, R.L.; Goldstein, S.L.; Kashani, K.B.; et al. Moving toward a contemporary classification of drug-induced kidney disease. Crit. Care 2023, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sampani, E.; Sarafidis, P.; Dimitriadis, C.; Kasimatis, E.; Daikidou, D.; Bantis, K.; Papanikolaou, A.; Papagianni, A. Severe euglycemic diabetic ketoacidosis of multifactorial etiology in a type 2 diabetic patient treated with empagliflozin: Case report and literature review. BMC Nephrol. 2020, 21, 276. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Luo, D.; Luo, N.; Li, B.; Fu, D.; Fan, L.; Li, Z.; Chen, W.; Mao, H. Serum Hepcidin-25 and Risk of Mortality in Patients on Peritoneal Dialysis. Front. Med. 2021, 8, 684548. [Google Scholar] [CrossRef] [PubMed]
- Schröppel, B.; Fischereder, M.; Wiese, P.; Segerer, S.; Huber, S.; Kretzler, M.; Heiss, P.; Sitter, T.; Schlöndorff, D. Expression of glucose transporters in human peritoneal mesothelial cells. Kidney Int. 1998, 53, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Schricker, S.; Oberacker, T.; Fritz, P.; Ketteler, M.; Alscher, M.D.; Schanz, M. Peritoneal Expression of SGLT-2, GLUT1, and GLUT3 in Peritoneal Dialysis Patients. Kidney Blood Press. Res. 2022, 47, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Martus, G.; Bergling, K.; de Arteaga, J.; Öberg, C.M. SGLT2 inhibition does not reduce glucose absorption during experimental peritoneal dialysis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2021, 41, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Shentu, Y.; Li, Y.; Xie, S.; Jiang, H.; Sun, S.; Lin, R.; Chen, C.; Bai, Y.; Zhang, Y.; Zheng, C.; et al. Empagliflozin, a sodium glucose cotransporter-2 inhibitor, ameliorates peritoneal fibrosis via suppressing TGF-β/Smad signaling. Int. Immunopharmacol. 2021, 93, 107374. [Google Scholar] [CrossRef]
- Wang, J.; Lv, X.; A-Ni-Wan, A.S.J.; Tian, S.S.; Wang, J.M.; Liu, H.Y.; Fan, X.G.; Zhou, S.J.; Yu, P. Canagliflozin alleviates high glucose-induced peritoneal fibrosis via HIF-1α inhibition. Front Pharmacol. 2023, 14, 1152611. [Google Scholar] [CrossRef]
| All (N = 16) | NoDM | DM | p-Value | |
|---|---|---|---|---|
| (N = 8) | (N = 8) | |||
| Age—years, mean (SD) | 67.3 (10.3) | 64.5 (9.9) | 70 (10.6) | 0.2 |
| Sex—female, n (%) | 6 (37.5) | 4 (50) | 2 (25) | 0.3 |
| Dry weight (Kg), mean (SD) | 75.7 (18.3) | 70.4 (21.3) | 81.1 (14.1) | 0.3 |
| BMI (kg/m2), mean (SD) | 28.4 (6.1) | 25.73 (6.31) | 31.1 (4.9) | 0.07 |
| Causes of CKD, n (%) | 0.1 | |||
| Unknown origin | 7 (43.8) | 2 (25) | 5 (62.5) | |
| Glomerular disease | 5 (31.3) | 2 (25) | 3 (37.5) | |
| CTIN | 1 (6.3) | 1 (12.5) | 0 | |
| ADPKD | 3 (18.8) | 3 (37.5) | 0 | |
| HbA1c level, n (%) | 6.0 (0.9) | 5.4 (0.6) | 6.6 (0.8) | <0.001 |
| Hypertension, n (%) | 16 (100) | 16 (100) | 16 (100) | |
| ACE inhibitors/ARB | 16 (100) | 8 (100) | 8 (100) | |
| ACC | 6 (37.5) | 3 (37.5) | 3 (37.5) | |
| Loop diuretics | 13 (81.3) | 6 (75) | 7 (87.5) | |
| MRA | 9 (56.3) | 1 (12.5) | 8 (100) | |
| Thiazides | 6 (37.5) | 4 (50) | 2 (25) | |
| Alpha blockers | 5 (31.3) | 2 (25) | 3 (37.5) | |
| Beta blockers | 7 (43.8) | 4 (50) | 3 (37.55) | |
| SGLT-2 inhibitors, n (%) | 0.5 | |||
| Empagliflozin | 13 (81.2) | 7 (87.5) | 6 (75) | |
| Dapagliflozin | 3 (18.8) | 1 (12.5) | 2 (25) | |
| PD vintage (months), mean (SD) | 21.1 (15.0) | 13.1 (8.1) | 29.1 (16.5) | 0.03 |
| Technical peritoneal dialysis, n (%) | ||||
| APD | 4 (25) | 3 (37.5) | 1 (12.5) | 0.2 |
| CAPD | 12 (75) | 5 (62.5) | 7 (87.5) | |
| Technical prescription, n (%) | ||||
| Icodextrin | 10 (62.5) | 5 (62.5) | 5 (62.5) | 1 |
| Peritoneal equilibrium test: | ||||
| D/P, mean (SD) | 0.76 (0.12) | 0.78 (0.11) | 0.74 (0.13) | 0.9 |
| D/D0, mean (SD) | 0.29 (0.1) | 0.29 (0.09) | 0.29 (0.09) | |
| Peritoneal membrane function classification: | 0.4 | |||
| Low transporter, number (%) | 1 (6.3) | 0 | 1 (12.5) | |
| Average transporter, number (%) | 11 (68.8) | 5 (62.5) | 6 (75) | |
| High transporter, number (%) | 4 (25.0) | 3 (37.5) | 1 (12.5) | |
| Ultrafiltration volume (mL/day), median [IQR] | 425 [250–600] | 525 [300–650] | 375 [250–550] | 0.3 |
| nPCR, mean (SD) | 0.94 (0.16) | 0.94 (0.16) | 0.94 (0.18) | 0.9 |
| Residual diuresis (L), mean (SD) | 1.5 (0.7) | 1.35 Lt (0.315) | 1.69 (0.9) | 0.3 |
| KrU (SD), mL/min, mean (SD) | 4.1 (2.5) | 3.67 (2.36) | 4.6 (2.8) | 0.5 |
| ClCr (SD), mL/min, mean (SD) | 7.45 (4.2) | 10.05 (6.6) | ||
| Kt/V week, mean (SD) | 2.1 (0.4) | 2.1 (0.2) | 2.1(0.6) | 0.9 |
| 24 h proteinuria (g/day), median [IQR] | 489.7 [192.6–1128] | 229 [172.8–955.3] | 622.9 [398.8–2130] | 0.3 |
| MAU 24 h urine (mg/day), median [IQR] | 283 [65.2–553] | 105.6 [47.6–450.0] | 408 [188.7–1414.8] | 0.2 |
| SBP (mmHg), mean (SD) | 139.8 (10.2) | 141 (9.9) | 138.6 (11.0) | 0.7 |
| DBP (mmHg), mean (SD) | 71.4 (8.1) | 73.6 (7.4) | 69.3 (8.6) | 0.3 |
| Bicarbonate, mean (SD) | 22.1 (2.1) | 22.0 (1.5) | 22.1 (2.7) | 0.9 |
| Baseline | 3 Months | 6 Months | p-Value ANOVA | |
|---|---|---|---|---|
| N | 16 | 13 | 12 | |
| Weight (kg), mean (SD) | 75.7 (18.3) | 73.6 (16.9) | 72.8 (16.7) | 0.2 |
| Hb (g/dL), mean (SD) | 12.2 (1.5) | 11.6 (1.6) | 11.8 (0.8) | 0.5 |
| Cr (g/dL), mean (SD) | 6.6 (2.3) | 7.2 (2) | 7.1 (1.9) | 0.9 |
| CKD-EPI 2021 (mL/min/1.73m2), mean (SD) | 8.3 (3.6) | 7.2 (2.9) | 7.5 (3.6) | 0.8 |
| Urea (mmol/mL), mean (SD) | 139.4 (32.9) | 135.8 (28) | 131.8 (21.2) | 0.2 |
| Sodium (mmol/L), mean (SD) | 137.1 (3) | 136.4 (3.2) | 137.1 (1.9) | 0.7 |
| Potassium (mmol/L), mean (SD) | 4.8 (0.7) | 4.4 (0.5) | 4.6 (0.6) | 0.1 |
| Chlorine (mmol/L), mean (SD) | 100.2 (5) | 99 (4.2) | 99.6 (3.3) | 0.8 |
| Calcium (mg/dL), mean (SD) | 9.3 (0.8) | 9 (0.4) | 9.2 (0.5) | 0.3 |
| Phosphorus (mg/dL), mean (SD) | 4.8 (1.2) | 4.5 (1.1) | 4.7 (1.1) | 0.6 |
| Magnesium (mg/dL), mean (SD) | 2 (0.5) | 2.2 (0.5) | 2.2 (0.5) | 0.7 |
| HbA1c (%), mean (SD) | 6 (0.9) | 6 (0.7) | 5.9 (0.6) | 0.8 |
| Uric acid (mg/dL), mean (SD) | 5.2 (1.4) | 5.1 (1.2) | 5.4 (1.3) | 0.5 |
| Cholesterol (mg/dL), mean (SD) | 143.9 (29.5) | 148.8 (47.1) | 150.2 (47.8) | 0.9 |
| Triglycerides (mg/dL), mean (SD) | 152.3 (81.5) | 163.5 (80) | 193.6 (213.9) | 0.7 |
| HDL (mg/dL), mean (SD) | 50.1 (15.8) | 46.9 (14.5) | 43.5 (17.8) | 0.5 |
| LDL (mg/dL), mean (SD) | 71.8 (17.2) | 70.2 (40.3) | 64.3 (27.2) | 0.6 |
| Bilirubin (mg/dL), mean (SD) | 0.4 (0.2) | 0.4 (0.1) | 0.4 (0.2) | 0.6 |
| AST (IU/L), mean (SD) | 20.9 (11.7) | 23.4 (22.1) | 20.5 (15.7) | 0.5 |
| ALT (IU/L), mean (SD) | 24 (15.8) | 27.1 (27.3) | 20.5 (12.4) | 0.3 |
| GGT (IU/L), mean (SD) | 28.2 (21.7) | 43.7 (55.3) | 36.7 (35.5) | 0.2 |
| KT/V weekly (Lt), mean (SD) | 2.1 (0.4) | 2.1 (0.4) | 2 (0.4) | 0.5 |
| nPCR (g Urea/Kg/d), mean (SD) | 0.9 (0.2) | 0.8 (0.1) | 0.8 (0.1) | 0.08 |
| Ultrafiltration PD (mL/day), median [IQR] | 425 [250–600] | 500 [250–550] | 475 [250–700] | 0.8 |
| KrU (mL/min), mean (SD) | 4.1 (2.5) | 3.7 (2.2) | 3.8 (2.7) | 0.5 |
| Diuresis (mL/day), mean (SD) | 1521.9 (698.8) | 1448.5 (837.8) | 1402.5 (810) | 0.8 |
| ClCr (mL/min), mean (SD) | 8.7 (5.5) | 7.5 (5.2) | 6.7 (5.2) | 0.2 |
| (ClCr + KrU/2) (mL/min), mean (SD) | 6.4 (3.6) | 5.6 (3.6) | 5.3 (3.8) | 0.2 |
| MAU 24 h (mg/24 h), median [IQR] | 283 [65.2–553] | 139.5 [42.3–300] | 114.6 [56.9–428.4] | 0.9 |
| Proteinuria (mg/24 h), median [IQR] | 489.7 [192.6–1128] | 257.4 [180–500] | 328.6 [179–765.4] | 0.5 |
| SBP (mmHg), mean (SD) | 139.8 (10.2) | 129.5 (5.2) | 129.7 (7.0) | 0.003 |
| DBP (mmHg), mean (SD) | 71.4 (8.1) | 68.2 (6.8) | 71.8(5.9) | 0.06 |
| Bicarbonate (mEq/L), mean (SD) | 22.1 (2.1) | 21.8 (1.3) | 21.4 (0.8) | 0.5 |
| NoDM | DM | p-Value | |
|---|---|---|---|
| (N = 6) | (N = 6) | ||
| KrU (mL/min), median [IQR] | −0.1 [−0.4 to 0.2] | 0.1 [0–0.6] | 0.5 |
| ClCr (mL/min), median [IQR] | −1.1 [−1.6 to −0.7] | −0.3 [−3.1 to 2.1] | 0.5 |
| Diuresis (mL), median [IQR] | 0 [−150 to 200] | −125 [−600 to 130] | 0.5 |
| Ultrafiltration PD (mL/day), median [IQR] | 25 [−50 to 50] | 50 [0–200] | 0.5 |
| HbA1c (%), median [IQR] | 0 [−0.07 to 0] | −0.04 [−0.5 to −0.3] | 0.04 |
| Proteinuria (mg/24 h), median [IQR] | −15.3 [−40 to 440] | −266.3 [−2209.2 to −10.2] | 0.2 |
| MAU (mg/24 h), median [IQR] | −26.9 [−20 to 28.9] | −189 [−1172.6 to −79] | 0.05 |
| SBP (mmHg), median [IQR] | −15 [−20 to −7] | −5 [−8 to 2] | 0.1 |
| DBP (mmHg), median [IQR] | 51 [51–51] | 64 [53–68] | 0.07 |
| nPCR (g Urea/Kg/d), median [IQR] | −0.1 [−0.31 to 0.01] | −0.05 [−0.3 to 0.1] | 0.5 |
| KT/V weekly (L), median [IQR] | 0 [−0.02 to 0] | −0.05 [−0.1 to 0] | 0.3 |
| Dry weight (kg), median [IQR] | −0.8 [−2.2 to 0.4] | −2.4 [−3.6 to −1.1] | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moral Berrio, E.; De La Flor, J.C.; Arambarri Segura, M.; Rodríguez-Doyágüez, P.; Martínez Calero, A.; Zamora, R.; Cieza-Terrones, M.; Yuste-Lozano, C.; Sánchez de la Nieta García, M.D.; Nieto Iglesias, J.; et al. Effects of Sodium–Glucose Cotransporter 2 Inhibitors in Diabetic and Non-Diabetic Patients with Advanced Chronic Kidney Disease in Peritoneal Dialysis on Residual Kidney Function: In Real-World Data. Medicina 2024, 60, 1198. https://doi.org/10.3390/medicina60081198
Moral Berrio E, De La Flor JC, Arambarri Segura M, Rodríguez-Doyágüez P, Martínez Calero A, Zamora R, Cieza-Terrones M, Yuste-Lozano C, Sánchez de la Nieta García MD, Nieto Iglesias J, et al. Effects of Sodium–Glucose Cotransporter 2 Inhibitors in Diabetic and Non-Diabetic Patients with Advanced Chronic Kidney Disease in Peritoneal Dialysis on Residual Kidney Function: In Real-World Data. Medicina. 2024; 60(8):1198. https://doi.org/10.3390/medicina60081198
Chicago/Turabian StyleMoral Berrio, Esperanza, José C. De La Flor, Minerva Arambarri Segura, Pablo Rodríguez-Doyágüez, Alberto Martínez Calero, Rocío Zamora, Michael Cieza-Terrones, Claudia Yuste-Lozano, María Dolores Sánchez de la Nieta García, Javier Nieto Iglesias, and et al. 2024. "Effects of Sodium–Glucose Cotransporter 2 Inhibitors in Diabetic and Non-Diabetic Patients with Advanced Chronic Kidney Disease in Peritoneal Dialysis on Residual Kidney Function: In Real-World Data" Medicina 60, no. 8: 1198. https://doi.org/10.3390/medicina60081198
APA StyleMoral Berrio, E., De La Flor, J. C., Arambarri Segura, M., Rodríguez-Doyágüez, P., Martínez Calero, A., Zamora, R., Cieza-Terrones, M., Yuste-Lozano, C., Sánchez de la Nieta García, M. D., Nieto Iglesias, J., & Vozmediano Poyatos, C. (2024). Effects of Sodium–Glucose Cotransporter 2 Inhibitors in Diabetic and Non-Diabetic Patients with Advanced Chronic Kidney Disease in Peritoneal Dialysis on Residual Kidney Function: In Real-World Data. Medicina, 60(8), 1198. https://doi.org/10.3390/medicina60081198






