Circulating Malondialdehyde Is a Potential Biomarker for Predicting All-Cause Mortality during Follow-Up by Reflecting Comprehensive Inflammation at Diagnosis in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Data
2.3. Measurement of cMDA
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Correlation Analysis
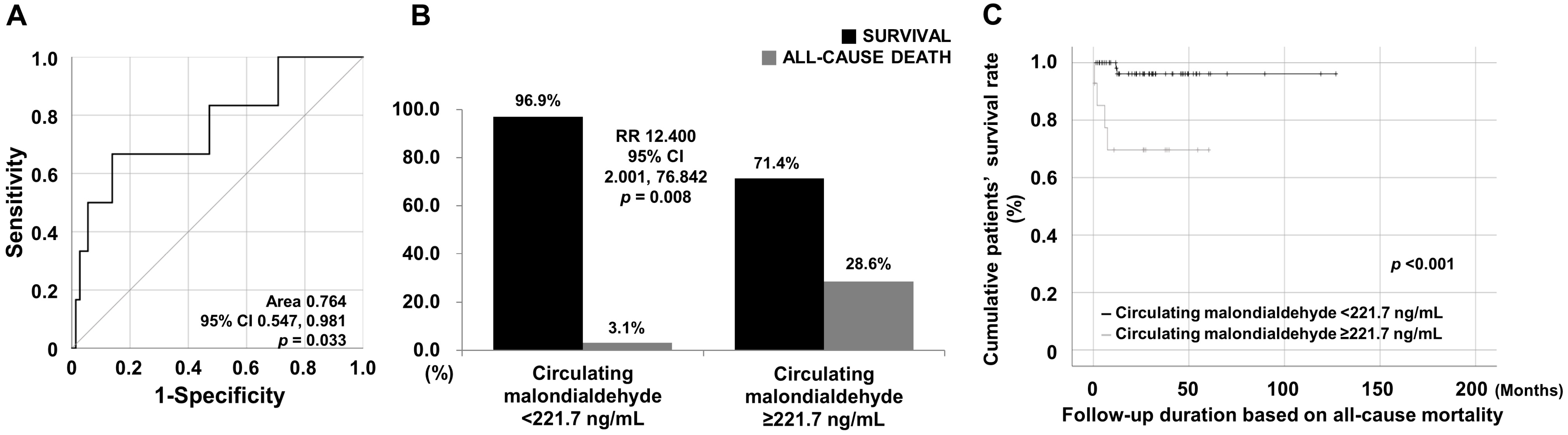
3.3. Cut-Off, RR, and Survival Rates of cMDA for Mortality
3.4. Cox Proportional Analyses for All-Cause Mortality during Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.J.; Binder, C.J. Malondialdehyde epitopes as mediators of sterile inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017, 1862, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Merino de Paz, N.; Quevedo-Abeledo, J.C.; Gómez-Bernal, F.; de Vera-González, A.; Abreu-González, P.; Martín-González, C.; González-Gay, M.; Ferraz-Amaro, I. Malondialdehyde Serum Levels in a Full Characterized Series of 430 Rheumatoid Arthritis Patients. J. Clin. Med. 2024, 13, 901. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.; Lane, S.; Hanslik, T.; Hauser, T.; Hellmich, B.; Koldingsnes, W.; Mahr, A.; Segelmark, M.; Cohen-Tervaert, J.W.; Scott, D. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 2007, 66, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Kronbichler, A.; Lee, K.H.; Denicolo, S.; Choi, D.; Lee, H.; Ahn, D.; Kim, K.H.; Lee, J.H.; Kim, H.; Hwang, M.; et al. Immunopathogenesis of ANCA-Associated Vasculitis. Int. J. Mol. Sci. 2020, 21, 7319. [Google Scholar] [CrossRef]
- Merino-Vico, A.; van Hamburg, J.P.; Tuijnenburg, P.; Frazzei, G.; Al-Soudi, A.; Bonasia, C.G.; Helder, B.; Rutgers, A.; Abdulahad, W.H.; Stegeman, C.A.; et al. Targeting NF-κB signaling in B cells as a potential new treatment modality for ANCA-associated vasculitis. J. Autoimmun. 2024, 142, 103133. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Meng, L.Q.; Xu, P.C.; Chen, M.; Zhao, M.H. p38MAPK, ERK and PI3K signaling pathways are involved in C5a-primed neutrophils for ANCA-mediated activation. PLoS ONE 2012, 7, e38317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suppiah, R.; Robson, J.C.; Grayson, P.C.; Ponte, C.; Craven, A.; Khalid, S.; Judge, A.; Hutchings, A.; Merkel, P.A.; Luqmani, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann. Rheum. Dis. 2022, 81, 321–326. [Google Scholar] [CrossRef]
- Robson, J.C.; Grayson, P.C.; Ponte, C.; Suppiah, R.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Watts, R.A.; Merkel, P.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann. Rheum. Dis. 2022, 81, 315–320. [Google Scholar] [CrossRef]
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, J.C.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Luqmani, R.A.; Watts, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann. Rheum. Dis. 2022, 81, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.Y.; Lee, L.E.; Park, Y.B.; Lee, S.W. Comparison of the 2022 ACR/EULAR Classification Criteria for Antineutrophil Cytoplasmic Antibody-Associated Vasculitis with Previous Criteria. Yonsei Med. J. 2023, 64, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.; Ha, J.W.; Ko, E.; Song, J.J.; Park, Y.-B.; Ahn, S.S.; Lee, S.-W. Vasculitis Activity-Predicting Ability of IL-12 Family Cytokines in Patients with Microscopic Polyangiitis and Granulomatosis with Polyangiitis. Yonsei Med. J. 2023, 64, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.; Pyo, J.Y.; Ahn, S.S.; Song, J.J.; Park, Y.B.; Lee, S.W. Serum soluble interleukin-7 receptor alpha levels are negatively correlated with the simultaneous activity of antineutrophil cytoplasmic antibody-associated vasculitis. Clin. Exp. Rheumatol. 2023, 41, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann. Rheum. Dis. 2009, 68, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Guillevin, L.; Pagnoux, C.; Seror, R.; Mahr, A.; Mouthon, L.; Toumelin, P.L.; French Vasculitis Study Group. The Five-Factor Score revisited: Assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 2011, 90, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Atkinson, C.; Bhalla, K.; Birbeck, G.; Burstein, R.; Chou, D.; Bartels, D.H.; Benjamin, E.J.; Bhalla, K.; Birbeck, G.; et al. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Kotani, T.; Matsuda, S.; Okazaki, A.; Nishioka, D.; Watanabe, R.; Gon, T.; Manabe, A.; Shoji, M.; Kadoba, K.; Hiwa, R.; et al. Risk prediction model for mortality in microscopic polyangiitis: Multicentre REVEAL cohort study. Arthritis Res. Ther. 2023, 25, 223. [Google Scholar] [CrossRef] [PubMed]
- Mun, C.H.; Yoo, J.; Jung, S.M.; Song, J.J.; Park, Y.B.; Lee, S.W. The initial predictors of death in 153 patients with ANCA-associated vasculitis in a single Korean centre. Clin. Exp. Rheumatol. 2018, 36 (Suppl. S111), 65–72. [Google Scholar]
- Dagostin, M.A.; Nunes, S.L.O.; Shinjo, S.K.; Pereira, R.M.R. Mortality predictors in ANCA-associated vasculitis: Experience of a Brazilian monocentric cohort of a rheumatology center. Medicine 2021, 100, e28305. [Google Scholar] [CrossRef]
- Watson, J.; Whiting, P.; Salisbury, C.; Banks, J.; Hamilton, W. Raised inflammatory markers as a predictor of one-year mortality: A cohort study in primary care in the UK using electronic health record data. BMJ Open 2020, 10, e036027. [Google Scholar] [CrossRef] [PubMed]
- Fest, J.; Ruiter, R.; Mooijaart, S.P.; Ikram, M.A.; van Eijck, C.H.J.; Stricker, B.H. Erythrocyte sedimentation rate as an independent prognostic marker for mortality: A prospective population-based cohort study. J. Intern. Med. 2019, 285, 341–348. [Google Scholar] [CrossRef] [PubMed]
| Variables | Values |
|---|---|
| At AAV diagnosis | |
| Demographic data | |
| Age (years) | 63.0 (51.8–73.3) |
| Male sex (n, (%)) | 32 (41.0) |
| Female sex (n, (%)) | 46 (59.0) |
| Ex-smoker (n, (%)) | 3 (3.8) |
| Body mass index (kg/m2) | 22.4 (20.8–24.7) |
| AAV subtypes (n, (%)) | |
| MPA | 38 (48.7) |
| GPA | 23 (29.5) |
| EGPA | 17 (21.8) |
| ANCA positivity (n, (%)) | |
| MPO-ANCA titre | 0 (0–20.5) |
| PR3-ANCA titre | 0 (0–0) |
| MPO-ANCA (or P-ANCA)-positive | 43 (55.1) |
| PR3-ANCA (or C-ANCA)-positive | 12 (15.4) |
| Both ANCA-positive | 3 (3.8) |
| AAV-specific indices | |
| BVAS | 5.0 (3.0–17.0) |
| FFS | 0 (0–1.0) |
| Comorbidities (n, (%)) | |
| Type 2 diabetes mellitus | 17 (21.8) |
| Hypertension | 25 (32.1) |
| Acute-phase reactants | |
| ESR (mm/h) | 24.5 (9.8–79.8) |
| CRP (mg/L) | 3.4 (0.8–19.4) |
| Laboratory results | |
| White blood cell count (/mm3) | 7,710.0 (5952.5–10,525.0) |
| Haemoglobin (g/dL) | 12.5 (10.3–13.6) |
| Platelet count (x1000/mm3) | 241.0 (191.5–356.5) |
| Fasting glucose (mg/dL) | 94.5 (87.8–109.3) |
| Total cholesterol (mg/dL) | 175.5 (140.0–212.3) |
| Blood urea nitrogen (mg/dL) | 19.3 (13.7–28.7) |
| Serum creatinine (mg/dL) | 0.8 (0.6–1.6) |
| Total serum protein (g/dL) | 6.8 (6.3–7.3) |
| Serum albumin (g/dL) | 4.2 (3.6–4.4) |
| cMDA (ng/mL) | 99.3 (4.0–196.8) |
| During AAV follow-up | |
| Mortality | |
| All-cause mortality | 6 (7.7) |
| Follow-up duration based on all-cause mortality | 26.7 (12.0–45.9) |
| Medications | |
| Glucocorticoids | 77 (98.7) |
| Cyclophosphamide | 51 (65.4) |
| Rituximab | 16 (20.5) |
| Mycophenolate mofetil | 20 (25.6) |
| Azathioprine | 48 (61.5) |
| Tacrolimus | 7 (9.0) |
| Methotrexate | 3 (3.8) |
| Variables | cMDA | |
|---|---|---|
| Correlation Coefficient (r) | p Value | |
| Age | 0.049 | 0.668 |
| Body mass index | −0.187 | 0.101 |
| MPO-ANCA titre | 0.078 | 0.498 |
| PR3-ANCA titre | 0.006 | 0.959 |
| BVAS | 0.117 | 0.310 |
| FFS | 0.163 | 0.154 |
| ESR | 0.251 | 0.027 |
| CRP | 0.222 | 0.058 |
| Variables | Univariable | Multivariable (with cMDA) | Multivariable (with cMDA ≥ 221.7 ng/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | 1.098 | 0.999, 1.207 | 0.052 | 1.169 | 1.024, 1.334 | 0.021 | 1.132 | 1.012, 1.266 | 0.030 |
| Male sex | 2.861 | 0.524, 15.618 | 0.225 | ||||||
| Ex-smoker | 0.046 | 0.000, 1,332,560.558 | 0.726 | ||||||
| Body mass index | 1.099 | 0.862, 1.400 | 0.446 | ||||||
| MPO-ANCA (or P-ANCA)-positive | 4.708 | 0.549, 40.404 | 0.158 | ||||||
| PR3-ANCA (or C-ANCA)-positive | 0.038 | 0.000, 516.628 | 0.501 | ||||||
| BVAS | 1.077 | 0.995, 1.165 | 0.065 | 1.028 | 0.915, 1.155 | 0.643 | 1.047 | 0.910, 1.206 | 0.520 |
| FFS | 2.512 | 0.831, 5.574 | 0.115 | ||||||
| Type 2 diabetes mellitus | 3.962 | 0.799, 19.640 | 0.092 | 1.650 | 0.202, 13.460 | 0.640 | 2.372 | 0.226, 24.923 | 0.472 |
| Hypertension | 1.124 | 0.206, 6.141 | 0.892 | ||||||
| ESR | 1.022 | 1.002, 1.042 | 0.029 | 1.012 | 0.984, 1.040 | 0.415 | 1.016 | 0.984, 1.050 | 0.324 |
| CRP | 1.019 | 1.001, 1.036 | 0.034 | 1.006 | 0.976, 1.037 | 0.701 | 1.008 | 0.971, 1.046 | 0.680 |
| cMDA | 1.006 | 1.001, 1.011 | 0.023 | 1.010 | 1.000, 1.019 | 0.055 | |||
| cMDA ≥ 221.7 ng/mL | 11.098 | 2.027, 60.745 | 24.076 | 2.422, 239.368 | 0.007 | ||||
| Variables | Multivariable (with cMDA) | Multivariable (with cMDA ≥ 221.7 ng/mL) | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| ESR (mm/h) | 1.015 | 0.990, 1.041 | 0.241 | 1.017 | 0.987, 1.048 | 0.268 |
| CRP (mg/L) | 1.007 | 0.984, 1.031 | 0.553 | 1.011 | 0.981, 1.042 | 0.482 |
| cMDA (ng/mL) | 1.005 | 1.000, 1.011 | 0.064 | |||
| cMDA ≥ 221.7 ng/mL | 13.462 | 2.235, 81.067 | 0.005 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, J.; Yoon, T.; Do, H.; Park, Y.-B.; Lee, S.-W. Circulating Malondialdehyde Is a Potential Biomarker for Predicting All-Cause Mortality during Follow-Up by Reflecting Comprehensive Inflammation at Diagnosis in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Medicina 2024, 60, 1182. https://doi.org/10.3390/medicina60071182
Chung J, Yoon T, Do H, Park Y-B, Lee S-W. Circulating Malondialdehyde Is a Potential Biomarker for Predicting All-Cause Mortality during Follow-Up by Reflecting Comprehensive Inflammation at Diagnosis in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Medicina. 2024; 60(7):1182. https://doi.org/10.3390/medicina60071182
Chicago/Turabian StyleChung, Jihye, Taejun Yoon, Hyunsue Do, Yong-Beom Park, and Sang-Won Lee. 2024. "Circulating Malondialdehyde Is a Potential Biomarker for Predicting All-Cause Mortality during Follow-Up by Reflecting Comprehensive Inflammation at Diagnosis in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis" Medicina 60, no. 7: 1182. https://doi.org/10.3390/medicina60071182
APA StyleChung, J., Yoon, T., Do, H., Park, Y.-B., & Lee, S.-W. (2024). Circulating Malondialdehyde Is a Potential Biomarker for Predicting All-Cause Mortality during Follow-Up by Reflecting Comprehensive Inflammation at Diagnosis in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Medicina, 60(7), 1182. https://doi.org/10.3390/medicina60071182






