Same Organ, Two Cancers: Complete Analysis of Renal Cell Carcinomas and Upper Tract Urothelial Carcinomas
Abstract
1. Introduction
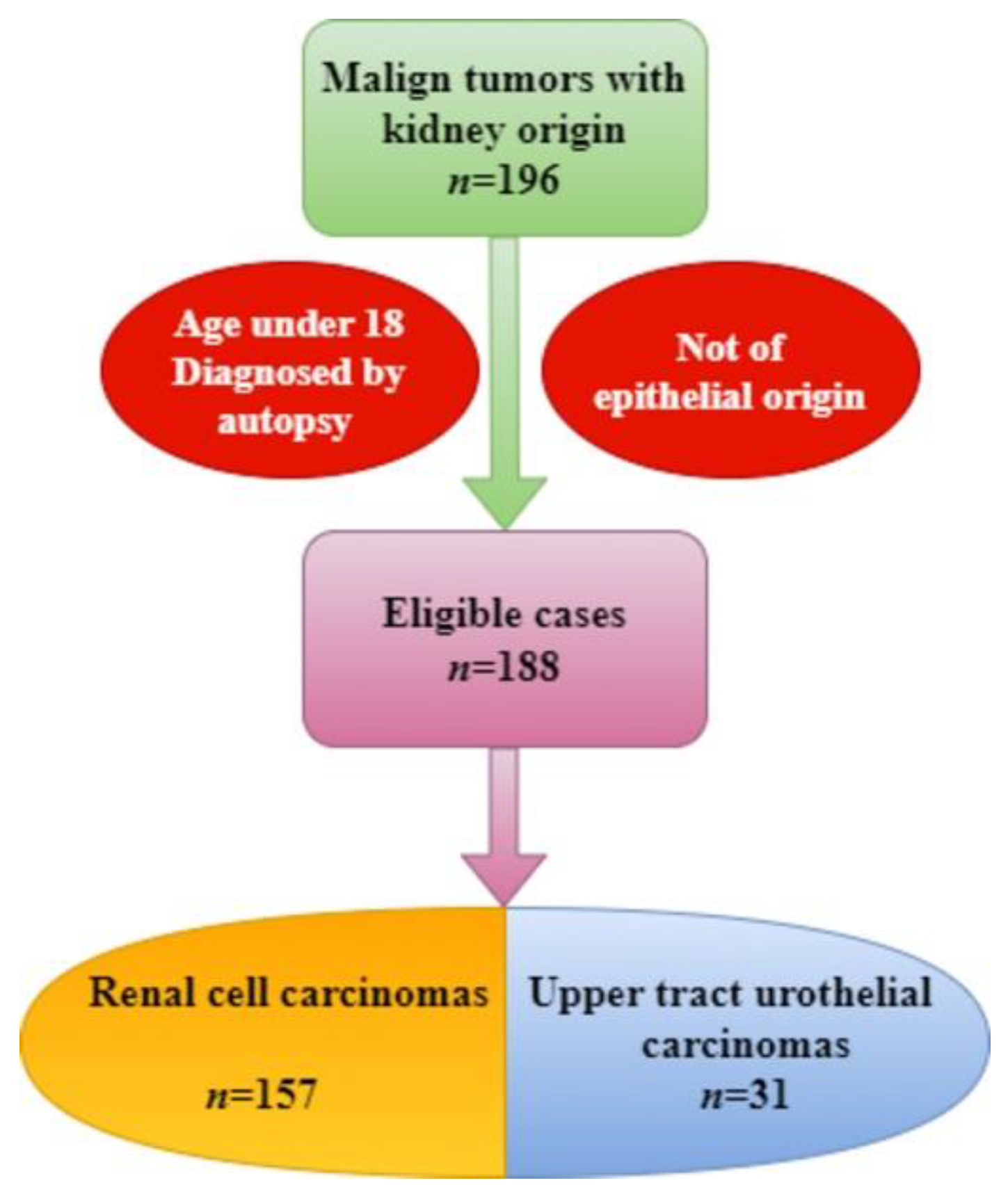
2. Materials and Methods
3. Results
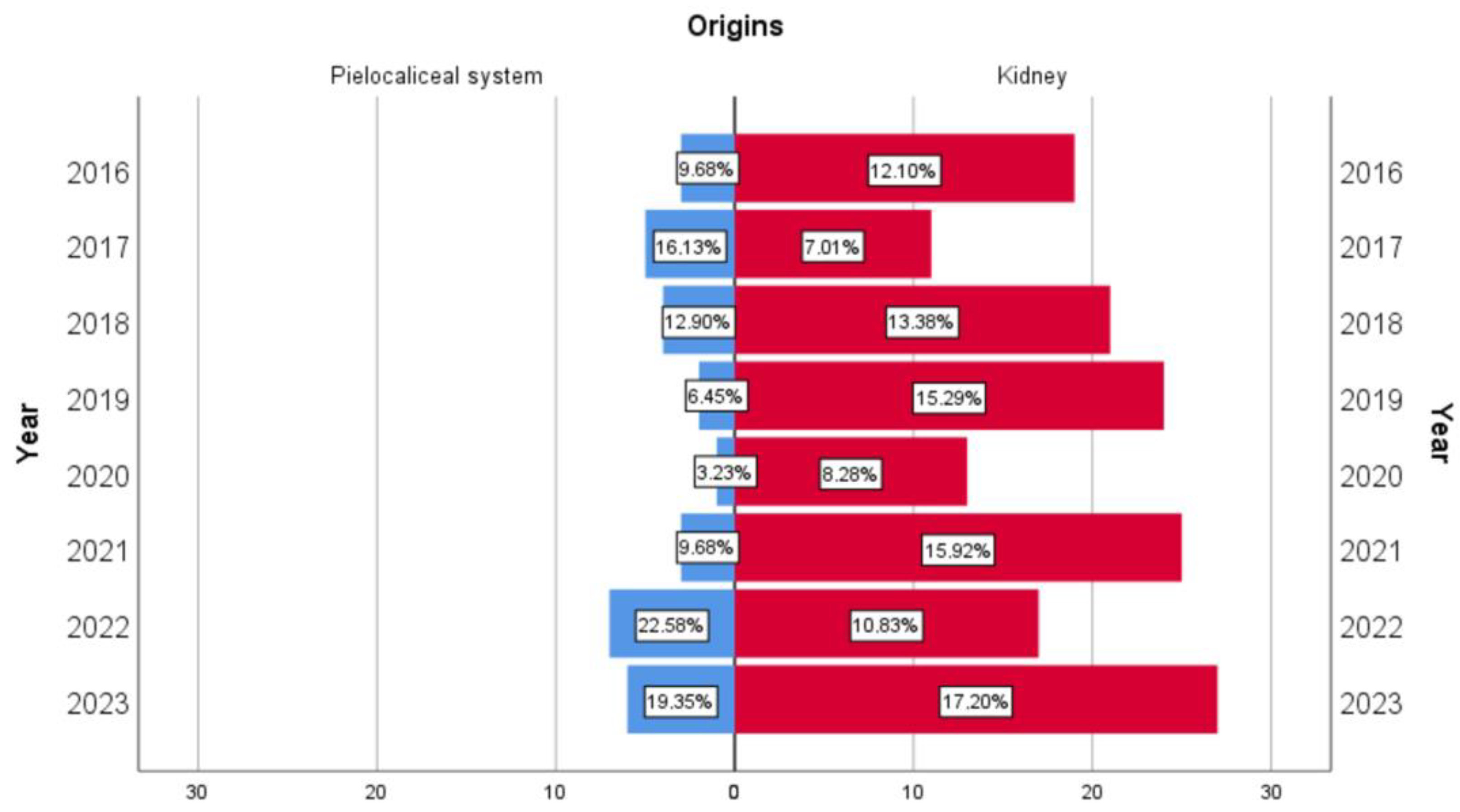
3.1. Demographic Aspects
3.2. Clinical and Paraclinical Characteristics
3.3. Morphometric Aspects
3.4. Macroscopic Aspects
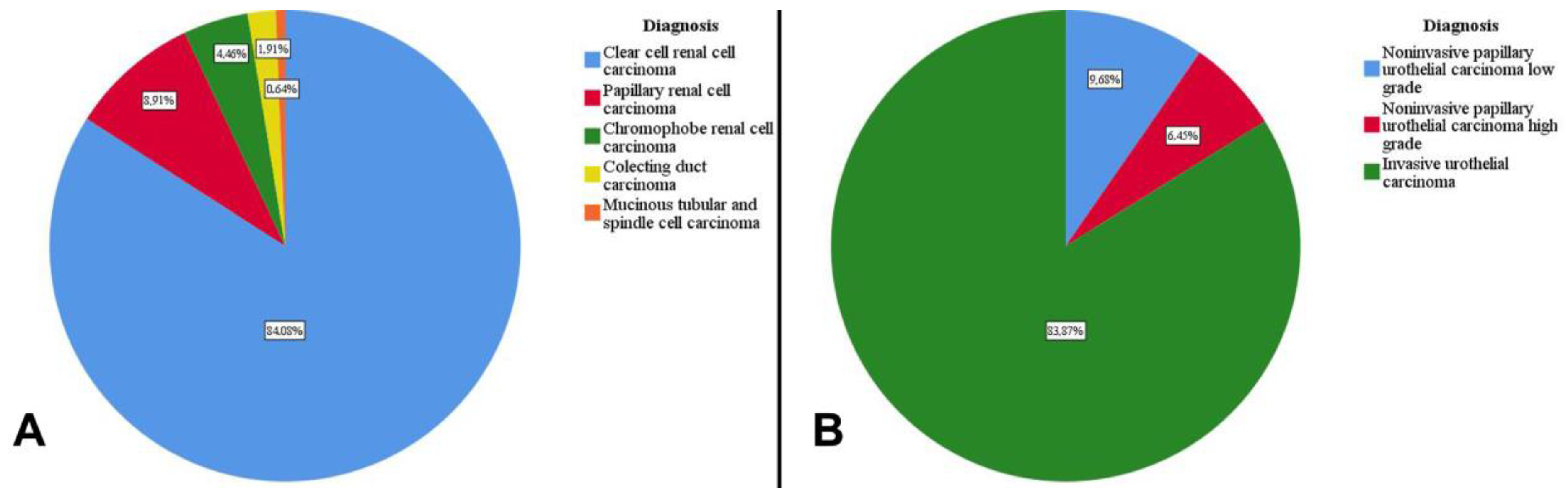
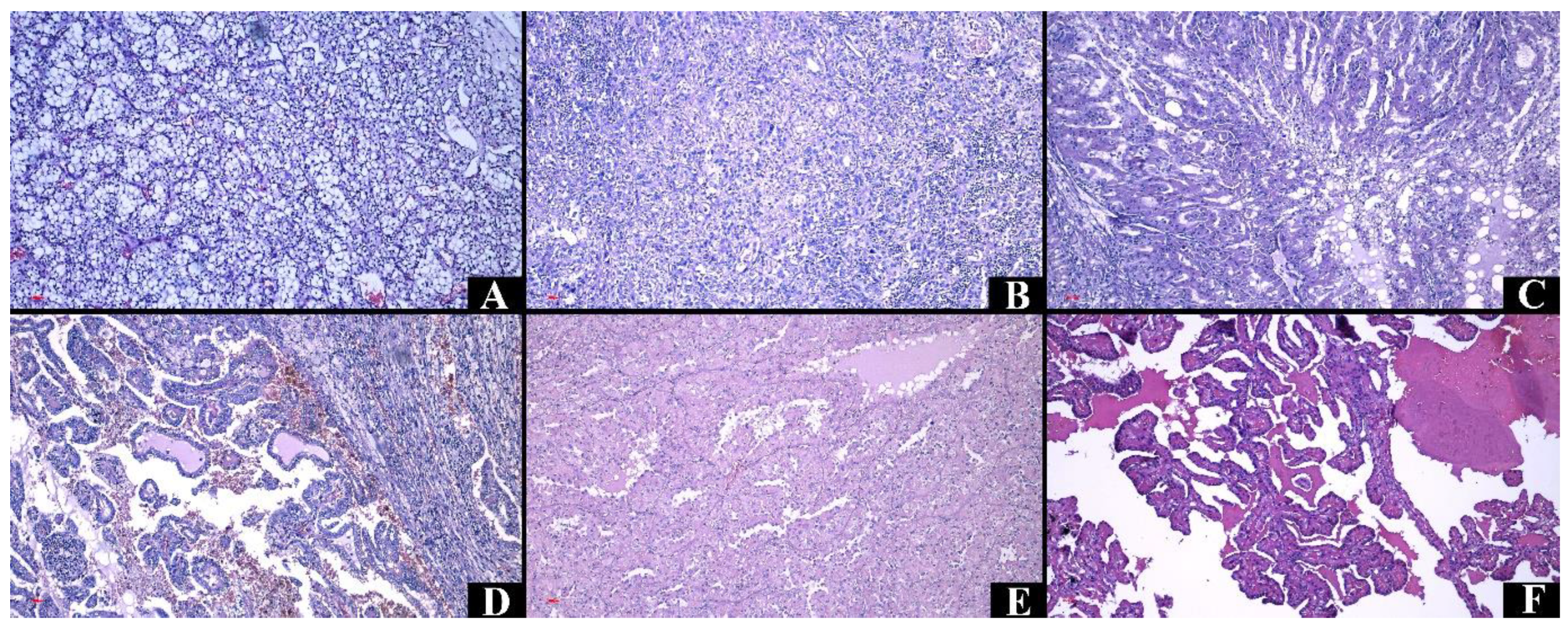
3.5. Microscopic Characteristics
3.6. Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Campbell, S.C.; Clark, P.E.; Chang, S.S.; Karam, J.A.; Souter, L.; Uzzo, R.G. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part I. J. Urol. 2021, 206, 199–208. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Baine, M.; Beckermann, K.; Carlo, M.I.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 71–90. [Google Scholar] [CrossRef]
- Froemming, A.; Potretzke, T.; Takahashi, N.; Kim, B. Upper tract urothelial cancer. Eur. J. Radiol. 2018, 98, 50–60. [Google Scholar] [CrossRef]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of renal cell carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Petros, F.G. Epidemiology, clinical presentation, and evaluation of upper-tract urothelial carcinoma. Transl. Androl. Urol. 2020, 9, 1794–1798. [Google Scholar] [CrossRef]
- Nuwatkrisin, K.; Itsaranujareankul, T.; Panumatrassamee, K.; Sowanthip, D.; Opanuraks, J.; Ratchanon, S.; Santingamkun, A.; Usawachintachit, M. Long-term survival of upper tract urothelial carcinoma patients in a tertiary care hospital. Insight Urol. 2022, 43, 25–32. [Google Scholar] [CrossRef]
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2016, 387, 894–906. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef]
- Cassell, A., 3rd; Manobah, B.; Willie, S. Diagnostic and Therapeutic Challenges of Rare Urogenital Cancers: Urothelial Carcinoma of the Renal Pelvis, Ureters and Urethra. World J. Oncol. 2021, 12, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Shao, I.H.; Chang, Y.H.; Pang, S.T. Recent advances in upper tract urothelial carcinomas: From bench to clinics. Int. J. Urol. 2019, 26, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Bensalah, K.; Axel, B.; Boorjian, S.A.; Bray, F.; Coleman, J. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Bukavina, L.; Bensalah, K.; Bray, F.; Carlo, M.; Challacombe, B.; Karam, J.A.; Kassouf, W.; Mitchell, T.; Montironi, R.; O’Brien, T.; et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022, 82, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.M.; Muhealdeen, D.N.; Fakhralddin, S.S.; Bapir, R.; Tahir, S.H.; Rashid, R.J.; Omer, C.S.; Abdullah, H.O.; Abdalla, B.A.; Mohammed, S.H.; et al. Prognostic factors in renal cell carcinoma: A single-center study. Mol. Clin. Oncol. 2023, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Patard, J.J. Prognostic factors in renal cell carcinoma. World J. Urol. 2010, 28, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Collà Ruvolo, C.; Nocera, L.; Stolzenbach, L.F.; Wenzel, M.; Cucchiara, V.; Tian, Z.; Shariat, S.F.; Saad, F.; Longo, N.; Montorsi, F.; et al. Incidence and Survival Rates of Contemporary Patients with Invasive Upper Tract Urothelial Carcinoma. Eur. Urol. Oncol. 2021, 4, 792–801. [Google Scholar] [CrossRef]
- Rouprêt, M.; Seisen, T.; Birtle, A.J.; Capoun, O.; Compérat, E.M.; Dominguez-Escrig, J.L.; Gürses Andersson, I.; Liedberg, F.; Mariappan, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2023 Update. Eur. Urol. 2023, 84, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rico, M.; Ramos, H.L.; Lobo, M.; Romo, J.; Prada, J.G. Epidemiology of renal cancer in developing countries: Review of the literature. Can. Urol. Assoc. J. 2018, 12, E154–E162. [Google Scholar] [CrossRef]
- Rossi, S.H.; Klatte, T.; Smith, J.U.; Steward, G.D. Epidemiology and screening for renal cancer. World J. Urol. 2018, 36, 1341–1353. [Google Scholar] [CrossRef]
- Soualhi, A.; Rammant, E.; George, G.; Russell, B.; Enting, D.; Nair, R.; Van Hemelrijck, M.; Bosco, C. The incidence and prevalence of upper tract urothelial carcinoma: A systematic review. BMC Urol. 2021, 21, 110. [Google Scholar] [CrossRef]
- Tsivian, M.; Moreira, D.M.; Caso, J.R.; Mouraviev, V.; Polascik, T.J. Cigarette smoking is associated with advanced renal cell carcinoma. J. Clin. Oncol. 2011, 29, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Du, J.; Xue, H.; Zhao, Y.; Li, C. The causal relationship between smoking, alcohol consumption, and renal clear cell carcinoma: A Mendelian randomization study. Front. Genet. 2024, 15, 1391542. [Google Scholar] [CrossRef]
- Gansler, T.; Fedewa, S.A.; Flanders, W.D.; Pollack, L.A.; Siegel, D.A.; Jemal, A. Prevalence of Cigarette Smoking among Patients with Different Histologic Types of Kidney Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1406–1412. [Google Scholar] [CrossRef]
- van Osch, F.H.; Jochems, S.H.; van Schooten, F.J.; Bryan, R.T.; Zeegers, M.P. Significant Role of Lifetime Cigarette Smoking in Worsening Bladder Cancer and Upper Tract Urothelial Carcinoma Prognosis: A Meta-Analysis. J. Urol. 2016, 195 Pt 1, 872–879. [Google Scholar] [CrossRef]
- Kumar, R.; Matulewicz, R.; Mari, A.; Moschini, M.; Ghodoussipour, S.; Pradere, B.; Rink, M.; Autorino, R.; Desai, M.M.; Gill, I.; et al. Impact of smoking on urologic cancers: A snapshot of current evidence. World J. Urol. 2023, 41, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Kadomoto, S.; Izumi, K.; Mizokami, A. Epidemiology and Prevention of Renal Cell Carcinoma. Cancers 2022, 14, 4059. [Google Scholar] [CrossRef]
- Zaitsu, M.; Kawachi, I.; Takeuchi, T.; Kobayashi, Y. Alcohol consumption and risk of upper-tract urothelial cancer. Cancer Epidemiol. 2017, 48, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Teissedre, P.L.; Rasines-Perea, Z.; Ruf, J.C.; Stockley, C.; Antoce, A.O.; Romano, R.; Fradera, U.; Kosti, R.I. Effects of alcohol consumption in general, and wine in particular, on the risk of cancer development: A review. OENO One 2020, 54, 813–832. [Google Scholar] [CrossRef]
- Perazella, M.A.; Dreicer, R.; Rosner, M.H. Renal cell carcinoma for the nephrologist. Kidney Int. 2018, 94, 471–483. [Google Scholar] [CrossRef]
- Barber, N.; Ali, A. Urologic Cancers; Exon Publications: Brisbane, Australia, 2022; pp. 63–67. [Google Scholar]
- Nabi, S.; Kessler, E.R.; Bernard, B.; Flaig, T.W.; Lam, E.T. Renal cell carcinoma: A review of biology and pathophysiology. F1000Research 2018, 7, 307. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Balan, D.G.; Tulin, A.; Stiru, O.; Vacaroiu, I.A.; Mihai, D.A.; Popa, C.C.; Papacocea, R.I.; Enyedi, M.; Sorin, N.A.; et al. PI3K/AKT/mTOR signalling pathway involvement in renal cell carcinoma pathogenesis (Review). Exp. Ther. Med. 2021, 21, 540. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumari, N.; Gupta, V.; Prasad, R. Renal Cell Carcinoma: Molecular Aspects. Indian J. Clin. Biochem. 2018, 33, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Peired, A.J.; Antonelli, G.; Angelotti, M.L.; Allinovi, M.; Guzzi, F.; Sisti, A.; Semeraro, R.; Conte, C.; Mazzinghi, B.; Nardi, S.; et al. Acute kidney injury promotes development of papillary renal cell adenoma and carcinoma from renal progenitor cells. Sci. Transl. Med. 2020, 12, eaaw6003. [Google Scholar] [CrossRef]
- Kang, Y.C.; Chen, M.H.; Lin, C.Y.; Lin, C.Y.; Chen, Y.T. Aristolochic acid-associated urinary tract cancers: An updated meta-analysis of risk and oncologic outcomes after surgery and systematic review of molecular alterations observed in human studies. Ther. Adv. Drug Saf. 2021, 12, 2042098621997727. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Margulis, V. Differences between Upper Tract Urothelial Carcinoma and Bladder Cancer. AUA News 2021, 26, 15–16. [Google Scholar] [PubMed]
- Aragon-Ching, J.B.; Nizam, A.; Henson, D.E. Carcinomas of the Renal Pelvis, Ureters, and Urinary Bladder Share a Carcinogenic Field as Revealed in Epidemiological Analysis of Tumor Registry Data. Clin. Genitourin. Cancer 2019, 17, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Almås, B.; Halvorsen, O.J.; Johannesen, T.B.; Beisland, C. Higher than expected and significantly increasing incidence of upper tract urothelial carcinoma. A population based study. World J. Urol. 2021, 39, 3385–3391. [Google Scholar] [CrossRef] [PubMed]
- Okbah, A.A.; Al-Shamahy, H.A.; Al-Sgamahi, E.H.; Al-Ankoshy, A.A.M. Renal lesions: Differentiation of malignant and benign tumors, sex and age distribution and variables associated with renal cell carcinoma in Sana’a City, Yemen. Univers. J. Pharm. Res. 2022, 7, 34–39. [Google Scholar] [CrossRef]
- Zaitsu, M.; Toyokawa, S.; Takeuchi, T.; Kobayashi, Y.; Kawachi, I. Sex-specific analysis of renal cell carcinoma histology and survival in Japan: A population-based study 2004 to 2016. Health Sci. Rep. 2019, 3, e142. [Google Scholar] [CrossRef]
- Huang, C.C.; Su, Y.L.; Luo, H.L.; Chen, Y.T.; Sio, T.T.; Hsu, H.C. Gender Is a Significant Prognostic Factor for Upper Tract Urothelial Carcinoma: A Large Hospital-Based Cancer Registry Study in an Endemic Area. Front. Oncol. 2019, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Deuker, M.; Rosiello, G.; Stolzenbach, L.F.; Martin, T.; Collà Ruvolo, C.; Nocera, L.; Tian, Z.; Roos, F.C.; Becker, A.; Kluth, L.A.; et al. Sex- and Age-Related Differences in the Distribution of Metastases in Patients with Upper Urinary Tract Urothelial Carcinoma. J. Natl. Compr. Cancer Netw. 2021, 19, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.J.; Xie, W.; Kroeger, N.; Lee, J.L.; Rini, B.I.; Knox, J.J.; Bjarnason, G.A.; Srinivas, S.; Pal, S.K.; Yuasa, T.; et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: A population-based study. Lancet Oncol. 2015, 16, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.H.; Huang, C.H. Unusual clinical presentation of upper urothelial carcinoma in Taiwan. Cancer 1999, 85, 1342–1344. [Google Scholar] [CrossRef]
- Ba, Z.; Xiao, Y.; He, M.; Liu, D.; Wang, H.; Liang, H.; Yuan, J. Risk Factors for the Comorbidity of Hypertension and Renal Cell Carcinoma in the Cardio-Oncologic Era and Treatment for Tumor-Induced Hypertension. Front. Cardiovasc. Med. 2022, 9, 810262. [Google Scholar] [CrossRef] [PubMed]
- Seretis, A.; Cividini, S.; Markozannes, G.; Tseretopoulou, X.; Lopez, D.S.; Ntzani, E.E.; Tsilidis, K.K. Association between blood pressure and risk of cancer development: A systematic review and meta-analysis of observational studies. Sci. Rep. 2019, 9, 8565. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Han, K.D.; Choi, H.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Association of Hypertension and Blood Pressure With Kidney Cancer Risk: A Nationwide Population-Based Cohort Study. Hypertension 2020, 75, 1439–1446. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, P.; Wang, M.; Zheng, Y.; Tian, T.; Yang, S.; Deng, Y.; Wu, Y.; Zhai, Z.; Hao, Q.; et al. Antihypertensive medications are associated with the risk of kidney and bladder cancer: A systematic review and meta-analysis. Aging 2020, 12, 1545–1562. [Google Scholar] [CrossRef]
- Lindsey, N. Hypertension Linked to Worse Upper Tract Urothelial Cancer Outcomes. Oncol. Times 2023, 45, 14–15. [Google Scholar]
- Lee, M.; Song, S.H.; Kim, H.; Lee, S.; Hong, S.K.; Byun, S.S.; Lee, S.E.; Oh, J.J. Effect of Body Mass Index and Hypertension on the Prognosis of Upper Tract Urothelial Carcinoma After Radical Nephroureterectomy. Korean J. Urol. Oncol. 2020, 18, 201–208. [Google Scholar] [CrossRef]
- Abudawood, M. Diabetes and cancer: A comprehensive review. J. Res. Med. Sci. 2019, 24, 94. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Yi, X.; Xu, Z.; Luo, H.; Ren, X.; Wei, Q.; Zhu, Z.; Jiang, Y.; Tang, Y.; He, H.; et al. Association of diabetes risk reduction diet with renal cancer risk in 101,755 participants: A prospective study. J. Transl. Med. 2023, 21, 684. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, L.; Ai, J.; Wang, W.; Di, X.; Peng, L. The Impact of Diabetes on the Prognosis of Upper Tract Urothelial Carcinoma After Radical Nephroureterectomy: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 741145. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Q.; Hou, H.; Zhu, K.; Wang, Q.; Liu, H.; Zhang, Q.; Ji, L.; Li, D. The association between BMI and kidney cancer risk: An updated dose-response meta-analysis in accordance with PRISMA guideline. Medicine 2018, 97, e12860. [Google Scholar] [CrossRef] [PubMed]
- van de Pol, J.A.A.; George, L.; van den Brandt, P.A.; Baldewijns, M.M.L.L.; Schouten, L.J. Etiologic heterogeneity of clear-cell and papillary renal cell carcinoma in the Netherlands Cohort Study. Int. J. Cancer 2021, 148, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, Z.; Yang, L.; Wang, X.; Yi, Z.; Zhou, L.; Chen, Y.; Yang, L.; Zhuo, H.; Bao, Y.; et al. Body Composition Parameters May Be Prognostic Factors in Upper Urinary Tract Urothelial Carcinoma Treated by Radical Nephroureterectomy. Front. Oncol. 2021, 11, 679158, reprinted in Front. Oncol. 2021, 11, 740572. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tan, P.; Zheng, X.; Ai, J.; Lin, T.; Jin, X. Metabolic syndrome and upper tract urothelial carcinoma: A retrospective analysis from a large Chinese cohort. Urol. Oncol. 2019, 37, 291.e19–291.e28. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef]
- Saly, D.L.; Eswarappa, M.S.; Street, S.E.; Deshpande, P. Renal Cell Cancer and Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2021, 28, 460–468.e1. [Google Scholar] [CrossRef]
- Peired, A.J.; Lazzeri, E.; Guzzi, F.; Anders, H.J.; Romagnani, P. From kidney injury to kidney cancer. Kidney Int. 2021, 100, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Janisch, F.; Mostafaei, H.; Lysenko, I.; Kimura, S.; Egawa, S. Prognostic value of preoperative blood-based biomarkers in upper tract urothelial carcinoma treated with nephroureterectomy: A systematic review and meta-analysis. Urol. Oncol. 2020, 38, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lai, S.; Wu, P.; Wang, J.; Wang, J.; Wang, J. Systematic oxidative stress indices predicts prognosis in patients with urothelial carcinoma of the upper urinary tract after radical nephroureterectomy. Eur. J. Med. Res. 2023, 28, 469. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Asakuma, J.; Horiguchi, A.; Kawaguchi, M.; Shinchi, M.; Masunaga, A. Chronic kidney disease and positive surgical margins as prognosticators for upper urinary tract urothelial carcinoma patients undergoing radical nephroureterectomy. Mol. Clin. Oncol. 2019, 10, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Hu, G.; Guzzo, T.J. Prognostic Significance of Preoperative Anemia in Patients Undergoing Surgery for Renal Cell Carcinoma: A Meta-analysis. Anticancer Res. 2017, 37, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, H.; Ma, X.; Gao, Y.; Chen, L.; Li, X.; Bao, X.; Du, Q.; Zhang, Y.; Zhang, X. Prognostic Significance of Hypoxia-Inducible Factor Expression in Renal Cell Carcinoma: A PRISMA-compliant Systematic Review and Meta-Analysis. Medicine 2015, 94, 1646. [Google Scholar] [CrossRef] [PubMed]
- Warli, S.M.; Andy, A.; Prapiska, F.F.; Siregar, G.P.; Sihombing, B. Poor prognosis of urothelial carcinoma in patients presented with persistent paraneoplastic leukocytosis with anemia. Urol. Ann. 2022, 14, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Weng, M.; Fan, H.; Peng, D.; Fang, D.; Xiong, G. The effect of preoperative anemia on the prognosis of upper urinary tract urothelial carcinoma! Retrospective study of single-center 608 patients. J. Peking Univ. Health Sci. 2019, 51, 1056–1061. [Google Scholar] [CrossRef]
- Luo, F.; Wang, Y.S.; Su, Y.H.; Zhang, Z.H.; Sun, H.H.; Li, J. Prognostic implications of preoperative anemia in urothelial carcinoma: A meta-analysis. PLoS ONE 2017, 12, e0171701. [Google Scholar] [CrossRef]
- Chang, Y.; An, H.; Xu, L.; Zhu, Y.; Yang, Y.; Lin, Z.; Xu, J. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br. J. Cancer 2015, 113, 626–633. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, K.; Lu, H.; Dong, X.; Min, F.; Zhuang, Q.; Yin, S.; He, X.; Xu, R. Systemic inflammation response index predicts prognosis in patients with clear cell renal cell carcinoma: A propensity score-matched analysis. Cancer Manag. Res. 2019, 11, 909–919. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, U.; Procopio, G.; Giannarelli, D.; Sabbatini, R.; Bearz, A.; Buti, S.; Basso, U.; Mitterer, M.; Ortega, C.; Bidoli, P.; et al. Association of Systemic Inflammation Index and Body Mass Index with Survival in Patients with Renal Cell Cancer Treated with Nivolumab. Clin. Cancer Res. 2019, 25, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- Jan, H.C.; Yang, W.H.; Ou, C.H. Combination of the Preoperative Systemic Immune-Inflammation Index and Monocyte-Lymphocyte Ratio as a Novel Prognostic Factor in Patients with Upper-Tract Urothelial Carcinoma. Ann. Surg. Oncol. 2019, 26, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Resch, I.; Miura, N.; Laukhtina, E.; Schuettfort, V.M.; Pradere, B.; Katayama, S.; D’Andrea, D.; Parizi, M.K.; Abufaraj, M.; et al. Prognostic role of the systemic immune-inflammation index in upper tract urothelial carcinoma treated with radical nephroureterectomy: Results from a large multicenter international collaboration. Cancer Immunol. Immunother. 2021, 70, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wu, Y.P.; Chen, S.H.; Ke, Z.B.; Liang, Y.C.; Xu, N. Prognosis and clinicopathological characteristics of renal cell carcinoma: Does bilateral occurrence influence overall and cancer-specific survival? Transl. Cancer Res. 2020, 9, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yao, K.; He, X.; Wu, S.; Ye, Y.; Chen, J.; Wu, C.L. Prognostic significance of laterality in renal cell carcinoma: A population-based study from the surveillance, epidemiology, and end results (SEER) database. Cancer Med. 2019, 8, 5629–5637. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, T.; Matsuyama, H.; Komura, K.; Ibuki, N.; Fujimoto, K.; Shiina, H.; Sakano, S.; Nagao, K.; Mastumoto, H.; Miyake, M.; et al. Tumor Location Based Segmentation in Upper-Tract Urothelial Carcinoma Impacts on the Urothelial Recurrence-Free Survival: A Multi-Institutional Database Study. Curr. Urol. 2020, 14, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, B.; Eble, J.N.; Samaratunga, H.; Thunders, M.; Yaxley, J.W.; Egevad, L. Staging of renal cell carcinoma: Current progress and potential advances. Pathology 2021, 53, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, K.M.; Rice-Stitt, T.; Wu, C.L. Updates in Staging and Reporting of Genitourinary Malignancies. Arch. Pathol. Lab. Med. 2020, 144, 305–319. [Google Scholar] [CrossRef]
- Mohd, A.B.; Ghannam, R.A.; Mohd, O.B.; Elayan, R.; Albakri, K.; Huneiti, N.; Daraghmeh, F.; Al-Khatatbeh, E.; Al-Thnaibat, M. Etiologies, Gross Appearance, Histopathological Patterns, Prognosis, and Best Treatments for Subtypes of Renal Carcinoma: An Educational Review. Cureus 2022, 14, e32338. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Muglia, V.F.; Prando, A. Renal cell carcinoma: Histological classification and correlation with imaging findings. Radiol. Bras. 2015, 48, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Eshghi, M. Urothelial Malignancies of the Upper Urinary Tract. In A Textbook of Step by Step Management; Springer: Phillapedhia, PA, USA, 2018; pp. 107–112. [Google Scholar]
- WHO Classification of Tumours Editorial Board. Urinary and Male Genital Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2022; Volume 8. [Google Scholar]
- Mori, K.; Janisch, F.; Parizi, M.K.; Mostafaei, H.; Lysenko, I.; Kimura, S.; Enikeev, D.V.; Egawa, S.; Shariat, S.F. Prognostic Value of Variant Histology in Upper Tract Urothelial Carcinoma Treated with Nephroureterectomy: A Systematic Review and Meta-Analysis. J. Urol. 2020, 203, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Monda, S.M.; Lui, H.T.; Pratsinis, M.A.; Chandrasekar, T.; Evans, C.P.; Dall’Era, M.A. The Metastatic Risk of Renal Cell Carcinoma by Primary Tumor Size and Subtype. Eur. Urol. 2023, 52, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zganjar, A.J.; Thiel, D.D.; Lyon, T.D. Diagnosis, workup, and risk stratification of upper tract urothelial carcinoma. Transl. Androl. Urol. 2023, 12, 1456–1468. [Google Scholar] [CrossRef] [PubMed]
- Foerster, B.; Abufaraj, M.; Mari, A.; Seisen, T.; Bandini, M.; Schweitzer, D.; Czech, A.K.; Moschini, M.; D’Andrea, D.; Bianchi, M.; et al. The Performance of Tumor Size as Risk Stratification Parameter in Upper Tract Urothelial Carcinoma (UTUC). Clin. Genitourin. Cancer 2021, 19, 272.e1–272.e7. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Loya, A.; Hameed, M.; Akhtar, N.; Mushtaq, S.; Hassan, U. Prognostic Significance of Percentage Necrosis in Clear Cell Renal Cell Carcinoma. Am. J. Clin. Pathol. 2021, 157, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Avulova, S.; Cheville, J.C.; Lohse, C.M.; Gupta, S.; Potretzke, T.A.; Tsivian, M.; Thompson, R.H.; Boorjian, S.A.; Leibovich, B.C.; Potretzke, A.M. Grading Chromophobe Renal Cell Carcinoma: Evidence for a Four-tiered Classification Incorporating Coagulative Tumor Necrosis. Eur. Urol. 2021, 79, 225–231. [Google Scholar] [CrossRef]
- Kuroe, T.; Watanabe, R.; Morisue, R.; Miyazaki, S.; Kojima, M.; Murata, S.C.; Nakai, T.; Taki, T.; Sakashita, S.; Sakamoto, N.; et al. Dirty necrosis in renal cell carcinoma is associated with NETosis and systemic inflammation. Cancer Med. 2023, 12, 4557–4567. [Google Scholar] [CrossRef]
- Bitaraf, M.; Ghafoori Yazdi, M.; Amini, E. Upper Tract Urothelial Carcinoma (UTUC) Diagnosis and Risk Stratification: A Comprehensive Review. Cancers 2023, 15, 4987. [Google Scholar] [CrossRef]
- Parmar, K.; Thummala, Y.; Kumar, S.; Kaundal, P.; Mandal, S. Massive intratumoral bleed in renal cell cancer: An unusual life-threatening event. Ann. R. Coll. Surg. Engl. 2022, 104, e168–e170. [Google Scholar] [CrossRef] [PubMed]
- Panaiyadiyan, S.; Singh, P.; Gurnani, N.; Nayak, B. Upper Tract Urothelial Carcinoma with Intra-renal Haemorrhage. Indian J. Surg. Oncol. 2021, 12, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jiang, X.; Guan, C.; Gu, M. The prognostic and predictive value of tumor infiltrating Macrophage and Neutrophil in patient with clear cell renal cell carcinoma: Tumor infiltrating lymphocytes in renal cell carcinoma. Medicine 2020, 99, e23181. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, E.; Long, J.; Hu, Z.; Peng, J.; Liu, L.; Tang, F.; Li, L.; Ouyang, Y.; Zeng, Z. Immune infiltration in renal cell carcinoma. Cancer Sci. 2019, 110, 1564–1572. [Google Scholar] [CrossRef]
- Cheng, S.; Zhong, W.; Xia, K.; Hong, P.; Lin, R.; Wang, B.; Li, X.; Chen, J.; Liu, Z.; Zhang, H.; et al. Prognostic role of stromal tumor-infiltrating lymphocytes in locally advanced upper tract urothelial carcinoma: A retrospective multicenter study (TSU-02 study). Oncoimmunology 2021, 10, 1861737. [Google Scholar] [CrossRef]
- Taneja, K.; Williamson, S.R. Updates in Pathologic Staging and Histologic Grading of Renal Cell Carcinoma. Surg. Pathol. Clin. 2018, 11, 797–812. [Google Scholar] [CrossRef]
- Dagher, J.; Delahunt, B.; Rioux-Leclercq, N.; Egevad, L.; Srigley, J.R.; Coughlin, G.; Dunglinson, N.; Gianduzzo, T.; Kua, B.; Malone, G.; et al. Clear cell renal cell carcinoma: Validation of World Health Organization/International Society of Urological Pathology grading. Histopathology 2017, 71, 918–925. [Google Scholar] [CrossRef]
- Kryvenko, O.N. Tumor necrosis adds prognostically significant information to grade in clear cell renal cell carcinoma: A study of 842 consecutive cases from a single institution. Khor LY, Dhakal HP, Jia X, Reynolds JP, McKenney JK, Rini BI, Magi-Galluzzi C, Przybycin CG.Am J Surg Pathol. September 2016;40(9):1224–1231. Urol. Oncol. 2017, 35, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Fojecki, G.; Magnusson, A.; Traxer, O.; Baard, J.; Osther, P.J.S.; Jaremko, G.; Seitz, C.; Knoll, T.; Giusti, G.; Brehmer, M. Consultation on UTUC, Stockholm 2018 aspects of diagnosis of upper tract urothelial carcinoma. World J. Urol. 2019, 37, 2271–2278. [Google Scholar] [CrossRef]
- Kanwal, R. Metastasis in renal cell carcinoma: Biology and treatment. Adv. Cancer Biol. Metastasis 2023, 7, 100094. [Google Scholar] [CrossRef]
- Lemelin, A.; Takemura, K.; Yick, D.; Ernst, M.S. Prognostic Models in Metastatic Renal Cell Carcinoma. Hematol. Oncol. Clin. N. Am. 2023, 37, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.H.; Chen, C.M.; Yu, W.L. Upper Tract Urothelial Carcinoma: Clinical Features and Management. SM J. Urol. 2020, 6, 11. [Google Scholar]
- Lwin, A.A.; Hsu, C.H.; Chipollini, J. Urothelial Carcinoma of the Renal Pelvis and Ureter: Does Location Make a Difference? Clin. Genitourin. Cancer 2020, 18, 45–49.e1. [Google Scholar] [CrossRef] [PubMed]
- Capek, S.; Krauss, W.E.; Amrami, K.K.; Parisi, J.E.; Spinner, R.J. Perineural Spread of Renal Cell Carcinoma: A Case Illustration with a Proposed Anatomic Mechanism and a Review of the Literature. World Neurosurg. 2016, 89, 728.e11–728.e17. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.W.; Lee, H.Y.; Yang, S.F.; Li, C.C.; Ke, H.L.; Li, W.M.; Li, C.Y.; Tu, H.P.; Wu, W.J.; Yeh, H.C. Perineural Invasion is a Powerful Prognostic Factor for Upper Tract Urothelial Carcinoma Following Radical Nephroureterectomy. Ann. Surg. Oncol. 2022, 29, 3306–3317. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Liang, P.; Lin, M.Y.; Yeh, S.M.; Zhen, Y.Y.; Chang, Y.H.; Huang, P.C.; Hung, C.C.; Kuo, I.C.; Lin, H.Y.; et al. Predominant global glomerulosclerosis in patients of upper urinary tract urothelial carcinoma with pre-existing renal function impairment is a predictor of poor renal outcomes. BMC Cancer 2019, 19, 337. [Google Scholar] [CrossRef] [PubMed]
- Roupret, M.; Gontero, P.; Birtle, A.; Comperat, E.M.; Dominguez-Escrig, J.L.; Liedberg, F.; Mariappan, P.; Lecomte, A.M.; Mostafid, A.H.; van Rhijn, B.W.G.; et al. EAU Guidelines on Upper Tract Urothelial Carcinoma; European Association of Urology: Milan, Italy, 2023; pp. 13–14. [Google Scholar]
- Kohada, Y.; Hayashi, T.; Goto, K.; Kobatake, K.; Abdi, H.; Honda, Y. Preoperative risk classification using neutrophil-lymphocyte ratio and hydronephrosis for upper tract urothelial carcinoma. Jpn. J. Clin. Oncol. 2018, 48, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Bahadoram, S.; Davoodi, M.; Hassanzadeh, S.; Bahadoram, M.; Barahman, M.; Mafakher, L. Renal cell carcinoma: An overview of the epidemiology, diagnosis, and treatment. G. Ital. Nefrol. 2022, 39, 2022-vol3. [Google Scholar] [PubMed]
- Chandrasekar, T.; Boorjian, S.A.; Capitanio, U.; Gershman, B.; Mir, M.C.; Kutikov, A. Collaborative Review: Factors Influencing Treatment Decisions for Patients with a Localized Solid Renal Mass. Eur. Urol. 2021, 80, 575–588. [Google Scholar] [CrossRef]
- Kim, S.P.; Campbell, S.C.; Gill, I.S.; Lane, B.R.; Van Poppel, H.; Smaldone, M.C.; Volpe, A.; Kutikov, A. Collaborative Review of Risk Benefit Trade-offs between Partial and Radical Nephrectomy in the Management of Anatomically Complex Renal Masses. Eur. Urol. 2017, 72, 64–75. [Google Scholar] [CrossRef]
- Verges, D.P.; Lallas, C.D.; Hubosky, S.G.; Bagley, D.H., Jr. Endoscopic Treatment of Upper Tract Urothelial Carcinoma. Curr. Urol. Rep. 2017, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Leow, J.J.; Liu, Z.; Tan, T.W.; Lee, Y.M.; Yeo, E.K.; Chong, Y.L. Optimal Management of Upper Tract Urothelial Carcinoma: Current Perspectives. OncoTargets Ther. 2020, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Cheriyan, S.K.; Peyton, C.C.; Foerster, B.; Shariat, S.F.; Spiess, P.E. Optimal Management of Upper Tract Urothelial Carcinoma: An Unmet Need. Curr. Treat. Options Oncol. 2019, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Unadkat, P.; Olumi, A.F.; Gershman, B. The Role of Lymphadenectomy in Patients with Advanced Renal Cell Carcinoma. Urol. Clin. N. Am. 2020, 47, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.W.; Wong, C.H.M.; Yuan, Y.; Teoh, J.Y. Lymph node dissection for upper tract urothelial carcinoma: A systematic review. Arab. J. Urol. 2020, 19, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Karmali, R.J.; Suami, H.; Wood, C.G.; Karam, J.A. Lymphatic drainage in renal cell carcinoma: Back to the basics. BJU Int. 2014, 114, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Carlson, P.; McGary, C.T. Educational Case: Renal Cell and Urothelial Carcinoma. Acad. Pathol. 2020, 7, 2374289520956363. [Google Scholar] [CrossRef]
- Singla, N.; Hutchinson, R.; Menegaz, C.; Haddad, A.Q.; Jiang, L.; Sagalowsky, A.I.; Cadeddu, J.A.; Lotan, Y.; Margulis, V. Comparing Changes in Renal Function After Radical Surgery for Upper Tract Urothelial Carcinoma and Renal Cell Carcinoma. Urology 2016, 96, 44–53. [Google Scholar] [CrossRef]
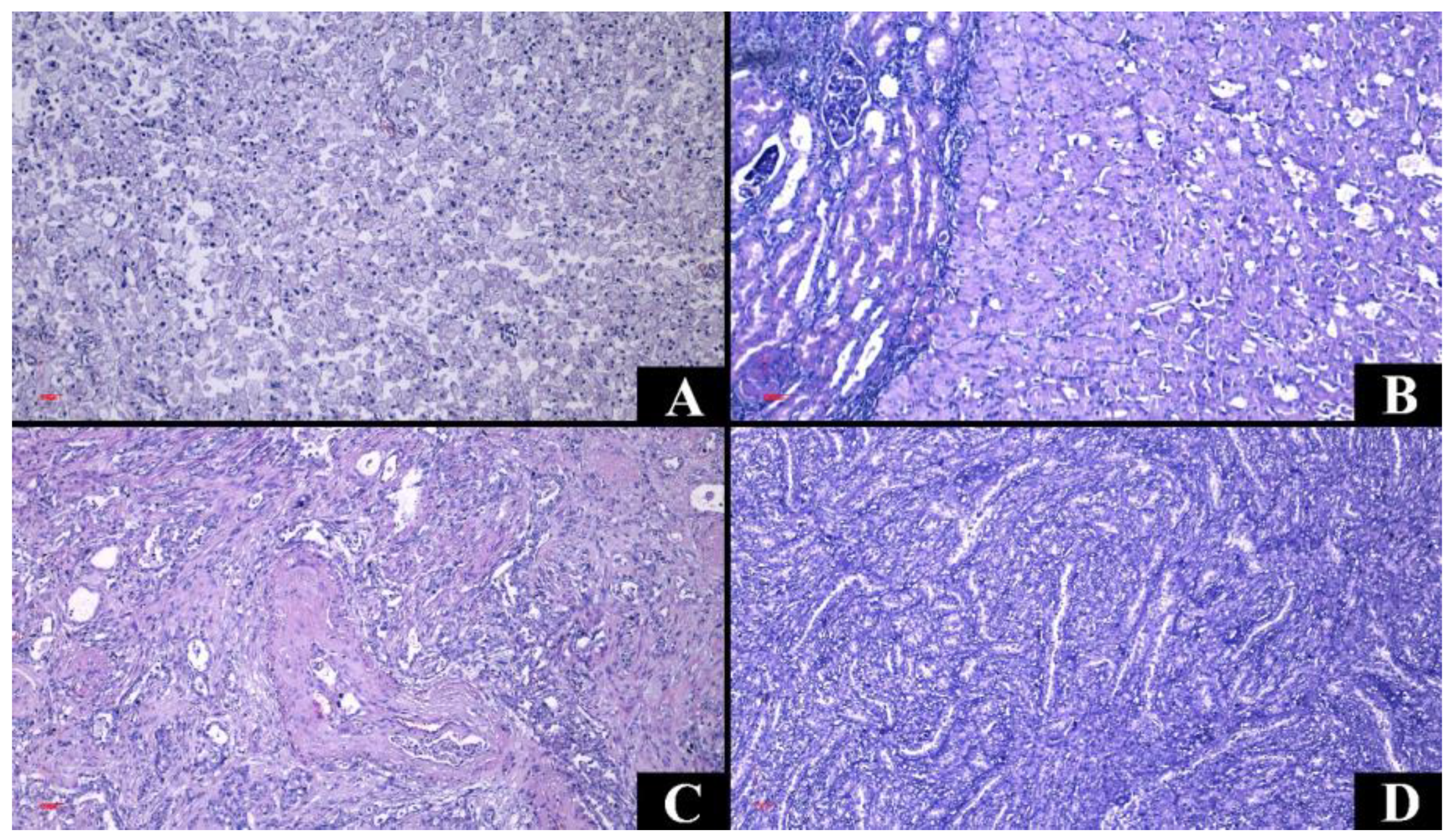
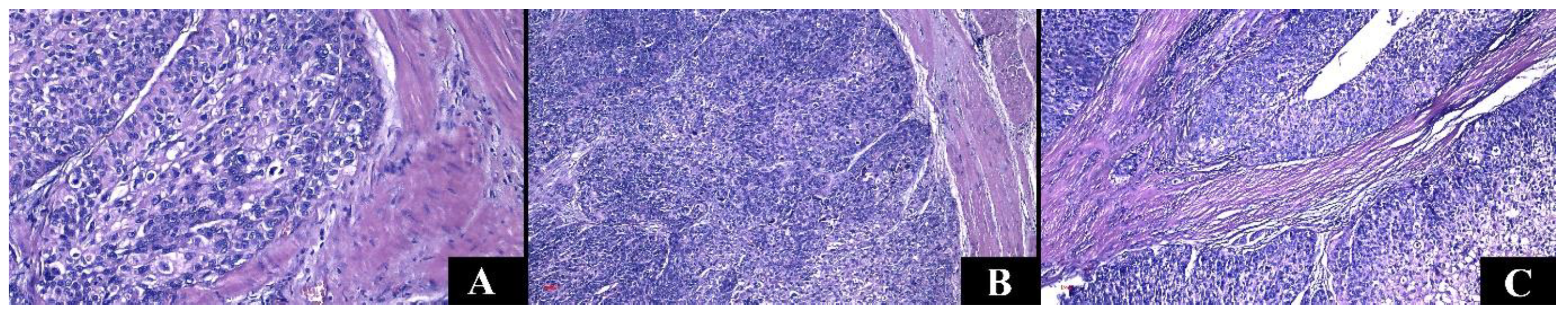
| Renal Cell Carcinomas | Upper Tract Urothelial Carcinomas | p-Value | |
|---|---|---|---|
| Age (years) | <0.001 | ||
| Median (percentiles 25–75) | 64 (56–70) | 73 (63–81) | |
| Min–max | 28–81 | 45–87 | |
| Decade (n, %) | 7, 38.85% | 9, 32.26% | <0.001 |
| Gender | 0.842 | ||
| Female | 42.68% | 38.71% | |
| Male | 57.32% | 61.29% | |
| Environment | 0.167 | ||
| Rural | 47.13% | 32.26% | |
| Urban | 52.87% | 67.74% | |
| Deaths | 24.85% | 45.16% | 0.029 |
| Renal Cell Carcinomas | Upper Tract Urothelial Carcinomas | p-Value | |
|---|---|---|---|
| Hematuria | 0.004 | ||
| Absence | 22.29% | 3.23% | |
| Microscopic | 36.94% | 25.81% | |
| Macroscopic | 40.77% | 70.97% | |
| Flank pain | 71.34% | 48.39% | 0.020 |
| Oligoanuria | 11.46% | 25.81% | 0.046 |
| Dysuria | 47.77% | 32.26% | 0.120 |
| High blood pressure | 59.23% | 70.97% | 0.313 |
| Diabetes mellitus | 24.84% | 25.81% | 0.910 |
| Obesity | 17.83% | 25.81% | 0.321 |
| Acute kidney injury | 27.39% | 64.52% | <0.001 |
| Serum creatinine (mg/dL) | <0.001 | ||
| Median (percentiles 25–75) | 0.88 (0.73–1.26) | 1.34 (1.02–1.72) | |
| Min–max | 0.29–12.62 | 0.69–26.16 | |
| Anemia | 26.75% | 70.97% | <0.001 |
| Mild | 52.38% | 40.91% | |
| Moderate | 33.33% | 40.91% | 0.721 |
| Severe | 14.29% | 18.18% | |
| Systemic inflammatory syndrome | 57.96% | 64.52% | 0.553 |
| Renal Cell Carcinomas | Upper Tract Urothelial Carcinomas | p-Value | |
|---|---|---|---|
| Weight (g) | 0.065 | ||
| Median (percentiles 25–75) | 339 (250–468) | 310 (230–350) | |
| Min–max | 120–1665 | 145–547 | |
| Length (cm) | 0.069 | ||
| Median (percentiles 25–75) | 12 (11–14) | 12 (11–13.50) | |
| Min–max | 7.5–21 | 8.5–18 | |
| Kidney | 0.845 | ||
| Left | 51.59% | 54.84% | |
| Right | 48.41% | 45.16% | |
| Localization | 0.065 | ||
| Superior | 40.13% | 25.81% | |
| Middle third/pelvis | 28.66% | 45.16% | |
| Inferior | 28.03% | 19.35% | |
| All | 3.18% | 9.68% |
| Renal Cell Carcinomas | Upper Tract Urothelial Carcinomas | p-Value | |
|---|---|---|---|
| Pseudoencapsulation | 91.08% | 0% | <0.001 |
| Cystic spaces | 52.87% | 6.45% | <0.001 |
| Infiltrative appearance | 24.20% | 87.10% | <0.001 |
| Exceeding the renal capsule | 17.19% | 6.45% | 0.176 |
| Invasion of the pyelocalyceal system | 14.65% | - | - |
| Maximum tumor diameter (cm) | |||
| Median (percentiles 25–75) | 5.5 (3.95–7.5) | 4.5 (3.5–5.8) | 0.025 |
| Min–max | 0.9–19 | 0.7–8 |
| Renal Cell Carcinomas | Upper Tract Urothelial Carcinomas | p-Value | |
|---|---|---|---|
| Tumor necrosis | 56.69% | 61.29% | 0.694 |
| Hemorrhagic infiltrate | 72.61% | 38.71% | 0.001 |
| Intratumoral inflammatory infiltrate | |||
| Acute | 2.55% | 0% | 0.41 |
| Mixed | 8.92% | 16.13% | |
| Chronic | 88.53% | 83.87% | |
| Angiolymphatic invasion | 22.92% | 48.39% | 0.007 |
| Perineural invasion | 7.64% | 29.03% | 0.002 |
| Histological grade | ISUP 2—41.40% | ||
| Low grade (1 and 2) | 55.41% | 38.71% | - |
| High grade (3 and 4) | 44.59% | 61.29% | 0.115 |
| pT | 3a—31.21% | 3—64.52% | - |
| Metastasis (n) | 2—lung | 2—bone | - |
| Complete resection | 98.73% | 87.10% | 0.007 |
| Adjacent renal parenchyma | 0.001 | ||
| Chronic pyelonephritis | 35.67% | 70.97% | |
| Normal | 31.85% | 3.23% | |
| Interstitial nephritis | 25.48% | 19.35% | |
| Hydronephrosis | 1.27% | 6.45% |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Parameter | Hazard Risk | p-Value | CI95% | Hazard Risk | p-Value | CI95% |
| Age | 1.073 | 0.002 | 1.027–1.121 | 1.084 | 0.002 | 1.031–1.139 |
| Exceeding the renal capsule | 2.504 | 0.040 | 1.045–6.003 | 1.255 | 0.693 | 0.407–3.871 |
| The lesion throughout the kidney | 10.000 | 0.046 | 1.045–95.683 | 5.110 | 0.238 | 0.341–76.590 |
| ILV | 2.444 | 0.029 | 1.096–5.448 | 1.635 | 0.344 | 0.591–4.519 |
| High grade | 2.172 | 0.039 | 1.039–4.537 | 2.242 | 0.076 | 0.919–5.469 |
| Acute kidney injury | 2.734 | 0.010 | 1.268–5.895 | 2.023 | 0.126 | 0.820–4.990 |
| Anemia | 2.875 | 0.007 | 1.329–6.219 | 2.838 | 0.028 | 1.122–7.179 |
| Univariate Analysis | |||
|---|---|---|---|
| Parameter | Hazard Risk | p-Value | CI95% |
| Perineural invasion | 7.500 | 0.029 | 1.228–45.807 |
| Acute kidney injury | 6.750 | 0.035 | 1.145–39.796 |
| Anemia | 11.556 | 0.033 | 1.223–109.185 |
| Hematuria | 10.448 | 0.038 | 1.138–95.926 |
| Tumor Type | Variants | Gross Aspect | Origin | Histologic Features |
|---|---|---|---|---|
| Clear cell renal cell carcinoma |
| Pseudoencapsulated Golden yellow Necrosis Hemorrhage | Tubular epithelium Proximal nephron | Nests and sheets of cells with clear cytoplasm. |
| Papillary renal cell carcinoma |
| Often pseudoencapsulated Solid or cystic Whitish Necrosis Hemorrhage | Tubular epithelium Distal nephron | Thin or thick papillae lined by uni- or pseudostratified cuboidal epithelium, foamy macrophages, and psammomatous bodies. |
| Chromophobe renal cell carcinoma |
| Well defined Gray to tan-brown Central scar | Intercalated cells of the distal tubules Distal nephron | Cells with prominent membrane and pale/eosinophilic cytoplasm. |
| Carcinoma of collecting ducts | Partially cystic Grayish-white | Collector tubes | Tubulopapillary architecture, hobnail cells, mucinous material, desmoplastic stroma. | |
| Noninvasive urothelial carcinoma |
| Flat or exophytic lesion | Urothelium | Varying degrees of cytoarchitectural atypia (fusion of papillae, disorganized tumor cells); cells with moderate or increased pleomorphism and mitotic activity. |
| Invasive urothelial carcinoma |
| Sessile, polypoid, nodular, or ulcerative lesion | Urothelium | Urothelial cells with high-grade atypia can associate divergent differentiation. Various architectures: papillary, micropapillary, nested, or tubular. |
| Therapeutic Management | Recommendations | Risks | |
|---|---|---|---|
| Active surveillance |
|
| |
| Ablative techniques |
|
|
|
| Surgical techniques |
|
|
|
|
|
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamesu, S.; Ursica, O.A.; Milea, S.E.; Deacu, M.; Aschie, M.; Mitroi, A.F.; Voinea, F.; Pundiche, M.B.; Orasanu, C.I.; Voda, R.I. Same Organ, Two Cancers: Complete Analysis of Renal Cell Carcinomas and Upper Tract Urothelial Carcinomas. Medicina 2024, 60, 1126. https://doi.org/10.3390/medicina60071126
Vamesu S, Ursica OA, Milea SE, Deacu M, Aschie M, Mitroi AF, Voinea F, Pundiche MB, Orasanu CI, Voda RI. Same Organ, Two Cancers: Complete Analysis of Renal Cell Carcinomas and Upper Tract Urothelial Carcinomas. Medicina. 2024; 60(7):1126. https://doi.org/10.3390/medicina60071126
Chicago/Turabian StyleVamesu, Sorin, Oana Andreea Ursica, Serban Eduard Milea, Mariana Deacu, Mariana Aschie, Anca Florentina Mitroi, Felix Voinea, Mihaela Butcaru Pundiche, Cristian Ionut Orasanu, and Raluca Ioana Voda. 2024. "Same Organ, Two Cancers: Complete Analysis of Renal Cell Carcinomas and Upper Tract Urothelial Carcinomas" Medicina 60, no. 7: 1126. https://doi.org/10.3390/medicina60071126
APA StyleVamesu, S., Ursica, O. A., Milea, S. E., Deacu, M., Aschie, M., Mitroi, A. F., Voinea, F., Pundiche, M. B., Orasanu, C. I., & Voda, R. I. (2024). Same Organ, Two Cancers: Complete Analysis of Renal Cell Carcinomas and Upper Tract Urothelial Carcinomas. Medicina, 60(7), 1126. https://doi.org/10.3390/medicina60071126






