Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in the Management of Colorectal Cancer with Peritoneal Metastasis: A Single-Center Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Technique
2.2. Statistical Analysis
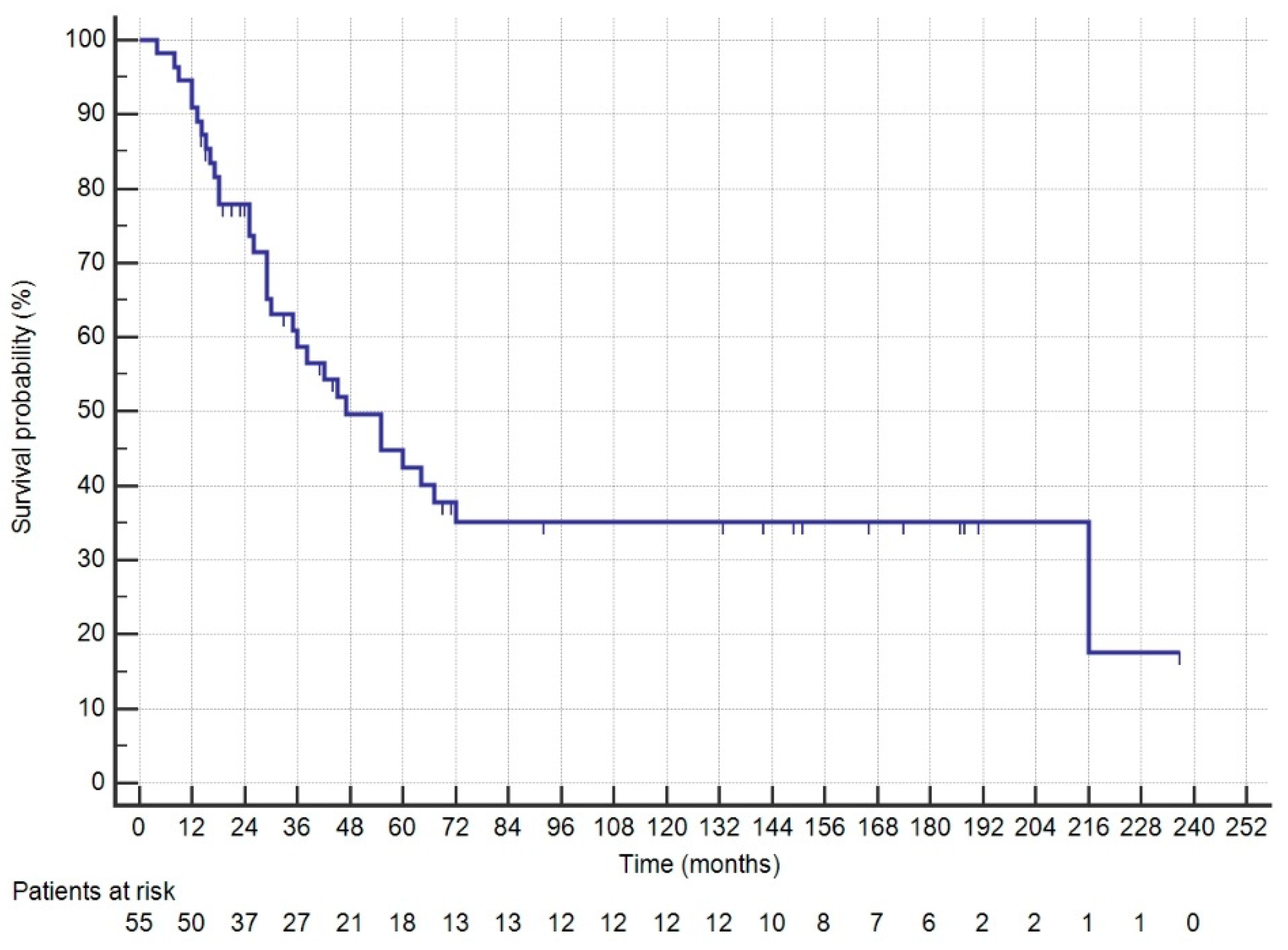
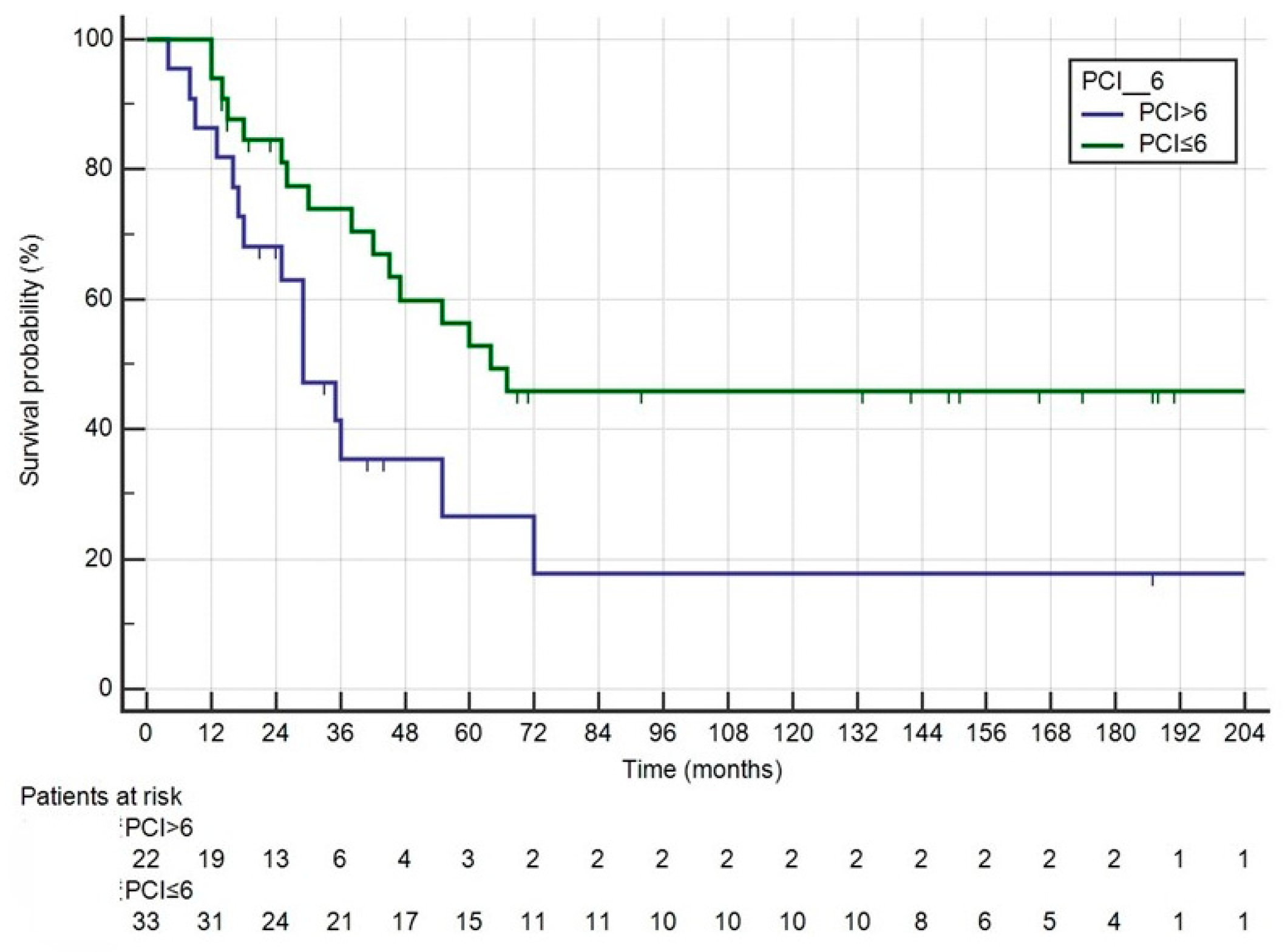
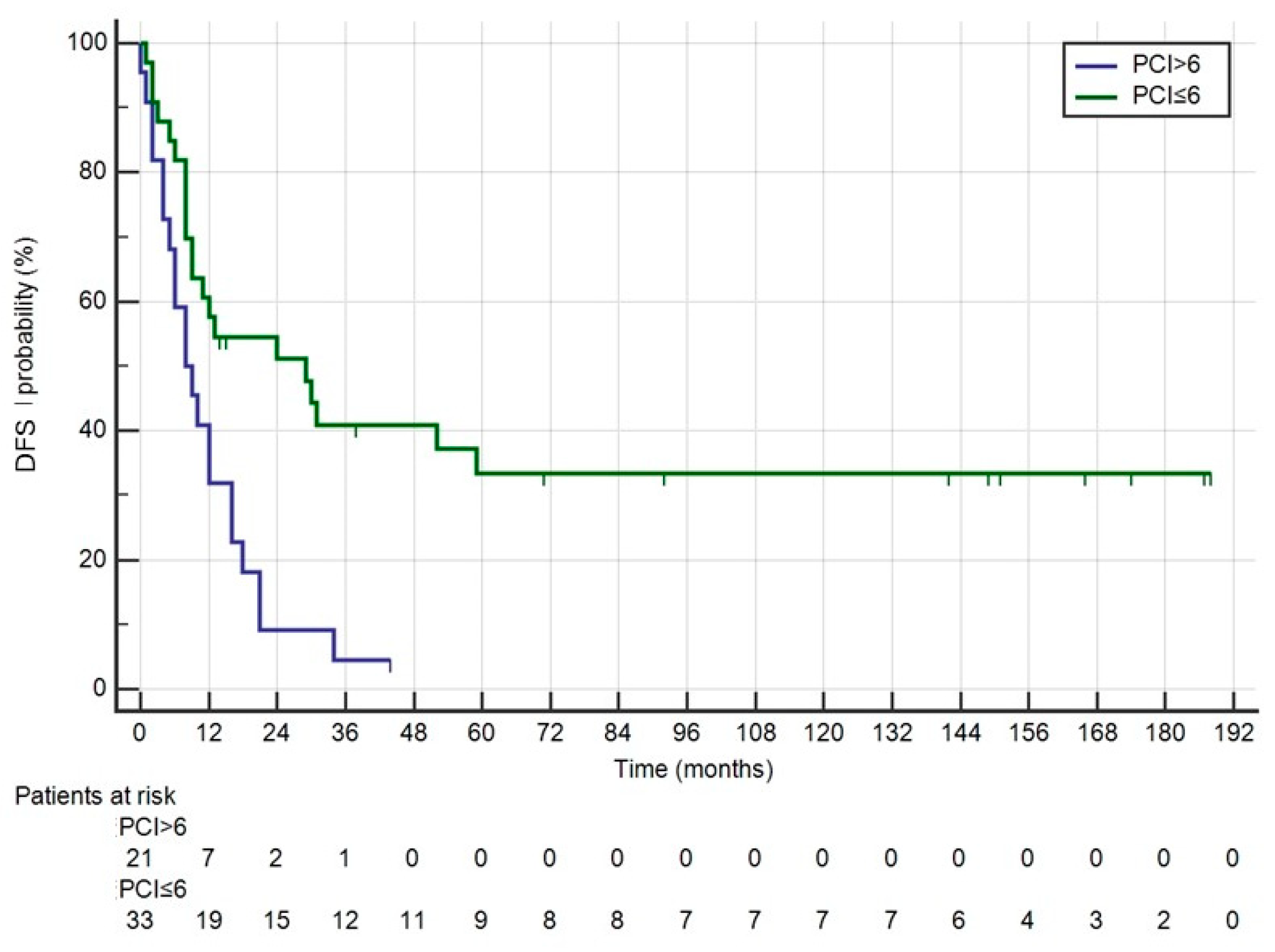
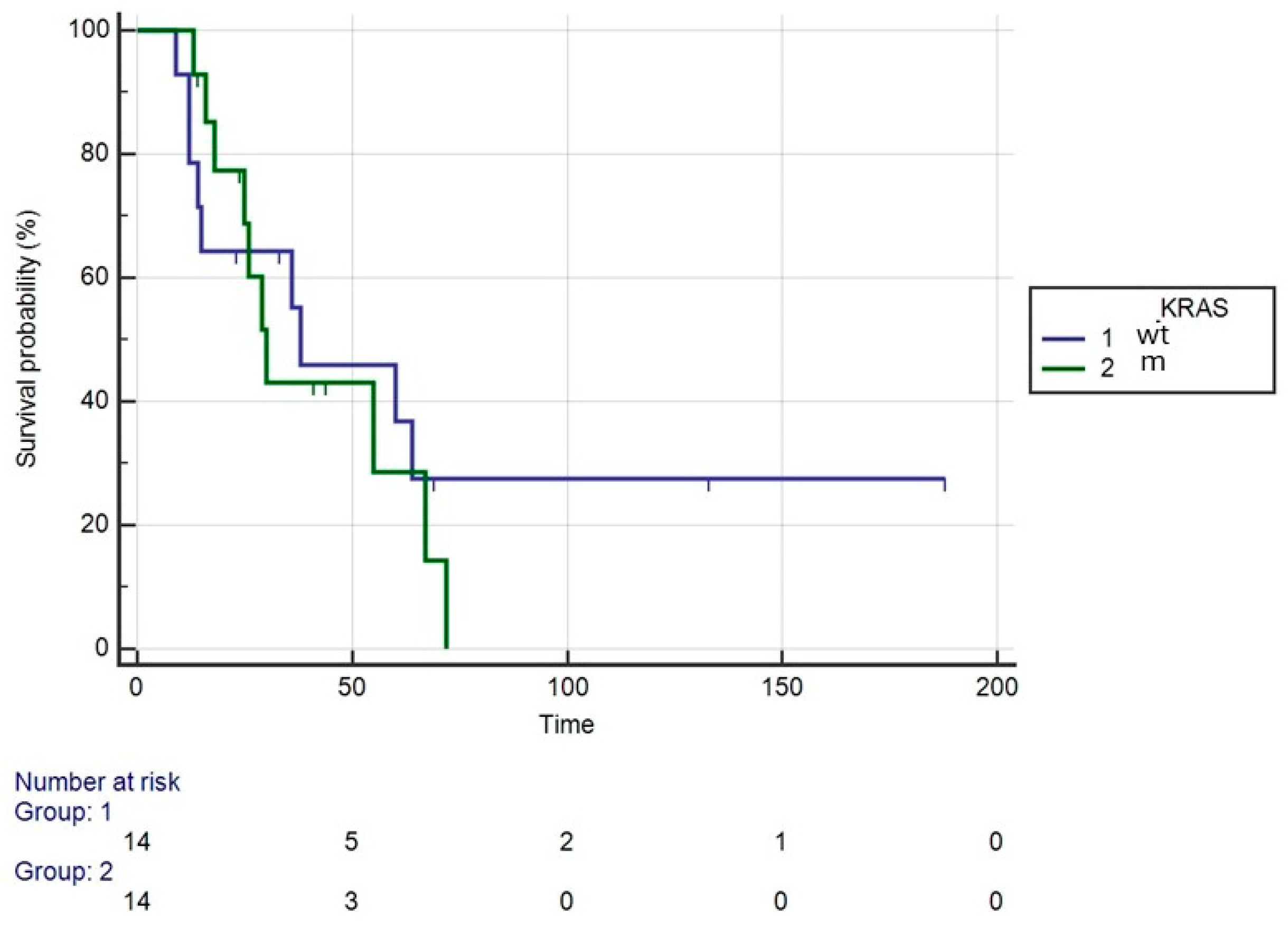
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, I.; Coebergh van den Braak, R.R.J.; Van Vugt, J.L.A.; Ijzermans, J.N.M.; Buettner, S. Actual survival after resection of primary colorectal cancer: Results from a prospective multicenter study. World J. Surg. Oncol. 2021, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Koppe, M.J.; Boerman, O.C.; Oyen, W.J.; Bleichrodt, R.P. Peritoneal carcinomatosis of colorectal origin: Incidence and current treatment strategies. Ann. Surg. 2006, 243, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, S.; Lepage, C.; Hatem, C.; Coatmeur, O.; Faivre, J.; Bouvier, A.M. Epidemiology and management of liver metastases from colorectal cancer. Ann. Surg. 2006, 244, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Liu, Q.; Yu, W.; Ma, Y.; Zhu, J.; Lian, P.; Cai, S.; Li, Q.; Li, X. Prognostic value of distant metastasis sites and surgery in stage IV colorectal cancer: A population-based study. Int. J. Colorectal Dis. 2018, 33, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, Y.; Qin, X.; Lv, Z.; Wang, H.; Wu, D.; Yuan, Z.; Wang, H. Development and Validation of a Prognostic Nomogram for Colorectal Cancer Patients with Synchronous Peritoneal Metastasis. Front. Oncol. 2021, 11, 615321. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Osinsky, D.; Beaujard, A.C.; Gilly, F.N. Natural history of peritoneal carcinomatosis from nongynecologic malignancies. Surg. Oncol. Clin. N. Am. 2003, 12, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Cunliffe, W.J.; Belliveau, J.; De Bruijn, E.A.; Graves, T.; Mullins, R.E.A.; Schlag, P. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin. Oncol. 1989, 16, 83–97. [Google Scholar] [PubMed]
- Sugarbaker, P.H. Peritonectomy procedures. Surg. Oncol. Clin. N. Am. 2003, 12, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Cotte, E.; Kusamura, S.; Deraco, M.; Baratti, D.; Passot, G.; Beaujard, A.C.; Noel, G.F. Hyperthermic intraperitoneal chemotherapy: Nomenclature and modalities of perfusion. J. Surg. Oncol. 2008, 98, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, N.; Kumar, B.R.; Pookunju, A.P.; Srinivasan, A.; Mahajan, V. Preliminary Experience and Morbidity Analysis of Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CRS/HIPEC) from a Tertiary Cancer Center in India. J. Clin. Diagn. Res. 2015, 9, Xc09–Xc13. [Google Scholar] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Cashin, P.; Sugarbaker, P.H. Hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal and appendiceal peritoneal metastases: Lessons learned from PRODIGE 7. J. Gastrointest. Oncol. 2021, 12, S8–S120. [Google Scholar] [CrossRef] [PubMed]
- Eftimie, M.A.; Potlog, G.; Alexandrescu, S.T. Surgical Options for Peritoneal Surface Metastases from Digestive Malignancies—A Comprehensive Review. Medicina 2023, 59, 255. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.M.; Ferron, G.; Guilloit, J.M.; Meeus, P.; Goéré, D.; et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J. Clin. Oncol. 2009, 27, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Solaini, L.; D’Acapito, F.; Passardi, A.; Framarini, M.; Tauceri, F.; Di Pietrantonio, D.; Frassineti, G.L.; Casadei Gardini, A.; Cucchetti, A.; Cavaliere, D.; et al. Cytoreduction plus hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis in colorectal cancer patients: A single-center cohort study. World J. Surg. Oncol. 2019, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Peritonectomy procedures. Cancer Treat. Res. 1996, 82, 235–253. [Google Scholar] [PubMed]
- Sugarbaker, P.H. A curative approach to peritoneal carcinomatosis from colorectal cancer. Semin. Oncol. 2005, 32, S68–S73. [Google Scholar] [CrossRef] [PubMed]
- Aziz, O.; Jaradat, I.; Chakrabarty, B.; Selvasekar, C.R.; Fulford, P.E.; Saunders, M.P.; Renehan, A.G.; Wilson, M.S.; O’Dwyer, S.T. Predicting Survival After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Appendix Adenocarcinoma. Dis. Colon. Rectum 2018, 61, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Quenet, F.; Elias, D.; Roca, L.; Goere, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J. Clin. Oncol. 2018, 36, 3503. [Google Scholar] [CrossRef]
- Esquivel, J.; Lowy, A.M.; Markman, M.; Chua, T.; Pelz, J.; Baratti, D.; Baumgartner, J.M.; Berri, R.; Bretcha-Boix, P.; Deraco, M.; et al. The American Society of Peritoneal Surface Malignancies (ASPSM) Multiinstitution Evaluation of the Peritoneal Surface Disease Severity Score (PSDSS) in 1013 Patients with Colorectal Cancer with Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2014, 21, 4195–4201. [Google Scholar] [CrossRef] [PubMed]
- Prada-Villaverde, A.; Esquivel, J.; Lowy, A.M.; Markman, M.; Chua, T.; Pelz, J.; Baratti, D.; Baumgartner, J.M.; Berri, R.; Bretcha-Boix, P.; et al. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with Mitomycin C versus Oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. J. Surg. Oncol. 2014, 110, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Cashin, P.H.; Esquivel, J.; Larsen, S.G.; Liauw, W.; Alzahrani, N.A.; Morris, D.L.; Kepenekian, V.; Sourrouille, I.; Dumont, F.; Tuech, J.J.; et al. Perioperative chemotherapy in colorectal cancer with peritoneal metastases: A global propensity score matched study. EClinicalMedicine 2023, 55, 101746. [Google Scholar] [CrossRef] [PubMed]
- Hentzen, J.; Rovers, K.P.; Kuipers, H.; van der Plas, W.Y.; Been, L.B.; Hoogwater, F.J.; van Ginkel, R.J.; Hemmer, P.H.; van Dam, G.M.; de Hingh, I.H.; et al. Impact of Synchronous Versus Metachronous Onset of Colorectal Peritoneal Metastases on Survival Outcomes After Cytoreductive Surgery (CRS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC): A Multicenter, Retrospective, Observational Study. Ann. Surg. Oncol. 2019, 26, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Fisher, O.M.; Brown, C.; Esquivel, J.; Larsen, S.G.; Liauw, W.; Alzahrani, N.A.; Morris, D.L.; Kepenekian, V.; Sourrouille, I.; Dumont, F.; et al. Hyperthermic intraperitoneal chemotherapy in colorectal cancer. BJS Open 2024, 8, zrae017. [Google Scholar] [CrossRef]
- Facy, O.; Combier, C.; Poussier, M.; Magnin, G.; Ladoire, S.; Ghiringhelli, F.; Chauffert, B.; Rat, P.; Ortega-Deballon, P. High pressure does not counterbalance the advantages of open techniques over closed techniques during heated intraperitoneal chemotherapy with oxaliplatin. Surgery 2015, 157, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Sánchez, A.; Espinosa-Redondo, E.; Gutiérrez-Calvo, A.; Segura-Sampedro, J.J.; Pérez-Viejo, E.; Concepción-Martín, V.; Sánchez-García, S.; García-Fadrique, A.; Prieto-Nieto, I.; Barrios-Sanchez, P.; et al. Efficacy and Safety of Intraoperative Hyperthermic Intraperitoneal Chemotherapy for Locally Advanced Colon Cancer: A Phase 3 Randomized Clinical Trial. JAMA Surg. 2023, 158, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Tonello, M.; Baratti, D.; Sammartino, P.; Di Giorgio, A.; Robella, M.; Sassaroli, C.; Framarini, M.; Valle, M.; Macrì, A.; Graziosi, L.; et al. Prognostic value of specific KRAS mutations in patients with colorectal peritoneal metastases. ESMO Open 2024, 9, 102976. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.M.; Mansvelt, B.; Lorimier, G.; Dube, P.; Glehen, O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Tonello, M.; Baratti, D.; Sammartino, P.; Di Giorgio, A.; Robella, M.; Sassaroli, C.; Framarini, M.; Valle, M.; Macrì, A.; Graziosi, L.; et al. Microsatellite and RAS/RAF Mutational Status as Prognostic Factors in Colorectal Peritoneal Metastases Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann. Surg. Oncol. 2022, 29, 3405–3417. [Google Scholar] [CrossRef] [PubMed]
- Wajekar, A.S.; Solanki, S.L.; Patil, V.P. Postoperative complications and critical care management after cytoreduction surgery and hyperthermic intraperitoneal chemotherapy: A systematic review of the literature. World J. Crit. Care Med. 2022, 11, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Macfie, R.C.; Cha, D.E.; Gleeson, E.; Yu, A.; Cohen, N.; Sarpel, U.; Golas, B.; Hiotis, S.; Labow, D. Hyperthermic intraperitoneal chemotherapy does not increase risk of major complication or failure to rescue in cytoreductive surgery. J. Surg. Oncol. 2022, 126, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.M.; Sleightholm, R.; Patel, A.; Shostrom, V.; Hall, B.; Neilsen, B.; Bartlett, D.; Smith, L. Morbidity and Mortality Rates Following Cytoreductive Surgery Combined with Hyperthermic Intraperitoneal Chemotherapy Compared with Other High-Risk Surgical Oncology Procedures. JAMA Netw. Open 2019, 2, e186847. [Google Scholar] [CrossRef] [PubMed]
| CRS + HIPEC Given to 55 Patients | CRS + HIPEC According to Prodige7 Criteria 52 Patients | Prodige7-Weighted Pseudopopulation CRS without HIPEC (n = 52) | p * | |
|---|---|---|---|---|
| Age (median) | 61 | 61.2 | 59 (50–66) | 0.510 |
| Sex | 0.844 | |||
| Male | 31 | 28 | 26 | |
| Female | 24 | 24 | 26 | |
| Primary tutor localization | 0.959 | |||
| Right | 20 | 19 | 20 | |
| Transverse | 2 | 2 | 3 | |
| Left | 29 | 27 | 25 | |
| Rectum | 4 | 4 | 4 | |
| No specific info | 0 | 0 | 0 | |
| Synchronous peritoneal metastases | 13 | 12 | 12 | |
| Previous surgery | 0.856 | |||
| For primary tumor | 35 | 33 | 40 | |
| For peritoneal metastases | 7 | 7 | 8 | |
| Previous cht | 0.662 | |||
| For primary tumor | 28 | 27 | 25 | |
| For peritoneal metastases | 0 | 0 | 0 | |
| OX in cht schedule | 22 | 19 | 23 | |
| Systemic cht-HIPEC | 0.008 | |||
| No cht | 6 | 6 | 2 | |
| Pre-operative | 11 | 10 | 9 | |
| Post-operative | 18 | 18 | 7 | |
| Both | 20 | 18 | 34 | |
| CCS | 1.000 | |||
| CC0 | 53 | 52 | 52 | |
| CC1 | 2 | 0 | 0 | |
| PCI | 0.070 | |||
| Median | 6 (1–26) | 5.5 (1–21) | ||
| <11 | 40 | 40 | 30 | |
| 11–15 | 10 | 10 | 21 | |
| >15 | 5 | 2 | 2 | |
| Time from diagnosis of PM to surgery, days (median) | 120 | 120 | 210 (60–330) | 0.042 |
| Duration of surgery (min, median) | 620 | 620 | 620 (540–680) | 0.865 |
| LOS (days, median) | 15 | 15 | 14 (12–18) | |
| Re-intervention | 3 | 3 | 3 | 1.000 |
| Mortality | 0 | 0 | 0 | 1.000 |
| Clavien–Dindo > 2 | 9 | 9 | 7 | 0.786 |
| Sites of Recurrence | Pts | % |
|---|---|---|
| No recurrence | 13 | 23.8 |
| Peritoneum only | 4 | 7.2 |
| Peritoneum + other sites | 33 | 60 |
| Liver only | 1 | 1.8 |
| Lung only | 1 | 1.8 |
| Nodes only | 1 | 1.8 |
| Multiple sites without peritoneum | 2 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Acapito, F.; Framarini, M.; Pietrantonio, D.D.; Tauceri, F.; Zucchini, V.; Pozzi, E.; Solaini, L.; Ercolani, G. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in the Management of Colorectal Cancer with Peritoneal Metastasis: A Single-Center Cohort Study. Medicina 2024, 60, 1058. https://doi.org/10.3390/medicina60071058
D’Acapito F, Framarini M, Pietrantonio DD, Tauceri F, Zucchini V, Pozzi E, Solaini L, Ercolani G. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in the Management of Colorectal Cancer with Peritoneal Metastasis: A Single-Center Cohort Study. Medicina. 2024; 60(7):1058. https://doi.org/10.3390/medicina60071058
Chicago/Turabian StyleD’Acapito, Fabrizio, Massimo Framarini, Daniela Di Pietrantonio, Francesca Tauceri, Valentina Zucchini, Eleonora Pozzi, Leonardo Solaini, and Giorgio Ercolani. 2024. "Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in the Management of Colorectal Cancer with Peritoneal Metastasis: A Single-Center Cohort Study" Medicina 60, no. 7: 1058. https://doi.org/10.3390/medicina60071058
APA StyleD’Acapito, F., Framarini, M., Pietrantonio, D. D., Tauceri, F., Zucchini, V., Pozzi, E., Solaini, L., & Ercolani, G. (2024). Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in the Management of Colorectal Cancer with Peritoneal Metastasis: A Single-Center Cohort Study. Medicina, 60(7), 1058. https://doi.org/10.3390/medicina60071058







