Drug-Related Problems and Recommendations Made during Home Medicines Reviews for Sick Day Medication Management in Australia
Abstract
1. Introduction
- (1)
- Describe the characteristics of DRP identified by pharmacists, especially those relating to SADMANS medications and inappropriate use of medications as per kidney function.
- (2)
- Describe recommendations made by pharmacists to general practitioners, including any recommendations to withhold medications during an acute illness.
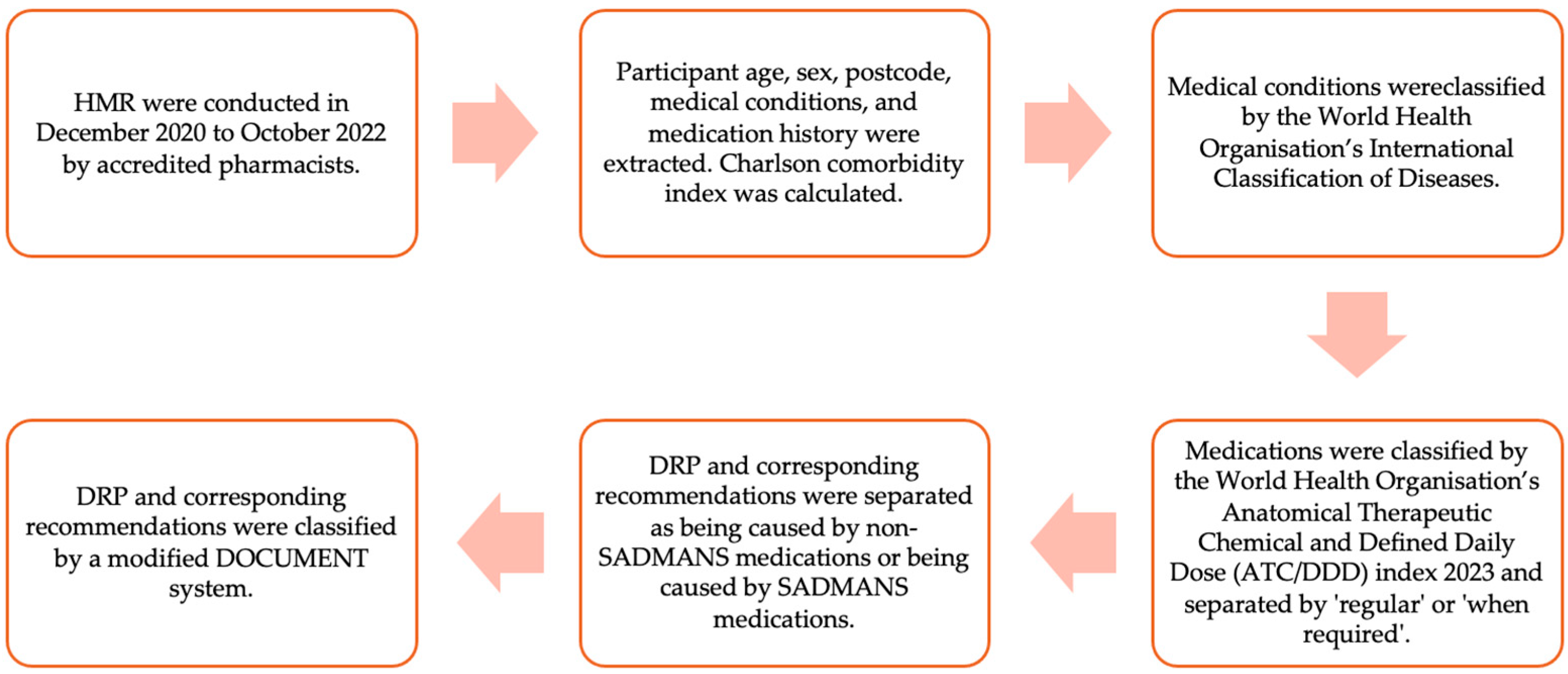
2. Materials and Methods
2.1. Sampling, Study Population, and Ethics
2.2. Data Extraction, Coding, and Exclusion Criteria
2.3. Data Handing
3. Results
3.1. Non-SADMANS DRP and Recommendations Made by Pharmacists
3.2. SADMANS Related DRP, Recommendations, and SDMG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, R.; Ellett, L.M.K.; Semple, S.; Roughead, E.E. The Extent of Medication-Related Hospital Admissions in Australia: A Review from 1988 to 2021. Drug Saf. 2022, 45, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Nivya, K.; Sri Sai Kiran, V.; Ragoo, N.; Jayaprakash, B.; Sonal Sekhar, M. Systemic review on drug related hospital admissions—A pubmed based search. Saudi Pharm. J. 2015, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ernst, F.R.; Grizzle, A.J. Drug-related morbidity and mortality: Updating the cost-of-illness model. J. Am. Pharm. Assoc. 2001, 41, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.; Villani, E.R.; Vetrano, D.L.; Cherubini, A.; Cruz-Jentoft, A.J.; Curtin, D.; Denkinger, M.; Gutiérrez-Valencia, M.; Guðmundsson, A.; Knol, W.; et al. Association of polypharmacy and hyperpolypharmacy with frailty states: A systematic review and meta-analysis. Eur. Geriatr. Med. 2019, 10, 9–36. [Google Scholar] [CrossRef] [PubMed]
- Dorj, G.; Nair, N.P.; Bereznicki, L.; Kelly, T.-L.; Pratt, N.; Kalisch-Ellett, L.; Andrade, A.; Rowett, D.; Whitehouse, J.; Widagdo, I.; et al. Risk factors predictive of adverse drug events and drug-related falls in aged care residents: Secondary analysis from the ReMInDAR trial. Drugs Aging 2023, 40, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Bárcena, P.; Lord, A.; Lizotte, A.; Berbiche, D.; Lalonde, L. Prevalence and Management of Drug-Related Problems in Chronic Kidney Disease Patients by Severity Level: A Subanalysis of a Cluster Randomized Controlled Trial in Community Pharmacies. J. Manag. Care Spec. Pharm. 2018, 24, 173–181. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, P.; Cherney, D.; Gilbert, R.E.; Senior, P. Chronic Kidney Disease in Diabetes. Can. J. Diabetes 2018, 42, S201–S209. [Google Scholar] [CrossRef] [PubMed]
- Subeesh, V.K.; Abraham, R.; Satya Sai, M.V.; Koonisetty, K.S. Evaluation of prescribing practices and drug-related problems in chronic kidney disease patients: A cross-sectional study. Perspect. Clin. Res. 2020, 11, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Alruqayb, W.S.; Price, M.J.; Paudyal, V.; Cox, A.R. Drug-Related Problems in Hospitalised Patients with Chronic Kidney Disease: A Systematic Review. Drug Saf. 2021, 44, 1041–1058. [Google Scholar] [CrossRef]
- Lea-Henry, T.N.; Carland, J.E.; Stocker, S.L.; Sevastos, J.; Roberts, D.M. Clinical pharmacokinetics in kidney disease: Fundamental principles. Clin. J. Am. Soc. Nephrol. 2018, 13, 1085–1095. [Google Scholar] [CrossRef]
- Tesfaye, W.H.; Castelino, R.L.; Wimmer, B.C.; Zaidi, S.T.R. Inappropriate prescribing in chronic kidney disease: A systematic review of prevalence, associated clinical outcomes and impact of interventions. Int. J. Clin. Pract. 2017, 71, e12960. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.E.; Dhaliwal, K.; McMurtry, E.; Donald, T.; Lamont, N.; Benterud, E.; Kung, J.Y.; Robertshaw, S.; Verdin, N.; Drall, K.M.; et al. Sick Day Medication Guidance for People With Diabetes, Kidney Disease, or Cardiovascular Disease: A Systematic Scoping Review. Kidney Med. 2022, 4, 100491. [Google Scholar] [CrossRef]
- Faber, S.J.; Scherpbier, N.D.; Peters, H.J.G.; Uijen, A.A. Preventing acute kidney injury in high-risk patients by temporarily discontinuing medication—An observational study in general practice. BMC Nephrol. 2019, 20, 449. [Google Scholar] [CrossRef] [PubMed]
- Lea-Henry, T.N.; Baird-Gunning, J.; Petzel, E.; Roberts, D.M. Medication management on sick days. Aust. Prescr. 2017, 40, 168–173. [Google Scholar] [CrossRef]
- Goldberg, R.; Dennen, P. Long-Term Outcomes of Acute Kidney Injury. Adv. Chronic Kidney Dis. 2008, 15, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G.; Yusuf, B.; Shlipak, M.G.; Garg, A.X.; Parikh, C.R. Long-term Risk of Mortality and Other Adverse Outcomes after Acute Kidney Injury: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2009, 53, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Kidney Health Australia. How to Sick Day Action Plan; Kidney Health Australia: Victoria, Australia, 2022; Available online: https://kidney.org.au/uploads/resources/KHA-How-To-Sick-Day-Action-Plan-FINAL.pdf (accessed on 2 November 2023).
- Kidneys, T. “Sick Day” Guidance in Patients at Risk of Acute Kidney Injury: A Position Statement from the Think Kidneys Board. Available online: https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf (accessed on 23 October 2023).
- Duong, H.; Tesfaye, W.; Van, C.; Sud, K.; Truong, M.; Krass, I.; Castelino, R.L. Sick day management in people with chronic kidney disease: A scoping review. J. Nephrol. 2022, 36, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Vinks, T.H.A.M.; Egberts, T.C.G.; de Lange, T.M.; de Koning, F.H.P. Pharmacist-Based Medication Review Reduces Potential Drug-Related Problems in the Elderly. Drugs Aging 2009, 26, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.F. Pharmacist-Led Home Medicines Review and Residential Medication Management Review: The Australian Model. Drugs Aging 2016, 33, 199–204. [Google Scholar] [CrossRef]
- Gudi, S.K.; Kashyap, A.; Chhabra, M.; Rashid, M.; Tiwari, K.K. Impact of pharmacist-led home medicines review services on drug-related problems among the elderly population: A systematic review. Epidemiol. Health 2019, 41, e2019020. [Google Scholar] [CrossRef]
- Duong, H.; Tesfaye, W.; Van, C.; Sud, K.; Castelino, R.L. Hospitalisation Due to Community-Acquired Acute Kidney Injury and the Role of Medications: A Retrospective Audit. J. Clin. Med. 2023, 12, 3347. [Google Scholar] [CrossRef] [PubMed]
- Tso, M.; Sud, K.; Van, C.; Tesfaye, W.; Castelino, R.L. Clinical characteristics and outcomes of community acquired-acute kidney injury. Int. Urol. Nephrol. 2023, 55, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- WHO. ICD-11 Coding Tool Mortality and Morbidity Statistics (MMS); WHO: Geneva, Switzerland, 2023; Available online: https://icd.who.int/ct11/icd11_mms/en/release (accessed on 1 March 2023).
- Pharmaceutical Society of Australia. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef] [PubMed]
- MDCalc. Charlson Comorbidity Index; MD Calc: New York, NY, USA, 2023; Available online: https://www.mdcalc.com/calc/3917/charlson-comorbidity-index-cci (accessed on 1 March 2023).
- Mthodology. WCCfDS. In Anatomical Therapeutic Chemical (ATC) Classification System; Norweigan Institute of Public Health: Oslo, Norway, 2022; Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 1 March 2023).
- Williams, M.; Peterson, G.M.; Tenni, P.C.; Bindoff, I.K.; Stafford, A.C. Document: A system for classifying drug-related problems in community pharmacy. Int. J. Clin. Pharm. 2012, 34, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Truong, M.; Tesfaye, W.; Sud, K.; Van, C.; Seth, S.; Croker, N.; Castelino, R.L. Drug-Related Problems and Sick Day Management Considerations for Medications that Contribute to the Risk of Acute Kidney Injury. J. Clin. Med. 2024, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chen, N.; Wang, R.R.; Li, L.; Jiang, S.P. Inappropriateness of medication prescriptions about chronic kidney disease patients without dialysis therapy in a Chinese tertiary teaching hospital. Ther. Clin. Risk Manag. 2016, 12, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Quek, H.W.; Etherton-Beer, C.; Page, A.; McLachlan, A.J.; Lo, S.Y.; Naganathan, V.; Kearney, L.; Hilmer, S.N.; Comans, T.; Mangin, D.; et al. Deprescribing for older people living in residential aged care facilities: Pharmacist recommendations, doctor acceptance and implementation. Arch. Gerontol. Geriatr. 2023, 107, 104910. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.E.; Spiers, G.; Kingston, A.; Todd, A.; Adamson, J.; Hanratty, B. Adverse Outcomes of Polypharmacy in Older People: Systematic Review of Reviews. J. Am. Med. Dir. Assoc. 2020, 21, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.H.; Wang, K.N.; Sluggett, J.K.; Ilomäki, J.; Hilmer, S.N.; Corlis, M.; Bell, J.S. Process, impact and outcomes of medication review in Australian residential aged care facilities: A systematic review. Australas. J. Ageing 2019, 38 (Suppl. S2), 9–25. [Google Scholar] [CrossRef]
- Al-Babtain, B.; Cheema, E.; Hadi, M.A. Impact of community-pharmacist-led medication review programmes on patient outcomes: A systematic review and meta-analysis of randomised controlled trials. Res. Soc. Adm. Pharm. 2022, 18, 2559–2568. [Google Scholar] [CrossRef]
- Nishtala, P.S.; Hilmer, S.N.; McLachlan, A.J.; Hannan, P.J.; Chen, T.F. Impact of Residential Medication Management Reviews on Drug Burden Index in Aged-Care Homes. Drugs Aging 2009, 26, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Stafford, A.C.; Tenni, P.C.; Peterson, G.M.; Jackson, S.L.; Hejlesen, A.; Villesen, C.; Rasmussen, M. Drug-related problems identified in medication reviews by Australian pharmacists. Pharm. World Sci. 2009, 31, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Bolmsjö, B.B.; Palagyi, A.; Keay, L.; Potter, J.; Lindley, R.I. Factors influencing deprescribing for residents in Advanced Care Facilities: Insights from General Practitioners in Australia and Sweden. BMC Fam. Pract. 2016, 17, 152. [Google Scholar] [CrossRef] [PubMed]
- Plácido, A.I.; Herdeiro, M.T.; Morgado, M.; Figueiras, A.; Roque, F. Drug-related Problems in Home-dwelling Older Adults: A Systematic Review. Clin. Ther. 2020, 42, 559–572.e514. [Google Scholar] [CrossRef] [PubMed]
- Runciman, W.B.; Roughead, E.E.; Semple, S.J.; Adams, R.J. Adverse drug events and medication errors in Australia. Int. J. Qual. Health Care 2003, 15 (Suppl. S1), i49–i59. [Google Scholar] [CrossRef] [PubMed]
- Castelino, R.L.; Hilmer, S.N.; Bajorek, B.V.; Nishtala, P.; Chen, T.F. Drug Burden Index and Potentially Inappropriate Medications in Community-Dwelling Older People. Drugs Aging 2010, 27, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Tabeefar, H.; Chang, F.; Cooke, M.; Patel, T. Community pharmacists and chronic pain: A qualitative study of experience, perception, and challenges. Can. J. Pain 2020, 4, 29–39. [Google Scholar] [CrossRef]
- Freeman, C.R.; Cottrell, W.N.; Kyle, G.; Williams, I.D.; Nissen, L. An evaluation of medication review reports across different settings. Int. J. Clin. Pharm. 2013, 35, 5–13. [Google Scholar] [CrossRef] [PubMed]
- San-José, A.; Agustí, A.; Vidal, X.; Formiga, F.; López-Soto, A.; Fernández-Moyano, A.; García, J.; Ramírez-Duque, N.; Torres, O.H.; Barbé, J. Inappropriate prescribing to older patients admitted to hospital: A comparison of different tools of misprescribing and underprescribing. Eur. J. Intern. Med. 2014, 25, 710–716. [Google Scholar] [CrossRef]
- Pepe, G.M.; Kaefer, T.N.; Goode, J.-V.K.R. Impact of pharmacist identification of medication-related problems in a nontraditional long-term care pharmacy. J. Am. Pharm. Assoc. 2018, 58, S51–S54. [Google Scholar] [CrossRef]
- van Boven, J.F.M.; Ryan, D.; Eakin, M.N.; Canonica, G.W.; Barot, A.; Foster, J.M. Enhancing Respiratory Medication Adherence: The Role of Health Care Professionals and Cost-Effectiveness Considerations. J. Allergy Clin. Immunol. Pract. 2016, 4, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical Society of Australia. Guidelines for Comprehensive Medication Management Reviews; Pharmaceutical Society of Australia: Canberra, Australia, 2020. [Google Scholar]
- Gheewala, P.A.; Peterson, G.M.; Curtain, C.M.; Nishtala, P.S.; Hannan, P.J.; Castelino, R.L. Impact of the Pharmacist Medication Review Services on Drug-Related Problems and Potentially Inappropriate Prescribing of Renally Cleared Medications in Residents of Aged Care Facilities. Drugs Aging 2014, 31, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Vicary, D.; Hutchison, C.; Aspden, T. Avoiding acute kidney injury in primary care: Attitudes and behaviours of general practitioners and community pharmacists in Hawke’s Bay. J. Prim. Health Care 2020, 12, 244–256. [Google Scholar] [CrossRef] [PubMed]
| Demographic Information (n = 201) | Value |
|---|---|
| Median age (years) (IQR) | 69 (24) |
| Sex (%) | |
| Female | 96 (47.8%) |
| Male | 105 (52.2%) |
| Remoteness (%) | |
| Major cities | 147 (73.1%) |
| Regional | 54 (26.9%) |
| Mean (±SD) number of medical conditions | 7.4 ± 3.0 |
| Top five medical conditions (n = 1482) | |
| 245 (16.5%) |
| 214 (14.4%) |
| 202 (13.6%) |
| 181 (12.2%) |
| 125 (8.4%) |
| Mean (±SD) number of regular medications | 10.7 ± 4.0 |
| Top five regular medications used (n = 2155) | |
| 735 (34.1%) |
| 448 (20.8%) |
| 445 (20.6%) |
| 107 (5.0%) |
| 86 (4.0%) |
| Mean (±SD) number of ‘when required’ medications | 2.2 ± 1.7 |
| Top five ’when required’ medications used (n = 434) | |
| 210 (48.4%) |
| 84 (19.4%) |
| 54 (12.4%) |
| 37 (8.5%) |
| 19 (4.4%) |
| Mean (±SD) Charlson comorbidity index | 3.1 ± 2.1 |
| Top Five Medications Associated with DRP | Incidence of DRP with Use | Four Most Frequent DRP Found with Use | Most Frequent Recommendation(s) to DRP |
|---|---|---|---|
| Nervous system | 28.0% (215/448) | Cautioning against toxicity (61) | Monitoring: laboratory test (33) |
| Condition undertreated (45) | Other referral required (13) | ||
| Preventative therapy required (19) | Drug change: initiate (19) | ||
| Other dose problem (16) | Dose decrease (5) | ||
| Alimentary tract and metabolism | 24.4% (161/667) | Cautioning against toxicity (25) | Monitoring: laboratory test (15) |
| No indication apparent (20) | Dose decrease (9) | ||
| Condition undertreated (19) | Drug change: initiate (5) | ||
| Other dose problem (17) | Dose decrease (11) | ||
| Cardiovascular | 28.5% (85/298) | Toxicity caused by dose (24) | Dose decrease (5) Drug change: cease and initiate (5) Review prescribed medicine (5) |
| Toxicity evident (12) | Dose frequency/schedule change (7) | ||
| Other dose problem (10) | Dose frequency/schedule change (7) | ||
| Contraindications apparent (9) | Dose decrease (3) | ||
| Blood and blood forming organs | 45.7% (32/70) | Cautioning against toxicity (12) | Monitoring: laboratory test (8) |
| Toxicity caused by dose (5) | Review prescribed medicine (2) | ||
| Prescribed dose too high (2) | Dose decrease (1) Monitoring: laboratory test (1) | ||
| Contraindications apparent (2) | Monitoring: laboratory test (2) | ||
| Respiratory | 29.1% (25/86) | Taking too little (7) | Education/counselling session (2) Drug change: cease and initiate (2) Refer to prescriber (2) |
| Condition undertreated (5) | Drug change: initiate (3) | ||
| Erratic use of medication (2) | Education/counselling session (2) | ||
| Other dose problem (2) | Drug change: cease (1) Refer to prescriber (1) |
| Medication or Drug Class | How the Medication Was Inappropriately Prescribed | Pharmacist Recommendation |
|---|---|---|
| Anticoagulants (3) | Prescribed dose too high (1) | Dose decrease (1) |
| Prescribed dose too low (1) | Dose increase (1) | |
| Contraindications apparent (1) | Refer to prescriber (1) | |
| DPP-4 inhibitors (2) | Prescribed dose too high (1) | Dose decrease (1) |
| Contraindications apparent (1) | Drug change: cease and initiate (1) | |
| Statins (2) | Cautioning against toxicity (2) | Drug change: cease and initiate (1) Dose decrease (1) |
| Atenolol (1) | Contraindications apparent (1) | Drug change: cease and initiate (1) |
| Cyclosporin (1) | Cautioning against toxicity (1) | Monitoring: laboratory test (1) |
| Digoxin (1) | Cautioning against toxicity (1) | Monitoring: laboratory test (1) |
| Fenofibrate (1) | Prescribed dose too high (2) | Dose decrease (2) |
| Hydroxychloroquine (1) | Toxicity caused by dose (1) | Monitoring: laboratory test (1) |
| Hydroxycarbamide (1) | Contraindications apparent (1) | Monitoring: laboratory test (1) |
| Nitrofurantoin (1) | Toxicity caused by dose (1) | Drug change: cease (1) |
| Sucralfate (1) | Contraindications apparent (1) | Refer to prescriber (1) |
| SADMANS Medication | Incidence of DRP with Use | Four Most Frequent DRP Found with Use | Most Frequent Recommendation(s) to DRP |
|---|---|---|---|
| Sulfonylureas | 38.5% (5/13) | Cautioning against toxicity (2) | Monitoring: laboratory test (2) |
| Laboratory monitoring (2) | Monitoring: non-laboratory test (1) | ||
| Toxicity caused by dose (1) | Dose decrease (1) | ||
| ACEis | 17.5% (7/40) | Toxicity caused by dose (4) | Dose decrease (1) Monitoring: non-laboratory test (1) Drug change: cease (1) Drug change: cease and initiate (1) |
| Contraindications apparent (1) | Drug change: cease and initiate (1) | ||
| Cautioning against toxicity (1) | Drug change: cease and initiate (I) | ||
| Condition undertreated (1) | Dose increase (1) | ||
| Diuretics | 69.4% (34/49) | Cautioning against toxicity (9) | Monitoring: laboratory test (7) |
| No indication apparent (6) | Drug change: cease (3) | ||
| Toxicity caused by dose (5) | Monitoring: laboratory test (3) | ||
| Condition undertreated (5) | Refer to prescriber (3) | ||
| Metformin | 70.5% (31/44) | Laboratory monitoring (6) | Monitoring: laboratory test (5) |
| Cautioning against toxicity (5) | Monitoring: laboratory test (4) | ||
| Prescribed dose too high (4) | Dose decrease (3) | ||
| Contraindications apparent (3) | Dose decrease (1) Drug change: cease (1) Drug change: cease and initiate (1) | ||
| Toxicity caused by dose (3) | Monitoring: laboratory test (1) Drug change: cease (1) Drug change: cease and initiate (1) | ||
| ARBs | 29.3% (17/58) | Cautioning against toxicity (5) | Monitoring: laboratory test (3) |
| Toxicity caused by dose (3) | Monitoring: non-laboratory test (2) | ||
| Contraindications apparent (2) | Monitoring: laboratory test (2) | ||
| Condition untreated (2) | Dose frequency/schedule change (1) Monitoring: non-laboratory test (1) | ||
| NSAIDS | 39.7% (25/63) | Cautioning against toxicity (6) | Drug change: cease and initiate (2) |
| No indication apparent (4) | Drug change: cease (3) | ||
| Preventative therapy required (4) | Drug change: initiate (3) | ||
| Toxicity evident (3) | Monitoring: laboratory test (1) Drug change: cease and initiate (1) Refer to prescriber (1) | ||
| SGLT2 inhibitors | 9.1% (1/11) | Contraindications apparent (1) | Drug change: cease (1) |
| Medication or Drug Class | How the Medication Was Inappropriately Prescribed | Pharmacist Recommendation |
|---|---|---|
| ACEis (2) | Contraindications apparent (1) | Drug change: cease and initiate (1) |
| Cautioning against toxicity (1) | Drug change: cease and initiate (1) | |
| Diuretic (3) | Contraindications apparent (2) | Refer to prescriber (1) Dose decrease (1) |
| Other drug selection problem (1) | Dose decrease (1) | |
| Metformin (8) | Contraindications apparent (3) | Dose decrease (1) Drug change: cease (1) Drug change: cease and initiate (1) |
| Prescribed dose too high (4) | Dose decrease (3) | |
| Toxicity caused by dose (1) | Drug change: cease (1) | |
| ARBs (6) | Cautioning against toxicity (2) | Monitoring: laboratory test (2) |
| Contraindications apparent (2) | Monitoring: laboratory test (2) | |
| Prescribed dose too high (1) | Dose decrease (1) | |
| Toxicity caused be dose (1) | Drug change: cease (1) | |
| NSAIDs (1) | Cautioning against toxicity (1) | Monitoring laboratory test (1) |
| SGLT2 inhibitors (1) | Contraindications apparent (1) | Drug change: cease (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, M.; Van, C.; Sud, K.; Tesfaye, W.; Croker, N.; Seth, S.; Castelino, R.L. Drug-Related Problems and Recommendations Made during Home Medicines Reviews for Sick Day Medication Management in Australia. Medicina 2024, 60, 798. https://doi.org/10.3390/medicina60050798
Truong M, Van C, Sud K, Tesfaye W, Croker N, Seth S, Castelino RL. Drug-Related Problems and Recommendations Made during Home Medicines Reviews for Sick Day Medication Management in Australia. Medicina. 2024; 60(5):798. https://doi.org/10.3390/medicina60050798
Chicago/Turabian StyleTruong, Mimi, Connie Van, Kamal Sud, Wubshet Tesfaye, Nerida Croker, Shrey Seth, and Ronald Lynel Castelino. 2024. "Drug-Related Problems and Recommendations Made during Home Medicines Reviews for Sick Day Medication Management in Australia" Medicina 60, no. 5: 798. https://doi.org/10.3390/medicina60050798
APA StyleTruong, M., Van, C., Sud, K., Tesfaye, W., Croker, N., Seth, S., & Castelino, R. L. (2024). Drug-Related Problems and Recommendations Made during Home Medicines Reviews for Sick Day Medication Management in Australia. Medicina, 60(5), 798. https://doi.org/10.3390/medicina60050798







