Effects of the Neuropeptides Pituitary Adenylate Cyclase Activating Polypeptide and Vasoactive Intestinal Peptide in Male Fertility
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Semen Analysis
- Fertile Men:
- Defined by having a sperm concentration of ≥20 × 106 cells/mL and sperm motility of >40%.
- 2.
- Sub-fertile Men:
- Oligospermia: Men with a low sperm count of <15 million.
- Asthenospermia: Men with sperm motility of <40%.
- Oligoasthenospermia: Men with both low sperm count of <15 million and low motility of <40%.
2.3. Measurement of VIP and PACAP Levels in Seminal Plasma
2.4. Statistical Analysis
3. Results
3.1. Subjects’ Demographic Characteristics, Semen Criteria, and Protein Levels
3.2. Comparison between Sub-Fertile Patients’ Groups and Fertile Males’ Group
3.3. Associations between Seminal Levels of VIP and PACAP and Seminal Parameters of Sub-Fertile Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Gnoth, C.; Godehardt, E.; Frank-Herrmann, P.; Friol, K.; Tigges, J.; Freundl, G. Definition and prevalence of subfertility and infertility. Hum. Reprod. 2005, 20, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Anwar, A. Infertility: A review on causes, treatment and management. Women’s Health Gynecol. 2016, 5, 2. [Google Scholar]
- Babakhanzadeh, E.; Nazari, M.; Ghasemifar, S.; Khodadadian, A. Some of the factors involved in male infertility: A prospective review. Int. J. Gen. Med. 2020, 13, 29–41. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization Infertility Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 19 January 2023).
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Koppan, M.; Nagy, Z.; Bosnyak, I.; Reglodi, D. Female reproductive functions of the neuropeptide PACAP. Front. Endocrinol. 2022, 13, 982551. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Smith, M.S. Regulation of hypothalamic neuropeptide Y messenger ribonucleic acid expression during lactation: Role of prolactin. Endocrinology 2004, 145, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.-M. Investigating the Effects of Neuropeptides on Human Sperm Function and Fertility. Ph.D. Thesis, Manchester Metropolitan University, Manchester, UK, 2020. [Google Scholar]
- Van Wielendaele, P.; Wynant, N.; Dillen, S.; Zels, S.; Badisco, L.; Broeck, J.V. Neuropeptide F regulates male reproductive processes in the desert locust, Schistocerca gregaria. Insect. Biochem. Mol. Biol. 2013, 43, 252–259. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Tusimin, M.; Karkada, I.R. Orexins and male reproduction. Asian Pac. J. Reprod. 2019, 8, 233–238. [Google Scholar] [CrossRef]
- Filippi, S.; Vannelli, G.; Granchi, S.; Luconi, M.; Crescioli, C.; Mancina, R.; Natali, A.; Brocchi, S.; Vignozzi, L.; Bencini, E. Identification, localization and functional activity of oxytocin receptors in epididymis. Mol. Cell. Endocrinol. 2002, 193, 89–100. [Google Scholar] [CrossRef]
- Bódis, J.; Sulyok, E.; Kőszegi, T.; Prémusz, V.; Várnagy, Á.; Koppán, M. Serum and follicular fluid levels of serotonin, kisspeptin, and brain-derived neurotrophic factor in patients undergoing in vitro fertilization: An observational study: Neurohormones in patients receiving IVF. J. Int. Med. Res. 2020, 48, 0300060519879330. [Google Scholar] [CrossRef]
- Csaba, Z.; Csernus, V.; Gerendai, I. Local effect of PACAP and VIP on testicular function in immature and adult rats. Peptides 1997, 18, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Kasson, B.G.; Lim, P.; Hsueh, A.J. Vasoactive intestinal peptide stimulates androgen biosynthesis by cultured neonatal testicular cells. Mol. Cell. Endocrinol. 1986, 48, 21–29. [Google Scholar] [CrossRef] [PubMed]
- El-Gehani, F.; Tena-Sempere, M.; Huhtaniemi, I. Vasoactive intestinal peptide stimulates testosterone production by cultured fetal rat testicular cells. Mol. Cell. Endocrinol. 1998, 140, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkrug, J. Gut/brain peptides in the genital tract: VIP and PACAP. Scand. J. Clin. Lab. Investig. 2001, 61, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Can, C.; Töre, F.; Tunçel, N.; Uysal, O.; Gürer, F.; Ak, D.; Tunçel, M. Protective effect of vasoactive intestinal peptide on testicular torsion-detorsion injury: Association with heparin-containing mast cells. Urology 2004, 63, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Huang, H.; Lu, S.; Ou, B.; Feng, J.; Shan, W.; Ma, Y. PACAP ameliorates fertility in obese male mice via PKA/CREB pathway-dependent Sirt1 activation and p53 deacetylation. J. Cell. Physiol. 2020, 235, 7465–7483. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, A.; Lelievre, V.; Roselli, C.E.; Salameh, W.; Lue, Y.-h.; Lawson, G.; Muller, J.-M.; Waschek, J.A.; Vilain, E. Delayed testicular aging in pituitary adenylate cyclase-activating peptide (PACAP) null mice. Proc. Natl. Acad. Sci. USA 2006, 103, 3793–3798. [Google Scholar] [CrossRef] [PubMed]
- Brubel, R.; Kiss, P.; Vincze, A.; Varga, A.; Varnagy, A.; Bodis, J.; Mark, L.; Jambor, E.; Maasz, G.; Hashimoto, H. Effects of pituitary adenylate cyclase activating polypeptide on human sperm motility. J. Mol. Neurosci. 2012, 48, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Köves, K.; Kántor, O.; Lakatos, A.; Szabó, E.; Kirilly, E.; Heinzlmann, A.; Szabó, F. Advent and recent advances in research on the role of pituitary adenylate cyclase-activating polypeptide (PACAP) in the regulation of gonadotropic hormone secretion of female rats. J. Mol. Neurosci. 2014, 54, 494–511. [Google Scholar] [CrossRef]
- Rosati, L.; Prisco, M.; Coraggio, F.; Valiante, S.; Scudiero, R.; Laforgia, V.; Andreuccetti, P.; Agnese, M. PACAP and PAC1 receptor in the reproductive cycle of male lizard Podarcis sicula. Gen. Comp. Endocrinol. 2014, 205, 102–108. [Google Scholar] [CrossRef]
- Rossato, M.; Nogara, A.; Gottardello, F.; Bordon, P.; Foresta, C. Pituitary adenylate cyclase activating polypeptide stimulates rat Leydig cell steroidogenesis through a novel transduction pathway. Endocrinology 1997, 138, 3228–3235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Argiolas, A.; Melis, M.R. Neuropeptides and central control of sexual behaviour from the past to the present: A review. Prog. Neurobiol. 2013, 108, 80–107. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Esteves, S.C.; Nizza, M.; Agarwal, A. Unexplained male infertility: Diagnosis and management. Int. Braz. J. Urol. 2012, 38, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Polit, D.F.; Cheryl, T.B. Nursing Research: Generating and Assessing Evidence for Nursing Practice, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2016. [Google Scholar]
- Björndahl, L.; Söderlund, I.; Kvist, U. Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum. Reprod. 2003, 18, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Unal, I. Defining an optimal cut-point value in ROC analysis: An alternative approach. Comput. Math. Methods Med. 2017, 2017, 3762651. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Arimura, A. Neuropeptides of the pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide/growth hormone-releasing hormone/secretin family in testis. Endocrine 2003, 20, 201–214. [Google Scholar] [CrossRef]
- Reglodi, D.; Cseh, S.; Somoskoi, B.; Fulop, B.; Szentléleky, E.; Szegeczki, V.; Kovacs, A.; Varga, A.; Kiss, P.; Hashimoto, H. Disturbed spermatogenic signaling in pituitary adenylate cyclase activating polypeptide-deficient mice. Reproduction 2018, 155, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Lu, S.; Ou, B.; Feng, J.; Wang, Z.; Li, H.; Lu, X.; Ma, Y. PACAP ameliorates the fertility of obese mice through PAC1/PKA/ERK/Nrf2 signal axis. J. Endocrinol. 2021, 248, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Arimura, A.; Somogyvári-Vigh, A.; Miyata, A.; Mizuno, K.; Coy, D.H.; Kitada, C. Tissue distribution of PACAP as determined by RIA: Highly abundant in the rat brain and testes. Endocrinology 1991, 129, 2787–2789. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Komaki, G.; Arimuraf, A. Pituitary adenylate cyclase activating polypeptide (PACAP) can cross the vascular component of the blood-testis barrier in the mouse. J. Androl. 1993, 14, 170–173. [Google Scholar] [CrossRef]
- Hannibal, J.; Fahrenkrug, J. Expression of pituitary adenylate cyclase activating polypeptide (PACAP) gene by rat spermatogenic cells. Regul. Pept. 1995, 55, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.O.; Kwak, S.D.; Kim, H.J.; Roh, G.; Kim, J.H.; Kang, S.S.; Choi, W.S.; Cho, G.J. Expression patterns of pituitary adenylate cyclase activating polypeptide and its type I receptor mRNAs in the rat placenta. Mol. Reprod. Dev. Inc. Gamete Res. 2003, 64, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Powell, C.J.; Paschall, C.S.; Arimura, A.; Culler, M.D. A novel hypothalamic peptide, pituitary adenylate cyclase activating peptide, modulates Sertoli cell function in vitro. Biol. Reprod. 1992, 47, 800–806. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hosoya, M.; Kimura, C.; Ogi, K.; Ohkubo, S.; Miyamoto, Y.; Kugoh, H.; Shimizu, M.; Onda, H.; Oshimura, M.; Arimura, A. Structure of the human pituitary adenylate cyclase activating polypeptide (PACAP) gene. Biochim. Biophys. Acta Gene Struct. Expr. 1992, 1129, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Gonzalez, B.J.; Basille, M.; Yon, L.; Fournier, A.; Vaudry, H. Pituitary adenylate cyclase-activating polypeptide and its receptors: From structure to functions. Pharmacol. Rev. 2000, 52, 269–324. [Google Scholar] [PubMed]
- Kononen, J.; Paavola, M.; Penttilä, T.; Parvinen, M.; Pelto-Huikko, M. Stage-specific expression of pituitary adenylate cyclase-activating polypeptide (PACAP) mRNA in the rat seminiferous tubules. Endocrinology 1994, 135, 2291–2294. [Google Scholar] [CrossRef] [PubMed]
- Yanaihara, H.; Vigh, S.; Kozicz, T.; Somogyvári-Vigh, A.; Arimura, A. Immunohistochemical demonstration of the intracellular localization of pituitary adenylate cyclase activating polypeptide-like immunoreactivity in the rat testis using the stamp preparation. Regul. Pept. Biochim. Biophys. Acta Gene Struct. 1998, 78, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.-M.; Cheng, D.-L.; Zhao, W.; Zhu, H. Pituitary adenylate cyclase-activating polypeptide mRNA expression in rat testis and epididymis during postnatal development and experimental cryptorchidism. Mol. Med. Rep. 2011, 4, 793–798. [Google Scholar] [PubMed]
- Li, M.; Funahashi, H.; Mbikay, M.; Shioda, S.; Arimura, A. Pituitary adenylate cyclase activating polypeptide-mediated intracrine signaling in the testicular germ cells. Endocr. Biochim. Biophys. Acta Gene Struct. 2004, 23, 59–75. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakamachi, T.; Endo, K.; Ito, K.; Machida, T.; Oka, T.; Hori, M.; Ishizaka, K.; Shioda, S. Distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) in the human testis and in testicular germ cell tumors. Andrologia 2014, 46, 465–471. [Google Scholar] [CrossRef]
- Tanii, I.; Aradate, T.; Matsuda, K.; Komiya, A.; Fuse, H. PACAP-mediated sperm-cumulus cell interaction promotes fertilization. Reproduction 2011, 141, 163. [Google Scholar] [CrossRef] [PubMed]
- Daniel, P.B.; Habener, J.F. Pituitary adenylate cyclase-activating polypeptide gene expression regulated by a testis-specific promoter in germ cells during spermatogenesis. Endocrinology 2000, 141, 1218–1227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rannikko, A.; Penttila, T.L.; Zhang, F.P.; Toppari, J.; Parvinen, M.; Huhtaniemi, I. Stage-specific expression of the FSH receptor gene in the prepubertal and adult rat seminiferous epithelium. J. Endocrinol. 1996, 151, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Themmen, A.P.; Blok, L.J.; Post, M.; Baarends, W.M.; Hoogerbrugge, J.W.; Parmentier, M.; Vassart, G.; Grootegoed, J.A. Follitropin receptor down-regulation involves a cAMP-dependent post- transcriptional decrease of receptor mRNA expression. Mol. Cell Endocrinol. 1991, 78, R7–R13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prisco, M.; Rosati, L.; Morgillo, E.; Mollica, M.P.; Agnese, M.; Andreuccetti, P.; Valiante, S. Pituitary adenylate cyclase-activating peptide (PACAP) and its receptors in Mus musculus testis. Gen. Comp. Endocrinol. 2020, 286, 113297. [Google Scholar] [CrossRef] [PubMed]
- El-Gehani, F.; Tena-Sempere, M.; Huhtaniemi, I. Evidence that pituitary adenylate cyclase-activating polypeptide is a potent regulator of fetal rat testicular steroidogenesis. Biol. Reprod. 2000, 63, 1482–1489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsumoto, S.; Arakawa, Y.; Ohishi, M.; Yanaihara, H.; Iwanaga, T.; Kurokawa, N. Suppressive action of pituitary adenylate cyclase activating polypeptide (PACAP) on proliferation of immature mouse Leydig cell line TM3 cells. Biomed. Res. 2008, 29, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Gozes, I.; Perl, O.; Zamostiano, R.; Rubinraut, S.; Fridkin, M.; Shochat, L.; Lewin, L.M. Multiple Actions of a Hybrid PACAP Antagonist: Neuronal Cell Killing and Inhibition of Sperm Motilitya. Ann. N. Y. Acad. Sci. 1998, 865, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, A.; Lelievre, V.; Roselli, C.E.; Muller, J.-M.; Waschek, J.A.; Vilain, E. Lack of vasoactive intestinal peptide reduces testosterone levels and reproductive aging in mouse testis. J. Endocrinol. 2007, 194, 153–160. [Google Scholar] [CrossRef][Green Version]
- El-Gehani, F.; Tena-Sempere, M.; Huhtaniemi, I. Vasoactive intestinal peptide is an important endocrine regulatory factor of fetal rat testicular steroidogenesis. Endocrinol. Biol. Reprod. 1998, 139, 1474–1480. [Google Scholar] [CrossRef][Green Version]
- Sweett, H.; Fonseca, P.; Suárez-Vega, A.; Livernois, A.; Miglior, F.; Cánovas, A. Genome-wide association study to identify genomic regions and positional candidate genes associated with male fertility in beef cattle. Sci. Rep. 2020, 10, 20102. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef] [PubMed]
| Fertile Males (n = 81) | Sub-Fertile Patients (n = 82) | p-Value | |
|---|---|---|---|
| Age | 31.63 ± 0.54 | 31.90 ± 0.55 | 0.724 |
| Height | 175.95 ± 0.60 | 177.49 ± 0.54 | 0.058 |
| Weight | 90.57 ± 1.25 | 89.87 ± 1.26 | 0.693 |
| BMI | 29.17 ± 0.29 | 28.46 ± 0.31 | 0.094 |
| Liquefaction time (min) | 29.14 ± 1.11 | 32.19 ± 1.09 | 0.052 |
| Total sperm count (106/mL) | 56.56 ± 2.83 | 11.20 ± 2.0 | <0.001 |
| Semen volume (mL) | 3.83 ± 0.17 | 3.15 ± 0.38 | 0.108 |
| Sperm concentration (106/mL) | 17.38 ± 1.22 | 4.46 ± 1.14 | <0.001 |
| Total motility (%) | 66.96 ± 1.07 | 23.98 ± 2.81 | <0.001 |
| Normal morphology (%) | 32.01 ± 3.13 | 31.00 ± 3.33 | 0.825 |
| Viscosity | 0.28 ± 0.08 | 0.44 ± 0.09 | 0.191 |
| Vitality, n (%) | <0.001 * | ||
| 79 (97.5) | 15 (18.3) | |
| 2 (2.5) | 56 (68.3) | |
| 0 (0.0) | 11 (13.4) | |
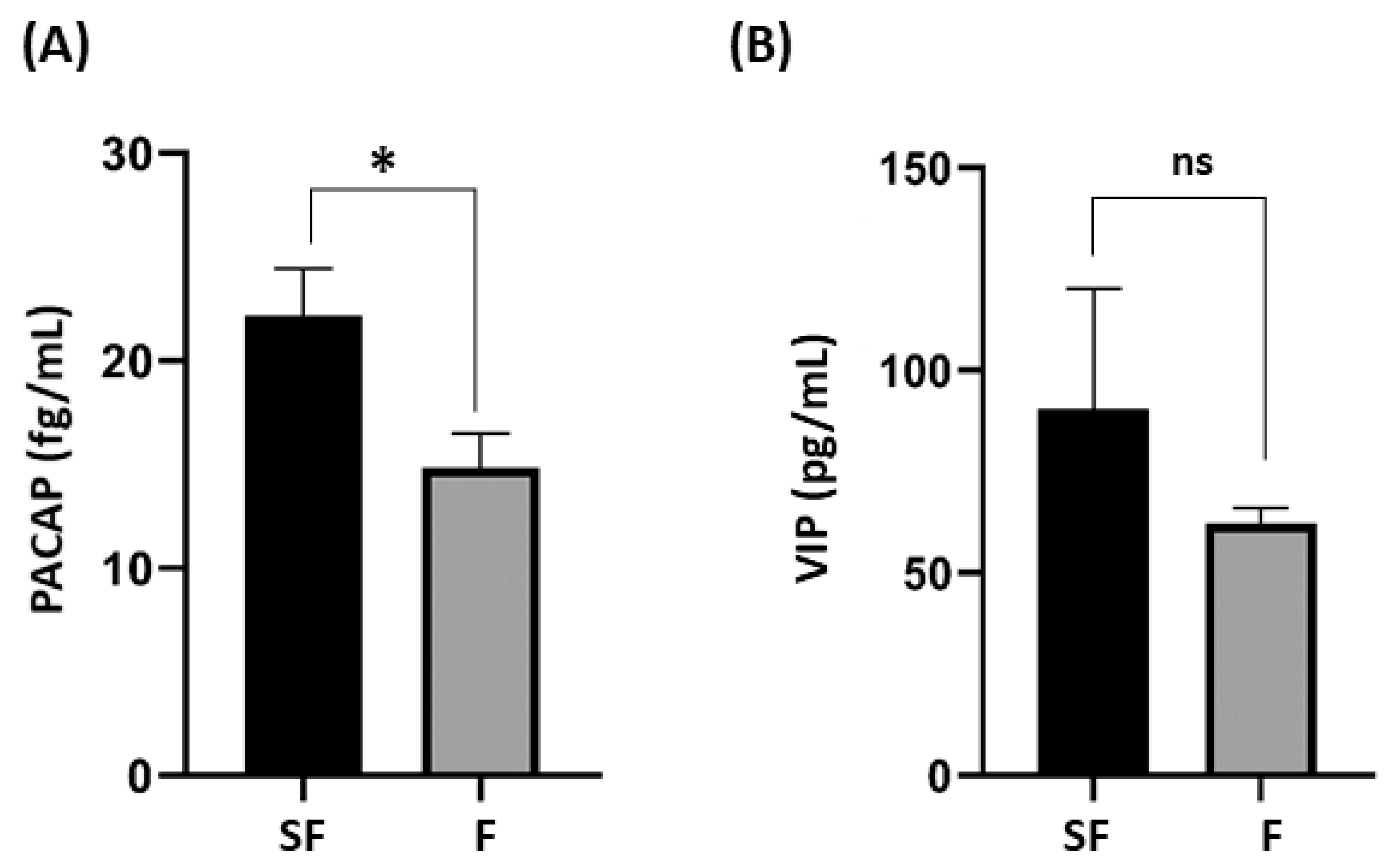
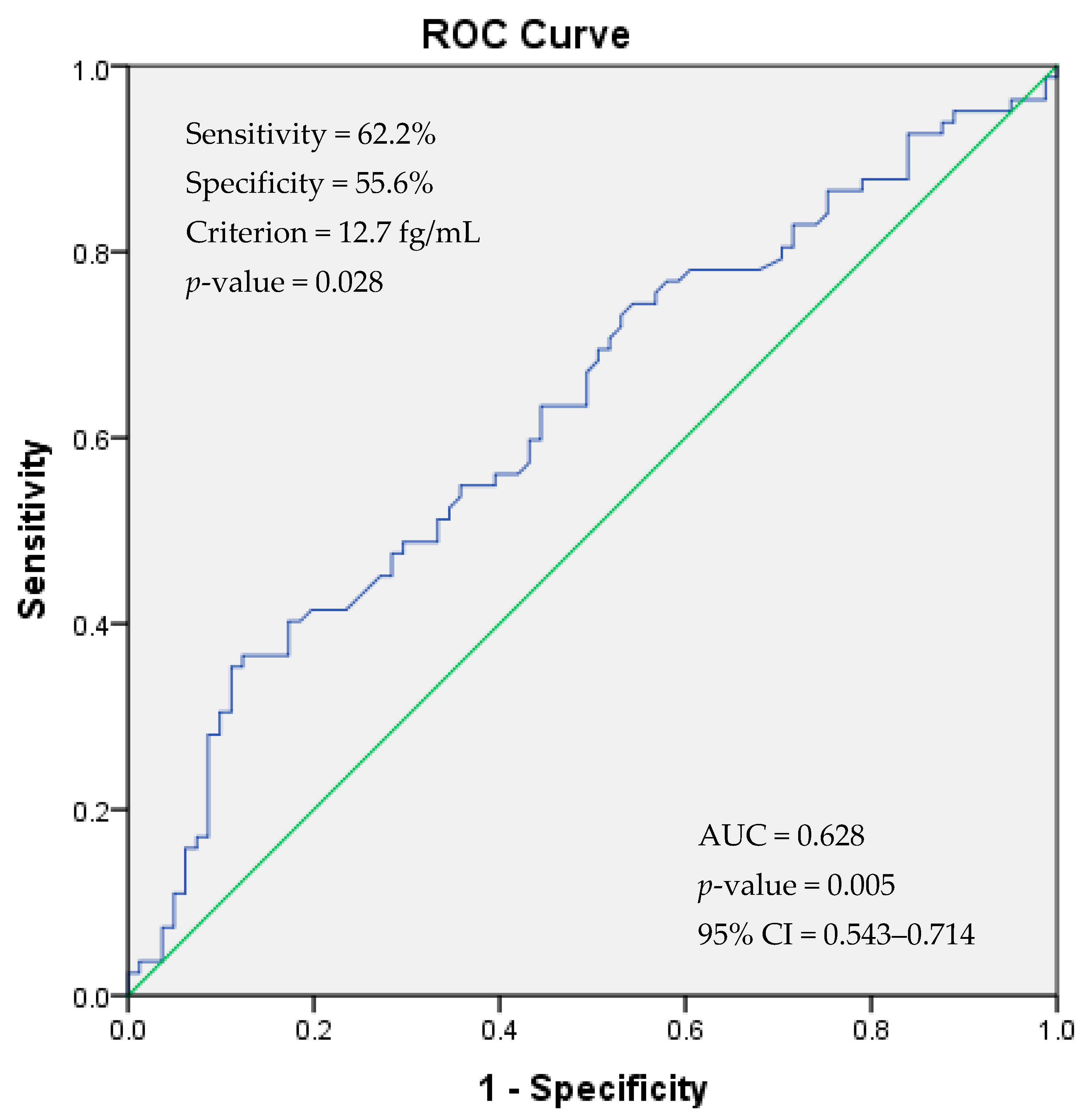
| PACAP | 14.87 ± 1.6 | 22.15 ± 2.30 | 0.011 |
| VIP | 62.18 ± 3.76 | 90.47 ± 29.74 | 0.350 |
| F (n = 81) | O (n = 19) | A (n = 12) | OA (n = 38) | Z (n = 13) | p-Value | |
|---|---|---|---|---|---|---|
| Age | 31.63 ± 0.54 | 30.42 ± 0.91 | 31.92 ± 1.25 | 31.63 ± 0.87 | 34.85 ± 1.40 | 0.154 |
| Liquefaction time (min) | 29.14 ± 1.11 | 31.32 ± 2.41 | 29.58 ± 2.34 | 34.34 ± 1.52 | 29.09 ± 3.36 | 0.108 |
| Total sperm count (106/mL) | 56.56 ± 2.83 | 10.98 ± 4.21 | 37.75 ± 7.16 | 6.16 ± 0.87 | 0 | <0.001 |
| Semen volume (mL) | 3.83 ± 0.17 | 4.85 ± 1.44 | 3.59 ± 0.58 | 2.62 ± 0.22 | 1.84 ± 0.60 | 0.004 |
| Sperm concentration (106/mL) | 17.38 ± 1.22 | 6.26 ± 4.38 | 12.20 ± 2.41 | 2.64 ± 0.33 | 0 | <0.001 |
| Total motility (%) | 66.96 ± 1.07 | 56.8 ± 2.41 | 25.14 ± 3.88 | 16.59 ± 2.62 | 0 | <0.001 |
| Normal morphology (%) | 32.01 ± 3.13 | 39.11 ± 7.26 | 21.50 ± 7.37 | 38.92 ± 4.57 | 0 | 0.001 |
| Viscosity | 0.28 ± 0.08 | 0.26 ± 0.13 | 0.83 ± 0.27 | 0.39 ± 0.13 | 0.44 ± 0.24 | 0.168 |
| Vitality, n (%) | <0.001 | |||||
| 79 (97.5) | 11 (57.9) | 0 (0.0) | 2 (5.3) | 2 (15.4) | |
| 2 (2.5) | 8 (42.1) | 12 (100) | 36 (94.7) | 11 (84.6) | |
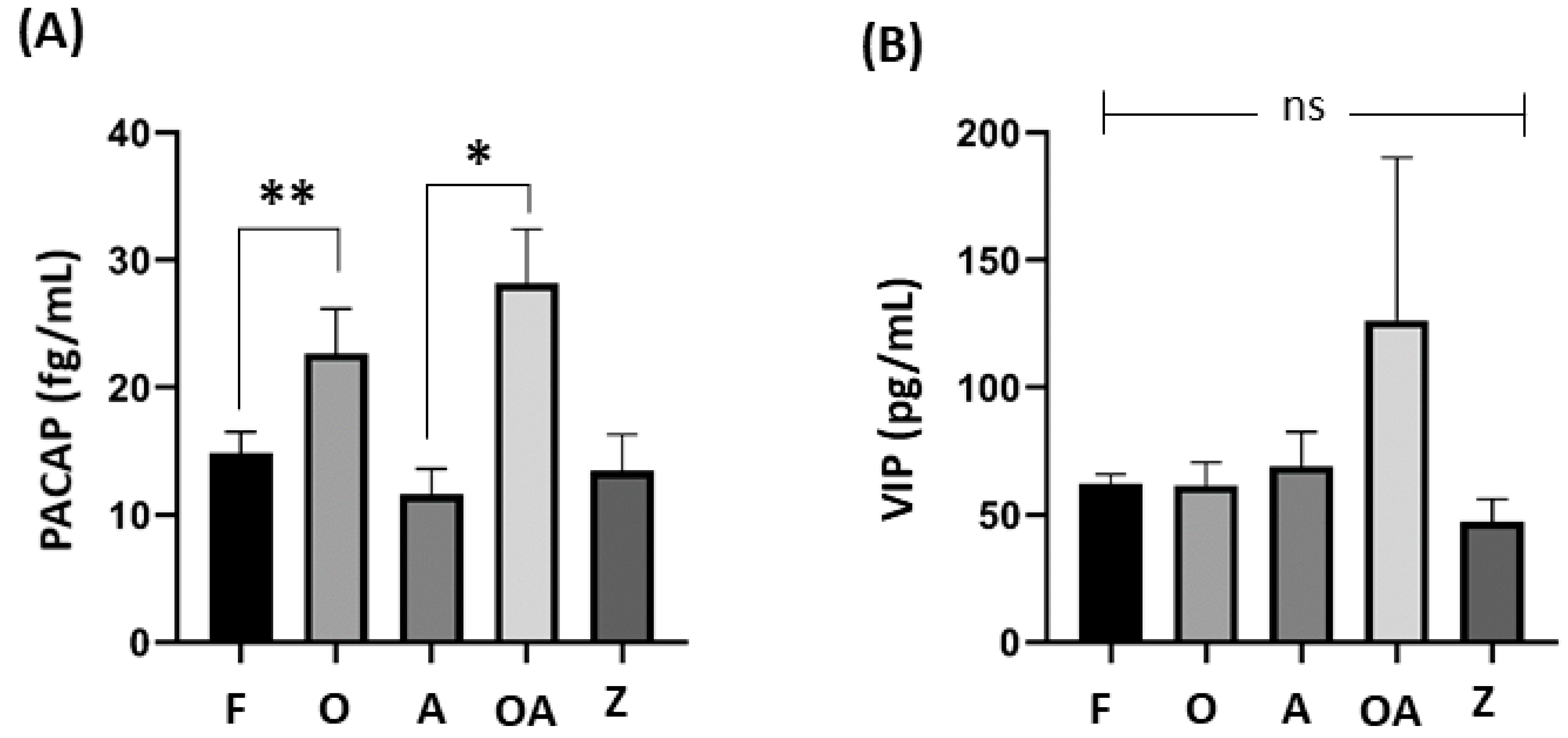
| PACAP | 14.87 ± 1.6 | 22.68 ± 3.50 | 11.62 ± 2.01 | 28.18 ± 4.23 | 13.48 ± 2.84 | 0.001 * |
| VIP | 62.18 ± 3.76 | 61.54 ± 9.03 | 69.07 ± 13.52 | 126.46 ± 63.77 | 47.34 ± 8.78 | 0.491 |
| Sub-Fertile Patients | ||
|---|---|---|
| PACAP | VIP | |
| Age | ||
| r | −0.142 | −0.082 |
| p-value | 0.204 | 0.465 |
| Liquefaction time (min) | ||
| r | 0.282 | 0.118 |
| p-value | 0.011 | 0.295 |
| Total sperm count (106/mL) | ||
| r | −0.189 | −0.067 |
| p-value | 0.094 | 0.554 |
| Semen volume (mL) | ||
| r | −0.066 | −0.047 |
| p-value | 0.558 | 0.673 |
| Sperm concentration (106/mL) | ||
| r | −0.135 | −0.041 |
| p-value | 0.226 | 0.715 |
| Total motility (%) | ||
| r | −0.063 | −0.122 |
| p-value | 0.618 | 0.331 |
| Normal morphology (%) | ||
| r | 0.392 | 0.130 |
| p-value | <0.001 | 0.251 |
| Viscosity | ||
| r | −0.168 | −0.065 |
| p-value | 0.142 | 0.570 |
| PACAP | ||
| PCC | -- | 0.626 |
| p-value | -- | <0.001 |
| VIP | ||
| r | 0.626 | -- |
| p-value | <0.001 | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bdeir, R.; Al-Keilani, M.S.; Khamaiseh, K. Effects of the Neuropeptides Pituitary Adenylate Cyclase Activating Polypeptide and Vasoactive Intestinal Peptide in Male Fertility. Medicina 2024, 60, 652. https://doi.org/10.3390/medicina60040652
Bdeir R, Al-Keilani MS, Khamaiseh K. Effects of the Neuropeptides Pituitary Adenylate Cyclase Activating Polypeptide and Vasoactive Intestinal Peptide in Male Fertility. Medicina. 2024; 60(4):652. https://doi.org/10.3390/medicina60040652
Chicago/Turabian StyleBdeir, Roba, Maha S. Al-Keilani, and Khaldoun Khamaiseh. 2024. "Effects of the Neuropeptides Pituitary Adenylate Cyclase Activating Polypeptide and Vasoactive Intestinal Peptide in Male Fertility" Medicina 60, no. 4: 652. https://doi.org/10.3390/medicina60040652
APA StyleBdeir, R., Al-Keilani, M. S., & Khamaiseh, K. (2024). Effects of the Neuropeptides Pituitary Adenylate Cyclase Activating Polypeptide and Vasoactive Intestinal Peptide in Male Fertility. Medicina, 60(4), 652. https://doi.org/10.3390/medicina60040652






