In-Stent Restenosis Overview: From Intravascular Imaging to Optimal Percutaneous Coronary Intervention Management
Abstract
1. Introduction
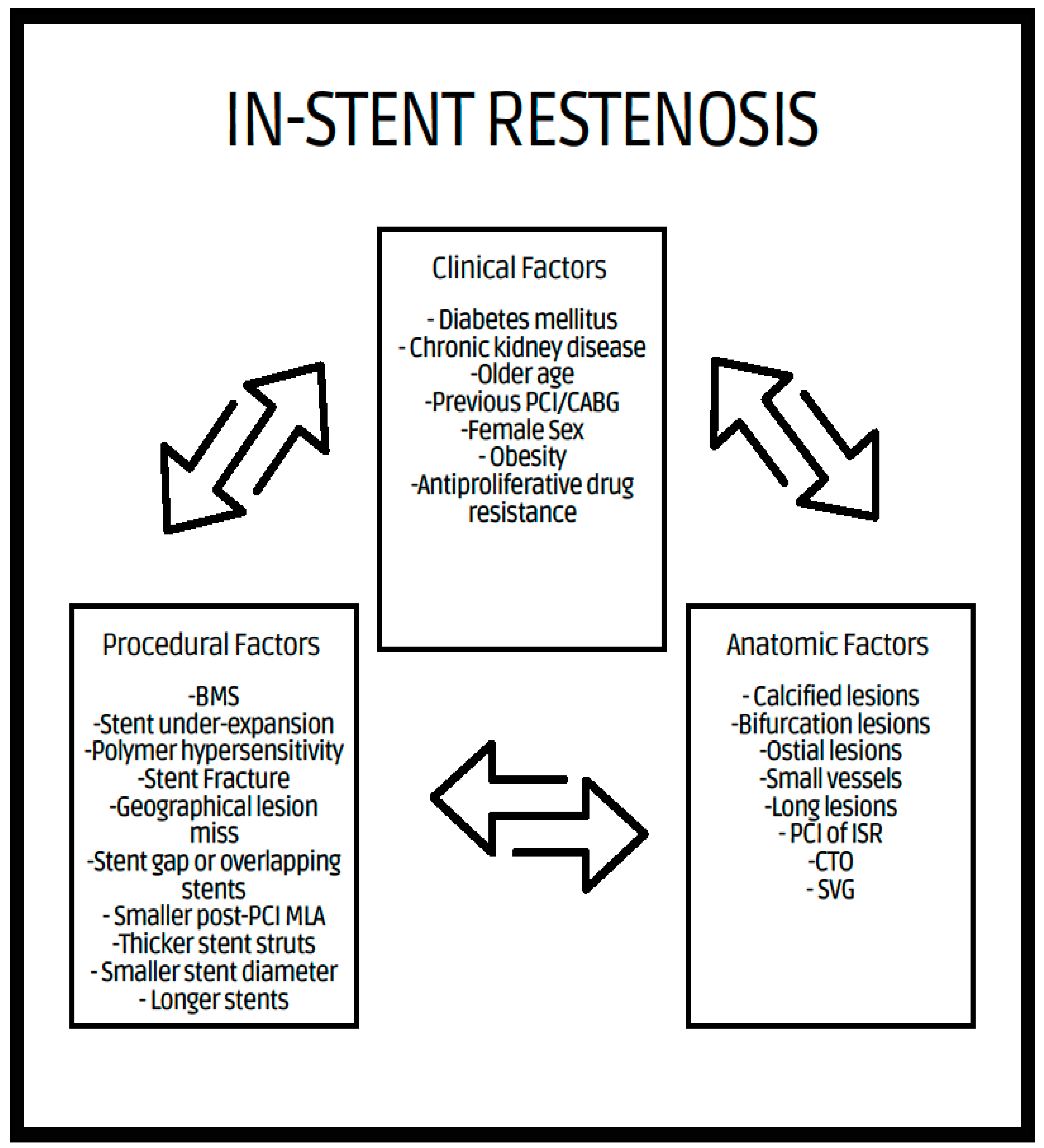
2. In-Stent Restenosis
2.1. Definition
2.2. Mechanism of ISR
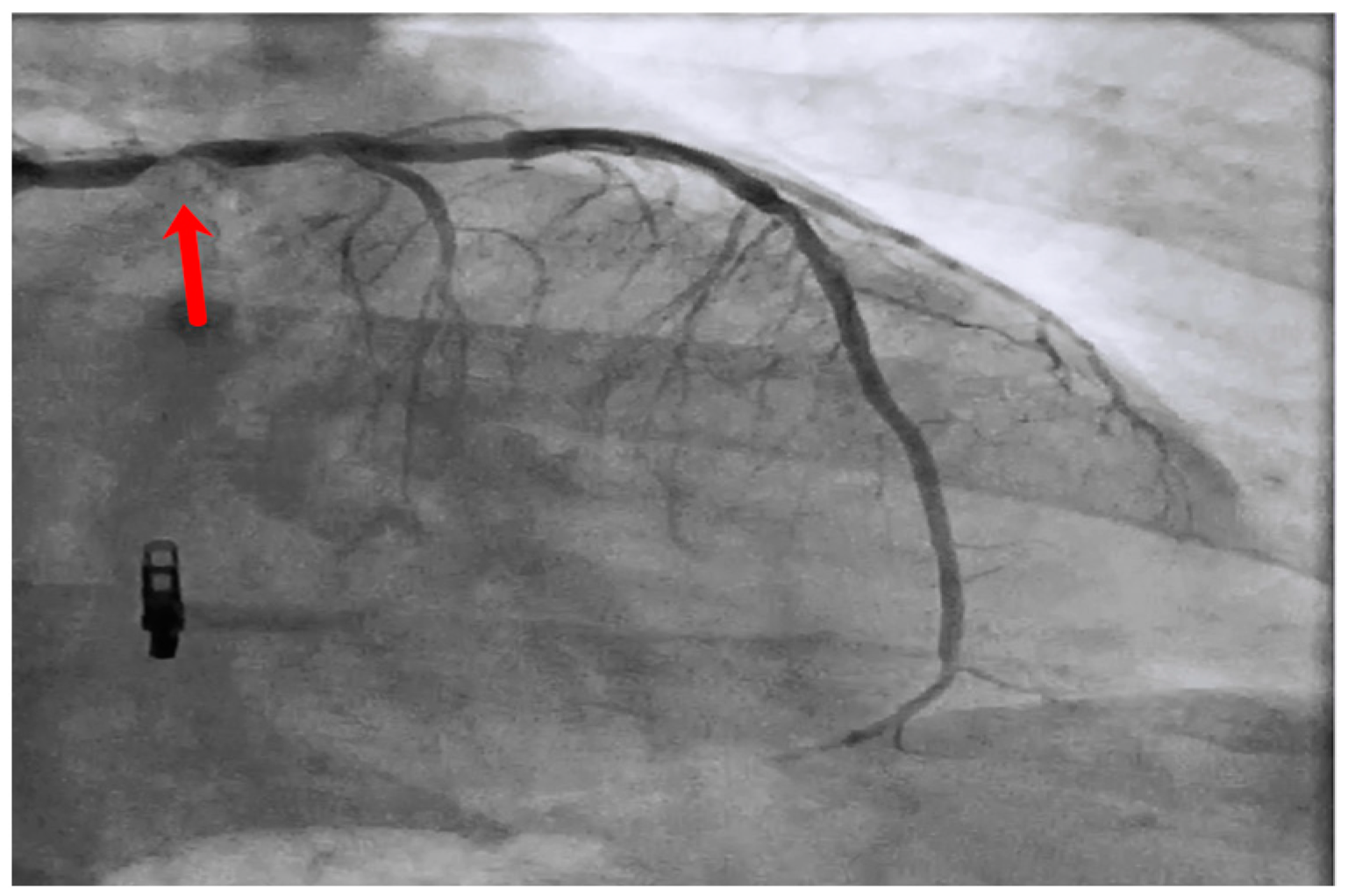
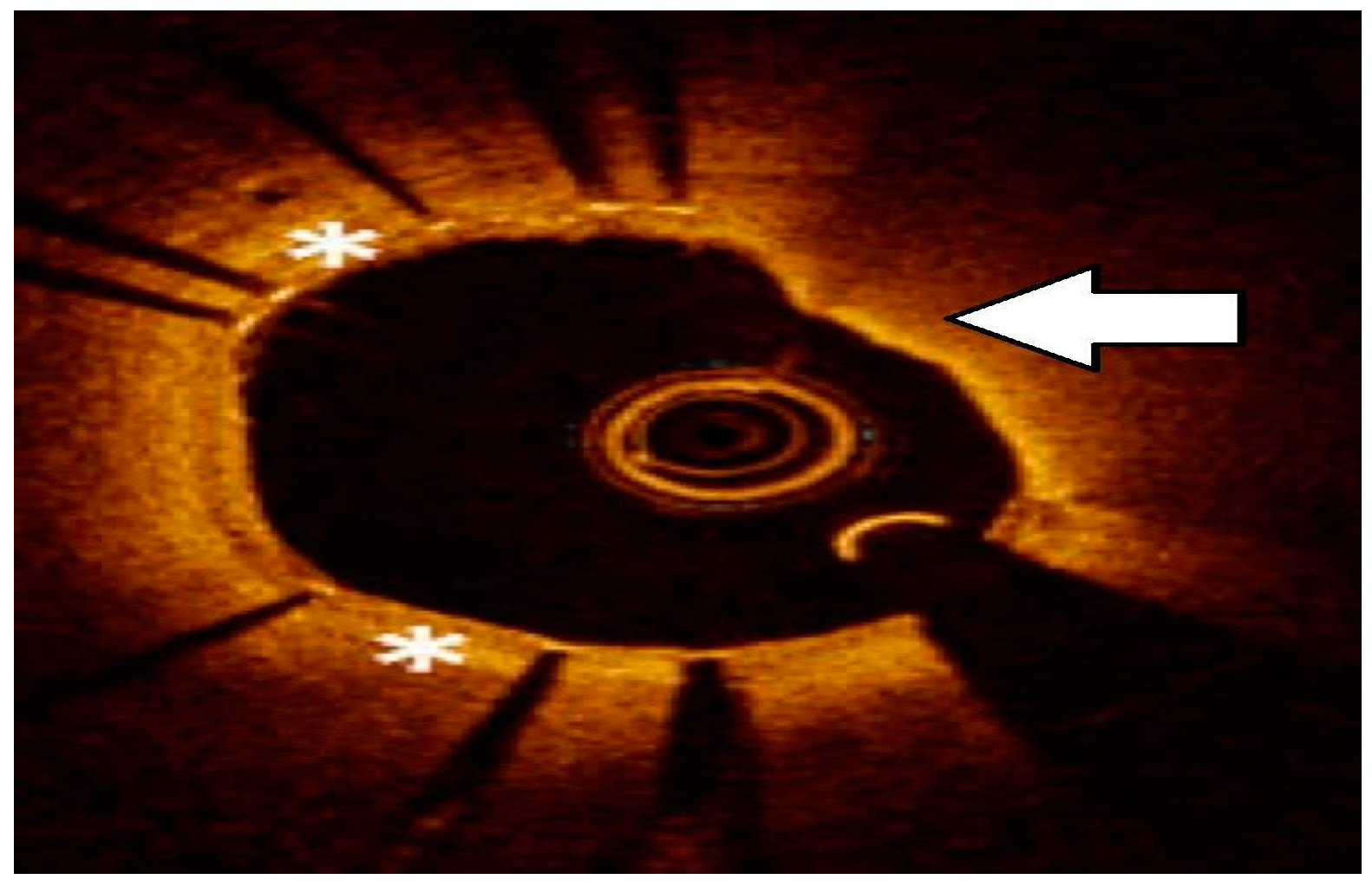
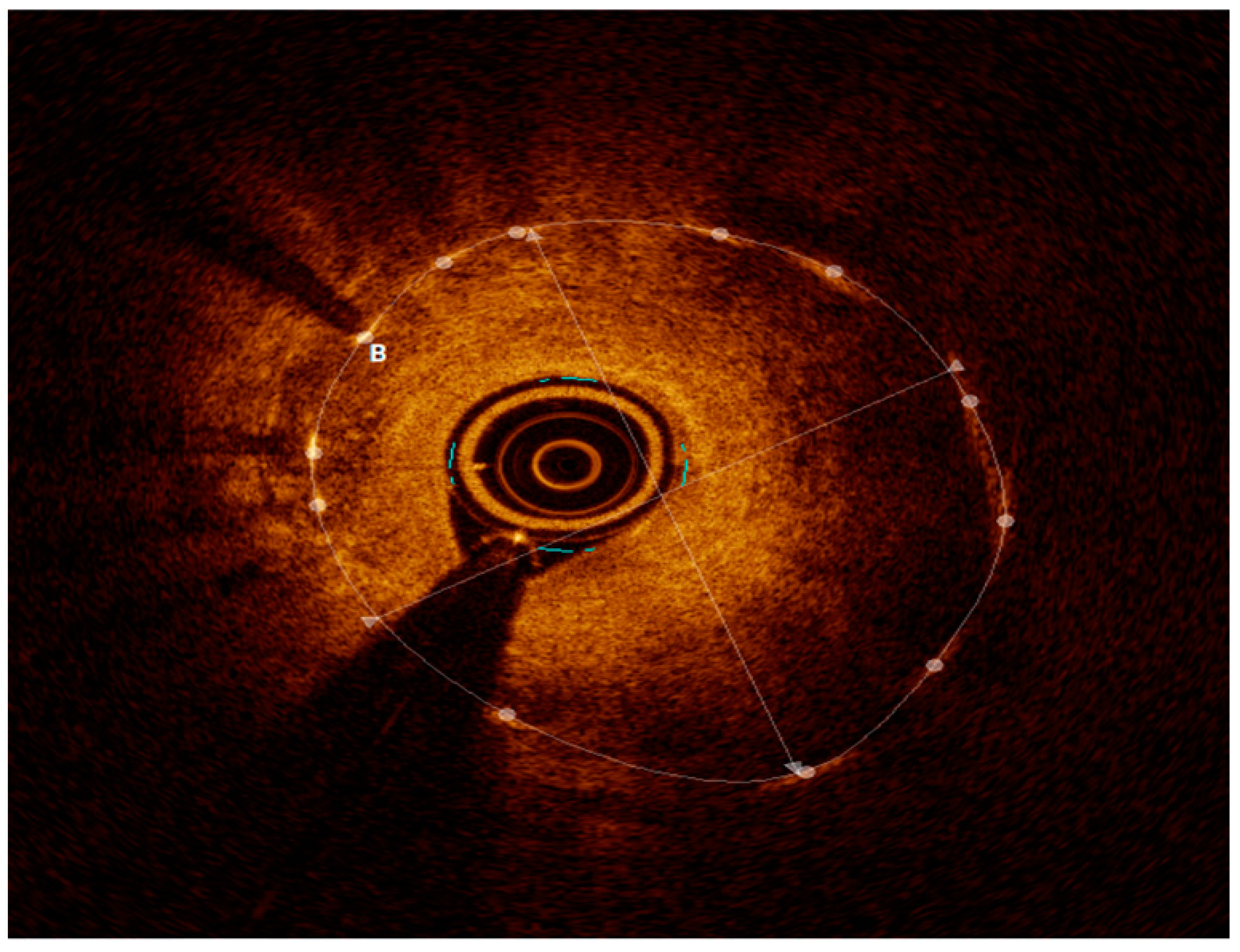
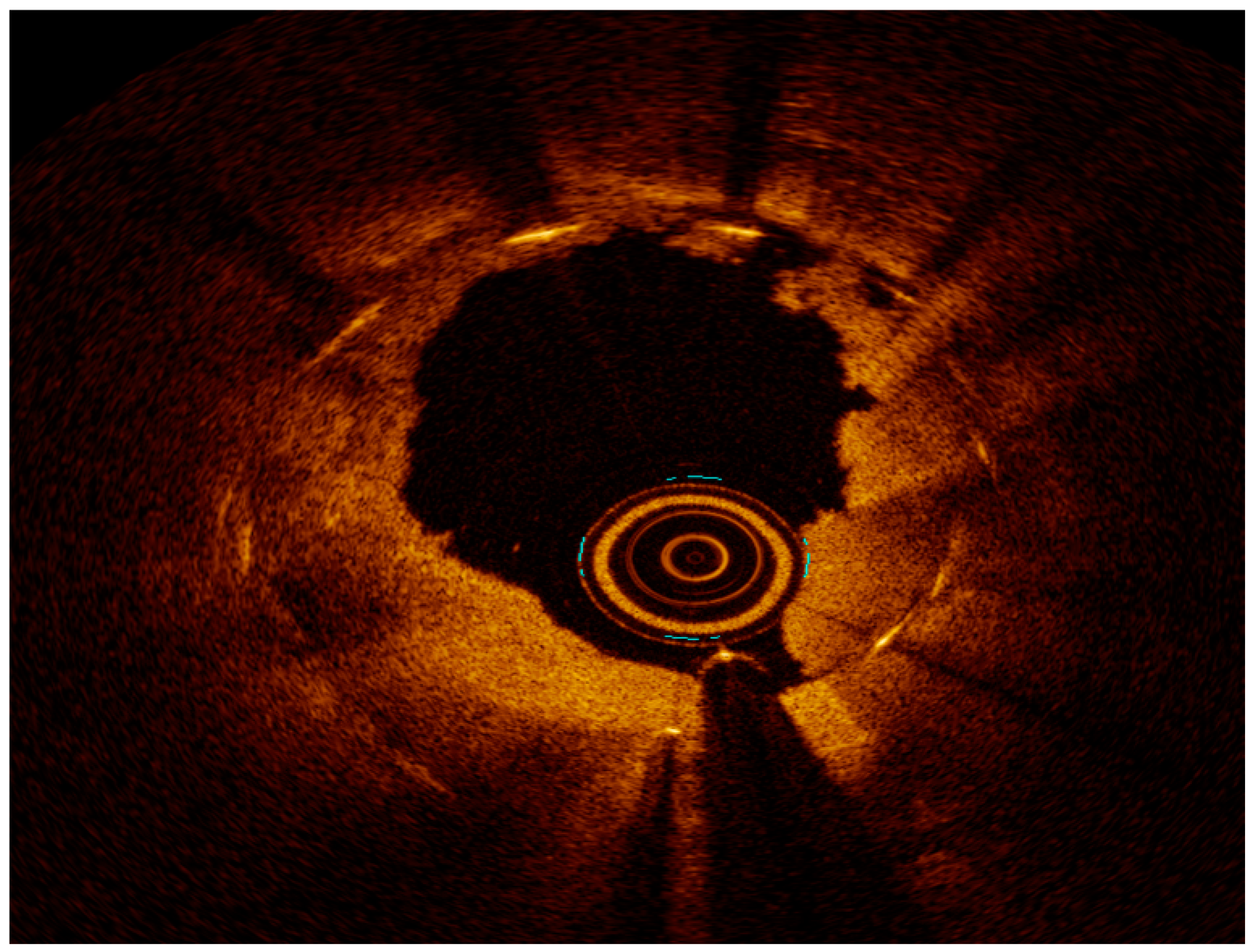
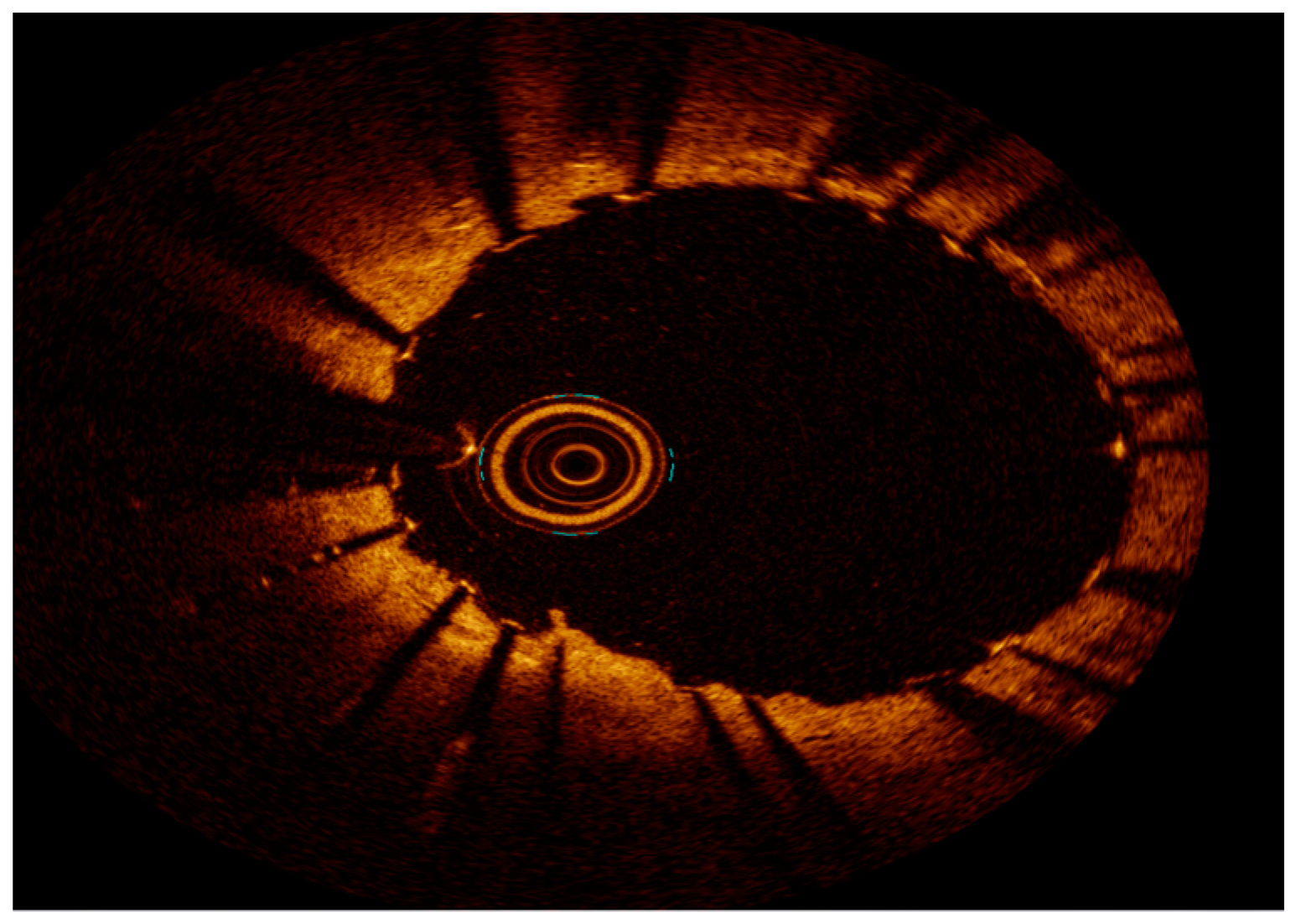
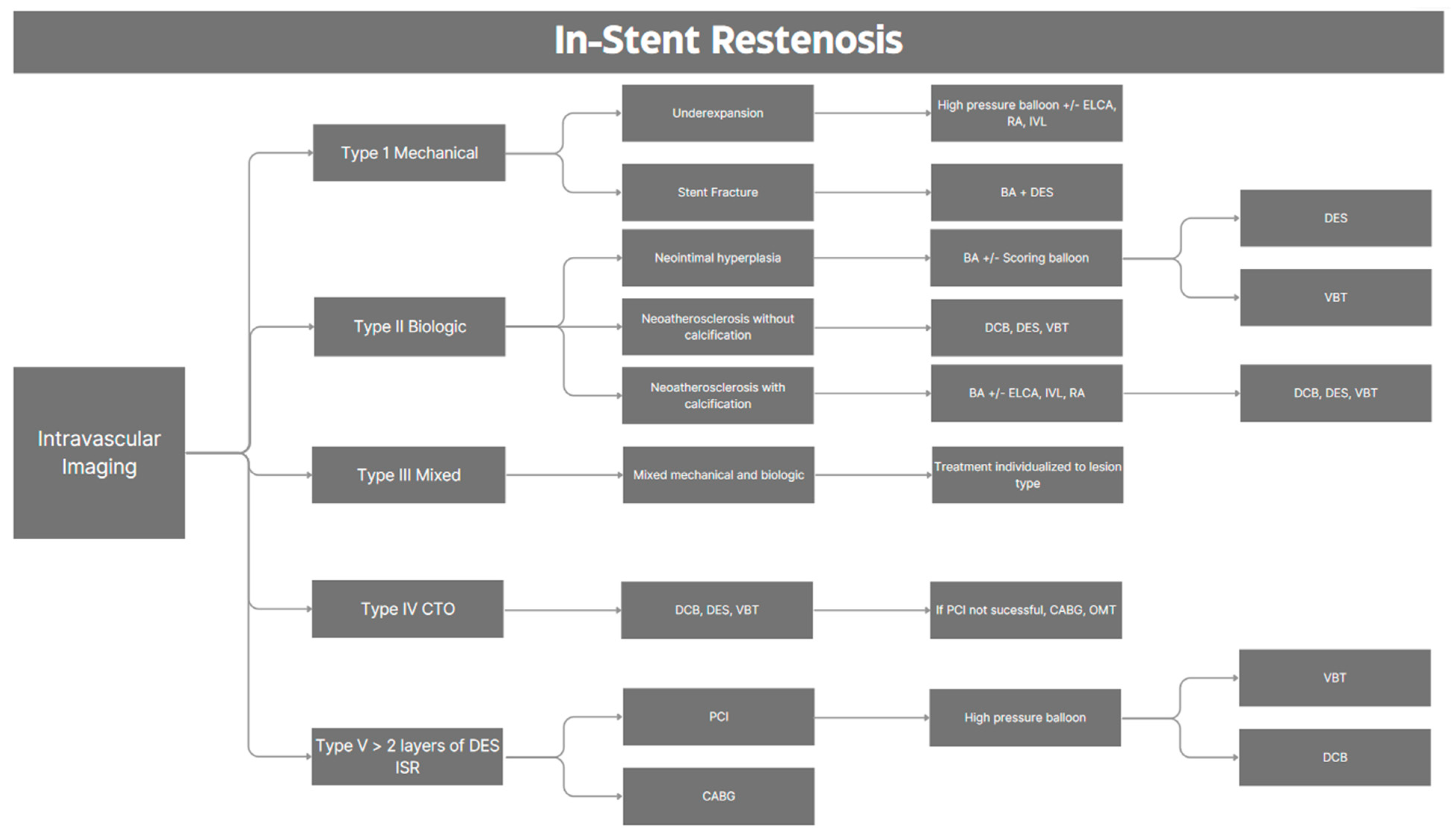
2.3. Intravascular Imaging
3. Invasive Physiology
4. Management Strategy
4.1. Approach to Severely Calcified Lesions—ISR
4.2. Excimer Laser Coronary Atherectomy (ELCA)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canfield, J.; Totary-Jain, H. 40 Years of Percutaneous Coronary Intervention: History and Future Directions. J. Pers. Med. 2018, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Colombo, A.; Camaj, A.; Yasumura, K.; Mehran, R.; Stone, G.W.; Kini, A.; Sharma, S.K. Coronary In-Stent Restenosis. J. Am. Coll. Cardiol. 2022, 80, 348–372. [Google Scholar] [CrossRef] [PubMed]
- Ghariani, A.; Ben Abdessalem, M.A.; Cheikh Sideya, K.; Fekih Romdhane, A.; Ben Ameur, Z.; Mosrati, H.; Bouraoui, H.; Mahdhaoui, A.; Jeridi, G. Outcomes and Prognostic Factors of Patients Treated for In-Stent Restenosis: A Retrospective Single-Center Experience. Egypt. Heart J. 2022, 74, 42. [Google Scholar] [CrossRef] [PubMed]
- Cassese, S.; Byrne, R.A.; Tada, T.; Pinieck, S.; Joner, M.; Ibrahim, T.; King, L.A.; Fusaro, M.; Laugwitz, K.-L.; Kastrati, A. Incidence and Predictors of Restenosis after Coronary Stenting in 10 004 Patients with Surveillance Angiography. Heart 2014, 100, 153–159. [Google Scholar] [CrossRef]
- Moussa, I.D.; Mohananey, D.; Saucedo, J.; Stone, G.W.; Yeh, R.W.; Kennedy, K.F.; Waksman, R.; Teirstein, P.; Moses, J.W.; Simonton, C. Trends and Outcomes of Restenosis After Coronary Stent Implantation in the United States. J. Am. Coll. Cardiol. 2020, 76, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.T.R.; Dallan, L.A.P.; Vergara-Martel, A.; Alaiti, M.A.; Bezerra, H.G. Treatment of In-Stent Restenosis Using Excimer Laser Coronary Atherectomy and Bioresorbable Vascular Scaffold Guided by Optical Coherence Tomography. Cardiovasc. Revasc. Med. 2021, 22, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, A.; Cassese, S. In-Stent Restenosis in the United States. J. Am. Coll. Cardiol. 2020, 76, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Teirstein, P.S.; Massullo, V.; Jani, S.; Popma, J.J.; Mintz, G.S.; Russo, R.J.; Schatz, R.A.; Guarneri, E.M.; Steuterman, S.; Morris, N.B.; et al. Catheter-Based Radiotherapy to Inhibit Restenosis after Coronary Stenting. N. Engl. J. Med. 1997, 336, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Dangas, G.; Abizaid, A.S.; Mintz, G.S.; Lansky, A.J.; Satler, L.F.; Pichard, A.D.; Kent, K.M.; Stone, G.W.; Leon, M.B. Angiographic Patterns of In-Stent Restenosis: Classification and Implications for Long-Term Outcome. Circulation 1999, 100, 1872–1878. [Google Scholar] [CrossRef]
- Mitra, A.K. In Stent Restenosis: Bane of the Stent Era. J. Clin. Pathol. 2006, 59, 232–239. [Google Scholar] [CrossRef]
- Nebeker, J.R.; Virmani, R.; Bennett, C.L.; Hoffman, J.M.; Samore, M.H.; Alvarez, J.; Davidson, C.J.; McKoy, J.M.; Raisch, D.W.; Whisenant, B.K.; et al. Hypersensitivity Cases Associated With Drug-Eluting Coronary Stents. J. Am. Coll. Cardiol. 2006, 47, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Shlofmitz, E.; Iantorno, M.; Waksman, R. Restenosis of Drug-Eluting Stents: A New Classification System Based on Disease Mechanism to Guide Treatment and State-of-the-Art Review. Circ. Cardiovasc. Interv. 2019, 12, e007023. [Google Scholar] [CrossRef] [PubMed]
- Her, A.-Y.; Shin, E.-S. Current Management of In-Stent Restenosis. Korean Circ. J. 2018, 48, 337. [Google Scholar] [CrossRef]
- Kang, S.-J.; Mintz, G.S.; Akasaka, T.; Park, D.-W.; Lee, J.-Y.; Kim, W.-J.; Lee, S.-W.; Kim, Y.-H.; Whan Lee, C.; Park, S.-W.; et al. Optical Coherence Tomographic Analysis of In-Stent Neoatherosclerosis After Drug–Eluting Stent Implantation. Circulation 2011, 123, 2954–2963. [Google Scholar] [CrossRef] [PubMed]
- Subban, V.; Raffel, O.C. Optical Coherence Tomography: Fundamentals and Clinical Utility. Cardiovasc. Diagn. Ther. 2020, 10, 1389–1414. [Google Scholar] [CrossRef] [PubMed]
- Nusca, A.; Viscusi, M.M.; Piccirillo, F.; De Filippis, A.; Nenna, A.; Spadaccio, C.; Nappi, F.; Chello, C.; Mangiacapra, F.; Grigioni, F.; et al. In Stent Neo-Atherosclerosis: Pathophysiology, Clinical Implications, Prevention, and Therapeutic Approaches. Life 2022, 12, 393. [Google Scholar] [CrossRef]
- Alfonso, F.; Coughlan, J.C.; Giacoppo, D.; Kastrati, A.; Byrne, R.B. Management of In-Stent Restenosis. EuroIntervention 2022, 18, e103–e123. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, G.; Otsuka, F.; Nakano, M.; Vorpahl, M.; Yazdani, S.K.; Ladich, E.; Kolodgie, F.D.; Finn, A.V.; Virmani, R. The Pathology of Neoatherosclerosis in Human Coronary Implants. J. Am. Coll. Cardiol. 2011, 57, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, E.; Bajaj, R.; Lansky, A.; Mathur, A.; Baumbach, A.; Bourantas, C.V. Intravascular Imaging for Guiding In-Stent Restenosis and Stent Thrombosis Therapy. J. Am. Heart Assoc. 2022, 11, e026492. [Google Scholar] [CrossRef]
- Abouelnour, A.; Gori, T. Intravascular Imaging in Coronary Stent Restenosis: Prevention, Characterization, and Management. Front. Cardiovasc. Med. 2022, 9, 843734. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Ali, Z.A.; Maehara, A.; Mintz, G.S.; Shlofmitz, R.; Jeremias, A. Intravascular Imaging-Guided Percutaneous Coronary Intervention: A Universal Approach for Optimization of Stent Implantation. Circ. Cardiovasc. Interv. 2020, 13, e008686. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e4–e17. [Google Scholar] [CrossRef] [PubMed]
- Buccheri, S.; Franchina, G.; Romano, S.; Puglisi, S.; Venuti, G.; D’Arrigo, P.; Francaviglia, B.; Scalia, M.; Condorelli, A.; Barbanti, M.; et al. Clinical Outcomes Following Intravascular Imaging-Guided Versus Coronary Angiography–Guided Percutaneous Coronary Intervention With Stent Implantation. JACC Cardiovasc. Interv. 2017, 10, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lo, S. Fundamentals and Role of Intravascular Ultrasound in Percutaneous Coronary Intervention. Cardiovasc. Diagn. Ther. 2020, 10, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, V.; Kalra, A.; Puri, R. When to Use Intravascular Ultrasound or Optical Coherence Tomography during Percutaneous Coronary Intervention? Cardiovasc. Diagn. Ther. 2020, 10, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, N.; Serruys, P.W.; Okamura, T.; Van Beusekom, H.M.; Garcia-Garcia, H.M.; Van Soest, G.; Van Der Giessen, W.; Regar, E. Optical Coherence Tomography Patterns of Stent Restenosis. Am. Heart J. 2009, 158, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-C.; Karanasos, A.; Regar, E. OCT Demonstrating Neoatherosclerosis as Part of the Continuous Process of Coronary Artery Disease. Herz 2015, 40, 845–854. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malle, C.; Tada, T.; Steigerwald, K.; Ughi, G.J.; Schuster, T.; Nakano, M.; Massberg, S.; Jehle, J.; Guagliumi, G.; Kastrati, A.; et al. Tissue Characterization After Drug-Eluting Stent Implantation Using Optical Coherence Tomography. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1376–1383. [Google Scholar] [CrossRef]
- Lopezpalop, R.; Pinar, E.; Lozano, I.; Saura, D.; Pico, F.; Valdes, M. Utility of the Fractional Flow Reserve in the Evaluation of Angiographically Moderate In-Stent Restenosis. Eur. Heart J. 2004, 25, 2040–2047. [Google Scholar] [CrossRef]
- Klein, L.W.; Nathan, S.; Maehara, A.; Messenger, J.; Mintz, G.S.; Ali, Z.A.; Rymer, J.; Sandoval, Y.; Al-Azizi, K.; Mehran, R.; et al. SCAI Expert Consensus Statement on Management of In-Stent Restenosis and Stent Thrombosis. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100971. [Google Scholar] [CrossRef]
- Escaned, J.; Ryan, N.; Mejía-Rentería, H.; Cook, C.M.; Dehbi, H.-M.; Alegria-Barrero, E.; Alghamdi, A.; Al-Lamee, R.; Altman, J.; Ambrosia, A.; et al. Safety of the Deferral of Coronary Revascularization on the Basis of Instantaneous Wave-Free Ratio and Fractional Flow Reserve Measurements in Stable Coronary Artery Disease and Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2018, 11, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Nam, C.-W.; Rha, S.-W.; Koo, B.-K.; Doh, J.-H.; Chung, W.-Y.; Yoon, M.-H.; Tahk, S.-J.; Lee, B.-K.; Lee, J.-B.; Yoo, K.-D.; et al. Usefulness of Coronary Pressure Measurement for Functional Evaluation of Drug-Eluting Stent Restenosis. Am. J. Cardiol. 2011, 107, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Pijls, N.H.J.; Van Schaardenburgh, P.; Manoharan, G.; Boersma, E.; Bech, J.-W.; Van’T Veer, M.; Bär, F.; Hoorntje, J.; Koolen, J.; Wijns, W.; et al. Percutaneous Coronary Intervention of Functionally Nonsignificant Stenosis. J. Am. Coll. Cardiol. 2007, 49, 2105–2111. [Google Scholar] [CrossRef]
- Chen, G.; Zrenner, B.; Pyxaras, S.A. Combined Rotational Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified In-Stent Neoatherosclerosis: A Mini-Review. Cardiovasc. Revasc. Med. 2019, 20, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, A.; Mehilli, J.; Von Beckerath, N.; Dibra, A.; Hausleiter, J.; Pache, J.; Schühlen, H.; Schmitt, C.; Dirschinger, J.; Schömig, A.; et al. Sirolimus-Eluting Stent or Paclitaxel-Eluting Stent vs Balloon Angioplasty for Prevention of Recurrences in Patients With Coronary In-Stent Restenosis: A Randomized Controlled Trial. JAMA 2005, 293, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mehilli, J.; Byrne, R.A.; Tiroch, K.; Pinieck, S.; Schulz, S.; Kufner, S.; Massberg, S.; Laugwitz, K.-L.; Schömig, A.; Kastrati, A. Randomized Trial of Paclitaxel- Versus Sirolimus-Eluting Stents for Treatment of Coronary Restenosis in Sirolimus-Eluting Stents. J. Am. Coll. Cardiol. 2010, 55, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Austin, D.; Gurbel, P.A.; Andreotti, F.; Tantry, U.; James, S.; Buffon, A.; Kozinski, M.; Obonska, K.; Bliden, K.; et al. Drug-Coated Balloons in Treatment of in-Stent Restenosis: A Meta-Analysis of Randomised Controlled Trials. Clin. Res. Cardiol. 2013, 102, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Baan, J.; Claessen, B.E.; Dijk, K.B.; Vendrik, J.; Van Der Schaaf, R.J.; Meuwissen, M.; Van Royen, N.; Gosselink, A.T.M.; Van Wely, M.H.; Dirkali, A.; et al. A Randomized Comparison of Paclitaxel-Eluting Balloon Versus Everolimus-Eluting Stent for the Treatment of Any In-Stent Restenosis. JACC Cardiovasc. Interv. 2018, 11, 275–283. [Google Scholar] [CrossRef]
- Varghese, M.J.; Bhatheja, S.; Baber, U.; Kezbor, S.; Chincholi, A.; Chamaria, S.; Buckstein, M.; Bakst, R.; Kini, A.; Sharma, S. Intravascular Brachytherapy for the Management of Repeated Multimetal-Layered Drug-Eluting Coronary Stent Restenosis. Circ. Cardiovasc. Interv. 2018, 11, e006832. [Google Scholar] [CrossRef]
- Tsutsui, R.S.; Sammour, Y.; Kalra, A.; Reed, G.; Krishnaswamy, A.; Ellis, S.; Nair, R.; Khatri, J.; Kapadia, S.; Puri, R. Excimer Laser Atherectomy in Percutaneous Coronary Intervention: A Contemporary Review. Cardiovasc. Revasc. Med. 2021, 25, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Iantorno, M. Refractory In-Stent Restenosis: Improving Outcomes by Standardizing Our Approach. Curr. Cardiol. Rep. 2018, 20, 140. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, E.; Abbott, J.D.; Bezerra, H.G. Optimizing Percutaneous Coronary Intervention in Calcified Lesions: Insights From Optical Coherence Tomography of Atherectomy. Circ. Cardiovasc. Interv. 2018, 11, e006813. [Google Scholar] [CrossRef]
- Mehran, R.; Mintz, G.S.; Popma, J.J.; Pichard, A.D.; Satler, L.F.; Kent, K.M.; Griffin, J.; Leon, M.B. Mechanisms and Results of Balloon Angioplasty for the Treatment of In-Stent Restenosis. Am. J. Cardiol. 1996, 78, 618–622. [Google Scholar] [CrossRef]
- Pleva, L.; Kukla, P.; Kusnierova, P.; Zapletalova, J.; Hlinomaz, O. Comparison of the Efficacy of Paclitaxel-Eluting Balloon Catheters and Everolimus-Eluting Stents in the Treatment of Coronary In-Stent Restenosis: The Treatment of In-Stent Restenosis Study. Circ. Cardiovasc. Interv. 2016, 9, e003316. [Google Scholar] [CrossRef]
- Wańha, W.; Bil, J.; Januszek, R.; Gilis-Malinowska, N.; Figatowski, T.; Milewski, M.; Pawlik, A.; Staszczak, B.; Wybraniec, M.; Tomasiewicz, B.; et al. Long-Term Outcomes Following Drug-Eluting Balloons Versus Thin-Strut Drug-Eluting Stents for Treatment of In-Stent Restenosis (DEB-Dragon-Registry). Circ. Cardiovasc. Interv. 2021, 14, e010868. [Google Scholar] [CrossRef] [PubMed]
- Kheifets, M.; Rahat, O.; Bental, T.; Levi, A.; Vaknin-Assa, H.; Greenberg, G.; Codner, P.; Witberg, G.; Kornowski, R.; Perl, L. Outcomes of Drug Eluting Balloons for In-Stent Restenosis: Large Cohort Analysis and Single Center Clinical Experience. Can. J. Cardiol. 2024, S0828282X24000114. [Google Scholar] [CrossRef] [PubMed]
- Boston Scientific Receives FDA Approval for the AGENT™ Drug-Coated Balloon. Available online: https://www.multivu.com/players/english/9029352-boston-scientific-fda-approval-agent-drug-coated-balloon/ (accessed on 1 March 2024).
- Albiero, D.R. Cutting Balloon Versus Conventional Balloon Angioplasty for the Treatment of Coronary Artery Disease. Eur. Cardiol. Rev. 2005, 1, 48. [Google Scholar] [CrossRef]
- Kufner, S.; Joner, M.; Schneider, S.; Tölg, R.; Zrenner, B.; Repp, J.; Starkmann, A.; Xhepa, E.; Ibrahim, T.; Cassese, S.; et al. Neointimal Modification With Scoring Balloon and Efficacy of Drug-Coated Balloon Therapy in Patients With Restenosis in Drug-Eluting Coronary Stents. JACC Cardiovasc. Interv. 2017, 10, 1332–1340. [Google Scholar] [CrossRef]
- Shah, M.; Najam, O.; Bhindi, R.; De Silva, K. Calcium Modification Techniques in Complex Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2021, 14, e009870. [Google Scholar] [CrossRef]
- Wańha, W.; Tomaniak, M.; Wańczura, P.; Bil, J.; Januszek, R.; Wolny, R.; Opolski, M.P.; Kuźma, Ł.; Janas, A.; Figatowski, T.; et al. Intravascular Lithotripsy for the Treatment of Stent Underexpansion: The Multicenter IVL-DRAGON Registry. J. Clin. Med. 2022, 11, 1779. [Google Scholar] [CrossRef] [PubMed]
- Dini, C.S.; Nardi, G.; Ristalli, F.; Mattesini, A.; Hamiti, B.; Di Mario, C. Contemporary Approach to Heavily Calcified Coronary Lesions. Interv. Cardiol. Rev. 2019, 14, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Brunner, F.J.; Becher, P.M.; Waldeyer, C.; Zengin-Sahm, E.; Schnabel, R.B.; Clemmensen, P.; Westermann, D.; Blankenberg, S.; Seiffert, M. Intravascular Lithotripsy for the Treatment of Calcium-Mediated Coronary In-Stent Restenoses. J. Invasive Cardiol. 2021, 33, E25–E31. [Google Scholar]
- Ben-Dor, I.; Maluenda, G.; Pichard, A.D.; Satler, L.F.; Gallino, R.; Lindsay, J.; Waksman, R. The Use of Excimer Laser for Complex Coronary Artery Lesions. Cardiovasc. Revasc. Med. 2011, 12, 69.e1–69.e8. [Google Scholar] [CrossRef]
- Lee, T.; Shlofmitz, R.A.; Song, L.; Tsiamtsiouris, T.; Pappas, T.; Madrid, A.; Jeremias, A.; Haag, E.S.; Ali, Z.A.; Moses, J.W.; et al. The Effectiveness of Excimer Laser Angioplasty to Treat Coronary In-Stent Restenosis with Peri-Stent Calcium as Assessed by Optical Coherence Tomography. EuroIntervention 2019, 15, e279–e288. [Google Scholar] [CrossRef] [PubMed]
- Dallan, L.A.P.; Pereira, G.T.R.; Alaiti, M.A.; Zimin, V.; Vergara-Martel, A.; Zago, E.I.; Pizzato, P.E.; Bezerra, H.G. Laser Imaging: Unraveling Laser Atherectomy Mechanisms of Action with Optical Coherence Tomography. Curr. Cardiovasc. Imaging Rep. 2019, 12, 33. [Google Scholar] [CrossRef]
- Ichimoto, E.; Kadohira, T.; Nakayama, T.; De Gregorio, J. Long-Term Clinical Outcomes after Treatment with Excimer Laser Coronary Atherectomy for In-Stent Restenosis of Drug-Eluting Stent. Int. Heart. J. 2018, 59, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mody, R.; Dash, D.; Mody, B.; Maligireddy, A.R.; Agrawal, A.; Rastogi, L.; Monga, I.S. Can Most Calcified Coronary Stenosis Be Optimized with Coronary Intravascular Lithotripsy? JACC Asia 2023, 3, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Manasrah, N.; Zghouzi, M.; Naughton, R.; Patel, D.; Osman, H.; Abdelrahman, A.K.; Halboni, A.; Deschamps, R.; Sattar, Y.; Alraies, M.C. Outcomes of Orbital Atherectomy for the Treatment of Severely Calcified Coronary Artery Lesions. Cureus 2023, 15, e37651. [Google Scholar] [CrossRef]
- Farb, A.; Roberts, D.K.; Pichard, A.D.; Kent, K.M.; Virmani, R. Coronary Artery Morphologic Features after Coronary Rotational Atherectomy: Insights into Mechanisms of Lumen Enlargement and Embolization. Am. Heart J. 1995, 129, 1058–1067. [Google Scholar] [CrossRef]
- Sakakura, K.; Ako, J.; Wada, H.; Naito, R.; Funayama, H.; Arao, K.; Kubo, N.; Momomura, S. Comparison of Frequency of Complications With On-Label Versus Off-Label Use of Rotational Atherectomy. Am. J. Cardiol. 2012, 110, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; White, R.L.; Chan, R.C.; Bass, B.G.; Geirlach, L.; Mintz, G.S.; Satler, L.F.; Mehran, R.; Serruys, P.W.; Lansky, A.J.; et al. Intracoronary Gamma-Radiation Therapy after Angioplasty Inhibits Recurrence in Patients with in-Stent Restenosis. Circulation 2000, 101, 2165–2171. [Google Scholar] [CrossRef]
- Budoff, M.J.; Shaw, L.J.; Liu, S.T.; Weinstein, S.R.; Tseng, P.H.; Flores, F.R.; Callister, T.Q.; Raggi, P.; Berman, D.S.; Mosler, T.P. Long-Term Prognosis Associated With Coronary Calcification. J. Am. Coll. Cardiol. 2007, 49, 1860–1870. [Google Scholar] [CrossRef]
- Tuzcu, E.M.; Berkalp, B.; De Franco, A.C.; Ellis, S.G.; Goormastic, M.; Whitlow, P.L.; Franco, I.; Raymond, R.E.; Nissen, S.E. The Dilemma of Diagnosing Coronary Calcification: Angiography versus Intravascular Ultrasound. J. Am. Coll. Cardiol. 1996, 27, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Matsumura, M.; Mintz, G.S.; Lee, T.; Zhang, W.; Cao, Y.; Fujino, A.; Lin, Y.; Usui, E.; Kanaji, Y.; et al. In Vivo Calcium Detection by Comparing Optical Coherence Tomography, Intravascular Ultrasound, and Angiography. JACC Cardiovasc. Imaging 2017, 10, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, B.; Pareek, N.; Macaya, F.; Yeoh, J.; Shlofmitz, E.; Gonzalo, N.; Hill, J.; Escaned, J. Contemporary Percutaneous Management of Coronary Calcification: Current Status and Future Directions. Open Heart 2023, 10, e002182. [Google Scholar] [CrossRef]
- Saito, Y.; Kobayashi, Y.; Fujii, K.; Sonoda, S.; Tsujita, K.; Hibi, K.; Morino, Y.; Okura, H.; Ikari, Y.; Honye, J. Clinical Expert Consensus Document on Intravascular Ultrasound from the Japanese Association of Cardiovascular Intervention and Therapeutics (2021). Cardiovasc. Interv. Ther. 2022, 37, 40–51. [Google Scholar] [CrossRef]
- Fujino, A.; Mintz, G.S.; Matsumura, M.; Lee, T.; Kim, S.-Y.; Hoshino, M.; Usui, E.; Yonetsu, T.; Haag, E.S.; Shlofmitz, R.A.; et al. A New Optical Coherence Tomography-Based Calcium Scoring System to Predict Stent Underexpansion. EuroIntervention 2018, 13, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shang, C.; Rong, Y.; Sun, J.; Cheng, Y.; He, B.; Wang, Z.; Li, M.; Ma, J.; Fu, B.; et al. Review on Laser Technology in Intravascular Imaging and Treatment. Aging Dis. 2022, 13, 246. [Google Scholar] [CrossRef]
- Mehran, R.; Mintz, G.S.; Satler, L.F.; Pichard, A.D.; Kent, K.M.; Bucher, T.A.; Popma, J.J.; Leon, M.B. Treatment of In-Stent Restenosis With Excimer Laser Coronary Angioplasty: Mechanisms and Results Compared with PTCA Alone. Circulation 1997, 96, 2183–2189. [Google Scholar] [CrossRef]
- Nishino, M.; Mori, N.; Takiuchi, S.; Shishikura, D.; Doi, N.; Kataoka, T.; Ishihara, T.; Kinoshita, N. Indications and Outcomes of Excimer Laser Coronary Atherectomy: Efficacy and Safety for Thrombotic Lesions—The ULTRAMAN Registry. J. Cardiol. 2017, 69, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, J.; Din, J.N.; Talwar, S.; O’Kane, P. Coronary Intervention with the Excimer Laser: Review of the Technology and Outcome Data. Interv. Cardiol. Rev. 2016, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Karacsonyi, J.; Danek, B.A.; Karatasakis, A.; Ungi, I.; Banerjee, S.; Brilakis, E.S. Laser Coronary Atherectomy During Contrast Injection for Treating an Underexpanded Stent. JACC Cardiovasc. Interv. 2016, 9, e147–e148. [Google Scholar] [CrossRef] [PubMed]
- Deckelbaum, L.I.; Natarajan, M.K.; Bittl, J.A.; Rohlfs, K.; Scott, J.; Chisholm, R.; Bowman, K.A.; Strauss, B.H. Effect of Intracoronary Saline Infusion on Dissection during Excimer Laser Coronary Angioplasty: A Randomized Trial. J. Am. Coll. Cardiol. 1995, 26, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Tsuchida, K.; Yuasa, S.; Taya, Y.; Koshikawa, T.; Tanaka, K.; Fujita, S.; Ikeda, Y.; Takahashi, M.; Okabe, M.; et al. The Effect of the Debulking by Excimer Laser Coronary Angioplasty on Long-Term Outcome Compared with Drug-Coating Balloon: Insights from Optical Frequency Domain Imaging Analysis. Lasers Med. Sci. 2020, 35, 403–412. [Google Scholar] [CrossRef]
- Ashikaga, T.; Yoshikawa, S.; Isobe, M. The Effectiveness of Excimer Laser Coronary Atherectomy with Contrast Medium for Underexpanded Stent: The Findings of Optical Frequency Domain Imaging. Catheter. Cardiovasc. Interv. 2015, 86, 946–949. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafiabadi Hassani, N.; Ogliari, L.C.; Vieira de Oliveira Salerno, P.R.; Pereira, G.T.R.; Ribeiro, M.H.; Palma Dallan, L.A. In-Stent Restenosis Overview: From Intravascular Imaging to Optimal Percutaneous Coronary Intervention Management. Medicina 2024, 60, 549. https://doi.org/10.3390/medicina60040549
Shafiabadi Hassani N, Ogliari LC, Vieira de Oliveira Salerno PR, Pereira GTR, Ribeiro MH, Palma Dallan LA. In-Stent Restenosis Overview: From Intravascular Imaging to Optimal Percutaneous Coronary Intervention Management. Medicina. 2024; 60(4):549. https://doi.org/10.3390/medicina60040549
Chicago/Turabian StyleShafiabadi Hassani, Neda, Lucas Carlini Ogliari, Pedro Rafael Vieira de Oliveira Salerno, Gabriel Tensol Rodrigues Pereira, Marcelo Harada Ribeiro, and Luis Augusto Palma Dallan. 2024. "In-Stent Restenosis Overview: From Intravascular Imaging to Optimal Percutaneous Coronary Intervention Management" Medicina 60, no. 4: 549. https://doi.org/10.3390/medicina60040549
APA StyleShafiabadi Hassani, N., Ogliari, L. C., Vieira de Oliveira Salerno, P. R., Pereira, G. T. R., Ribeiro, M. H., & Palma Dallan, L. A. (2024). In-Stent Restenosis Overview: From Intravascular Imaging to Optimal Percutaneous Coronary Intervention Management. Medicina, 60(4), 549. https://doi.org/10.3390/medicina60040549







