The Jack-in-the-Box: Pericardial Decompression Syndrome Managed by a Multidisciplinary Approach with Early Initiation of Veno-Arterial Extracorporeal Membrane Oxygenation: A Case Report
Abstract
1. Introduction
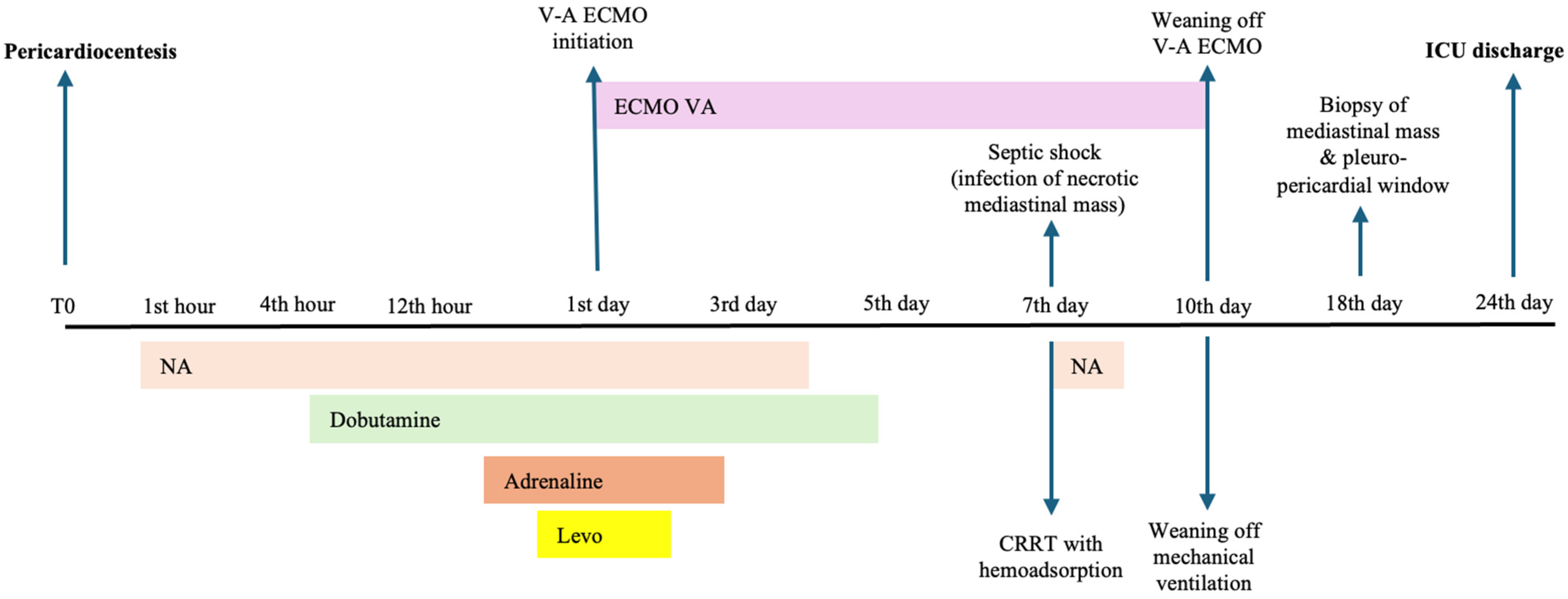
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vandyke, W.H.; Cure, J.; Chakko, C.S.; Gheorghiade, M. Pulmonary edema after pericardiocentesis for cardiac tamponade. N. Engl. J. Med. 1983, 309, 595–596. [Google Scholar] [CrossRef]
- Sarode, K.; Patel, A.; Arrington, K.; Makhija, R.; Mukherjee, D. Pericardial Decompression Syndrome: A Comprehensive Review of a Controversial Entity. Int. J. Angiol. 2024, 33, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, Y.; Goyal, A.; Khalid, N.; Sharma, N.; Nayyar, R.; Spodick, D.H.; Chhabra, L. Pericardial decompression syndrome: A comprehensive review. World J. Cardiol. 2019, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.W.; Edelman, E.R. Transient systolic dysfunction after relief of cardiac tamponade. Ann. Intern. Med. 1993, 119, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Hamaya, Y.; Dohi, S.; Ueda, N.; Akamatsu, S. Severe circulatory collapse immediately after pericardiocentesis in a patient with chronic cardiac tamponade. Anesth. Analg. 1993, 77, 1278–1281. [Google Scholar] [CrossRef]
- Nelson, D.M.; Brennan, A.P.; Burns, A.T.; MacIsaac, A.I. Pericardial decompression syndrome with acute right ventricular failure: A case series. Eur. Heart J. Case Rep. 2023, 7, ytad275. [Google Scholar] [CrossRef]
- Skalidis, E.I.; Kochiadakis, G.E.; Chrysostomakis, S.I.; Igoumenidis, N.E.; Manios, E.G.; Vardas, P.E. Effect of pericardial pressure on human coronary circulation. Chest 2000, 117, 910–912. [Google Scholar] [CrossRef]
- Chamoun, A.; Cenz, R.; Mager, A.; Rahman, A.; Champion, C.; Ahmad, M.; Birnbaum, Y. Acute left ventricular failure after large volume pericardiocentesis. Clin. Cardiol. 2003, 26, 588–590. [Google Scholar] [CrossRef]
- Amro, A.; Mansoor, K.; Amro, M.; Sobeih, A.; Suliman, M.; Okoro, K.; El-Hamdani, R.; Vilchez, D.; El-Hamdani, M.; Shweihat, Y.R. A comprehensive systemic literature review of pericardial decompression syndrome: Often unrecognized and potentially fatal syndrome. Curr. Cardiol. Rev. 2021, 17, 101–110. [Google Scholar] [CrossRef]
- Angouras, D.C.; Dosios, T. Pericardial decompression syndrome: A term for a well-defined but rather underreported complication of pericardial drainage. Ann. Thorac. Surg. 2010, 89, 1702–1703. [Google Scholar] [CrossRef]
- Imazio, M. Pericardial decompression syndrome: A rare but potentially fatal complication of pericardial drainage to be recognized and prevented. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, M.T.; Kampka, Z.; Drabczyk, M.; Zielonka, M.; Urbaniec, P.; Wypych, G.; Cichoń, M.; Szatan, T.; Jastrzębski, P.; Mizia-Stec, K. Clinical characteristics and risk factors of in-hospital mortality among patients undergoing percutaneous pericardiocentesis. Front. Cardiovasc. Med. 2023, 10, 1252525. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.L.; McAleer, E.; Stillwell, E.; Bott, M.; Rusch, V.W.; Schaffer, W.; Huang, J. Pericardial effusions in the cancer population: Prognostic factors after pericardial window and the impact of paradoxical hemodynamic instability. J. Thorac. Cardiovasc. Surg. 2011, 141, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, C.; Lekhakul, A.; Assawakawintip, C.; Fenstad, E.; Pislaru, S.; Sinak, L.; Garvan, K.C. Pericardial Decompression Syndrome: Incidence in a Large Consecutive Series of Echocardiographic-Guided Pericardiocentesis Procedures. Circulation 2018, 138 (Suppl. 1), A17022. [Google Scholar] [CrossRef]
- Pradhan, R.; Okabe, T.; Yoshida, K.; Angouras, D.C.; DeCaro, M.V.; Marhefka, G.D. Patient characteristics and predictors of mortality associated with pericardial decompression syndrome: A comprehensive analysis of published cases. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 113–120. [Google Scholar] [CrossRef]
- Sinnaeve, P.R.; Adriaenssens, T. A contemporary look at pericardiocentesis. Trends Cardiovas Med. 2019, 29, 375–383. [Google Scholar] [CrossRef]
- Ristić, A.D.; Imazio, M.; Adler, Y.; Anastasakis, A.; Badano, L.P.; Brucato, A.; Caforio, A.L.; Dubourg, O.; Elliott, P.; Gimeno, J.; et al. Triage strategy for urgent management of cardiac tamponade: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2014, 35, 2279–2284. [Google Scholar] [CrossRef]
- Johny, D.; Subramanyam, K.; Baikunje, N.; Hosmane, G.B. Cardiac tamponade and massive pleural effusion in a young COVID-19-positive adult. BMJ Case Rep. 2021, 14, e244518. [Google Scholar] [CrossRef]
- Walker, C.; Peyko, V.; Farrell, C.; Awad-Spirtos, J.; Adamo, M.; Scrocco, J. Pericardial effusion and cardiac tamponade requiring pericardial window in an otherwise healthy 30-year-old patient with COVID-19: A case report. J. Med. Case Rep. 2020, 14, 158. [Google Scholar] [CrossRef]
- Trimarchi, G.; Zito, C.; Pelaggi, G.; Carerj, S.; Di Bella, G. Pericardial agenesis: A case report of a rare congenital heart disease. Eur. Heart J. Case Rep. 2024, 8, ytae200. [Google Scholar] [CrossRef]
- Kuroda, M.; Amano, M.; Enomoto, S.; Miyake, M.; Kondo, H.; Tamura, T.; Kaitani, K.; Izumi, C.; Nakagawa, Y. Severe right ventricular and tricuspid valve dysfunction after pericardiocentesis. J. Med. Ultrason. 2001, 43, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Al Banna, R.; Husain, A. Reversible severe biventricular dysfunction postpericardiocentesis for tuberculous pericardial tamponade. BMJ Case Rep. 2011, 2011, bcr0220113837. [Google Scholar] [CrossRef]
- Laimoud, M.; Machado, P.; Zadra, A.R.; Maghirang, M.; Alenazy, A. Emergency Veno-Arterial Extracorporeal Membrane Oxygenation for Pericardial Decompression Syndrome. Case Rep. Cardiol. 2022, 1, 5440635. [Google Scholar] [CrossRef] [PubMed]
- Adi, O.; Fong, C.P.; Ahmad, A.H.; Panebianco, N. Worsening cardiac tamponade after pericardiocentesis in a patient with anterior mediastinum mass: A case report. Eur. Heart J.-Case Rep. 2022, 6, ytac329. [Google Scholar] [CrossRef] [PubMed]
- Veiras, S.; Bolón, A.; Aneiros, F. Ventricular Failure After Pericardial Decompression-Successful Outcome By Early ECMO Implant. Surg. Res. 2019, 1, 1–3. [Google Scholar]
- Matic, T.; Bakos, M.; Saric, D.; Cvitkovic, M.; Salek, Z.; Mestrovic, D.; Bilic, E. Low cardiac output syndrome requiring extracorporeal membrane oxygenation following pericardiocentesis in an adolescent with Hodgkin Lymphoma: A case report. Perfusion 2021, 36, 529–531. [Google Scholar] [CrossRef]
- Bratti, J.P.R.; Brunette, V.; Lebon, J.S.; Pellerin, M.; Lamarche, Y. Venoarterial extracorporeal membrane oxygenation support for severe pericardial decompression syndrome: A case report. Crit. Care Med. 2020, 48, e74–e75. [Google Scholar] [CrossRef]
- Kapoor, A.; McWhorter, Y.; Polce, D.R. Acute right ventricular failure immediately following subxiphoid pericardial window requiring temporary cardiopulmonary bypass support: A case report of pericardial decompression syndrome. Cureus 2022, 14, e26286. [Google Scholar] [CrossRef]
- Beckmann, E.; Ismail, I.; Cebotari, S.; Busse, A.; Martens, A.; Shrestha, M.; Kühn, C.; Haverich, A.; Fegbeutel, C. Right-sided heart failure and extracorporeal life support in patients undergoing pericardiectomy for constrictive pericarditis: A risk factor analysis for adverse outcome. J. Thorac. Cardiovasc. Surg. 2017, 65, 662–670. [Google Scholar]
| Parameter | ICU Admission | ICU Day 1 | ICU Day 3 | ICU Day 5 | ICU Day 7 | ICU Day 10 | ICU Discharge |
|---|---|---|---|---|---|---|---|
| Cardiovascular parameters | |||||||
| Noradrenaline (mcg/kg/min) | 0.1 | 0.75 | 0.5 | - | - | - | - |
| Adrenaline (mcg/kg/min) | - | 0.06 | - | - | - | - | - |
| Dobutamine (mcg/kg/min) | - | 10 | 6 | - | - | - | - |
| Levosimendan (mcg/kg/min) | - | 0.1 | - | - | - | - | - |
| Urine output (mL/kg/h) | 1.35 | 1.36 | <0.1 | 0.1 | <0.1 | <0.1 | 1.66 |
| Mean HR (beats/min) | 136 | 108 | 106 | 108 | 104 | 124 | 109 |
| BP: Sys/Dia/Mean (mmHg) | 136/102/113 | 60/40/46 | 98/52/67 | 90/52/64 | 112/73/86 | 106/50/68 | 128/73/91 |
| EF-TTE (%) | NA | 15 | 15 | 25 | 30 | 35 | 45 |
| CI (L/min/m2) | NA | 1.0 | 1.8 | NA | NA | NA | NA |
| ELWI (mL/kg) | NA | 15 | 16 | NA | NA | NA | NA |
| GEDI (mL/m2) | NA | 729 | 620 | NA | NA | NA | NA |
| SVRI (dyn·s·cm−5·m2) | NA | 5400 | 2800 | NA | NA | NA | NA |
| Paraclinical tests | |||||||
| ALT (U/L) | 284 | 400 | 606 | 374 | 140 | 33 | 14 |
| AST (U/L) | 347 | 569 | 607 | 170 | 50 | 30 | 40 |
| TB (mg/dL) | 2.67 | 2.16 | 2.84 | 3.45 | 2.54 | 2.9 | 1.84 |
| WBCs (×103/µL) | 10.6 | 11 | 13.1 | 10.6 | 9.5 | 8.9 | 7.4 |
| Hb (mg/dL) | 13.3 | 12.3 | 11.6 | 10.7 | 11.9 | 8.9 | 8.9 |
| PLTs (×103/µL) | 329 | 265 | 224 | 169 | 176 | 102 | 587 |
| SCr (mg/dL) | 1.04 | 1.5 | 1.45 | 1.41 | 1.02 | 3.83 | 1.17 |
| pH | 7.40 | 7.26 | 7.46 | 7.44 | 7.38 | 7.35 | 7.45 |
| PaO2/FiO2 | 304 | 194 | 322 | 356 | 280 | 255 | 480 |
| Lactate (mmol/L) | 2.80 | 2.29 | 1.58 | 1.35 | 0.72 | 1.02 | 1.32 |
| HCO3 (mmol/L) | 22.0 | 17.8 | 21.0 | 22.2 | 23.4 | 19.0 | 22.7 |
| Parameter | After Pericardial Drainage | At V-A ECMO Initiation | 5th Day on V-A ECMO | 7th Day on V-A ECMO | After V-A ECMO Weaning |
|---|---|---|---|---|---|
| LVEF (%) | 10 | 10 | 15 | 30 | 40 |
| TAPSE (mm) | 6 | 6 | 9 | 12 | 16 |
| LVOT-VTI (m/s) | 9 | 8 | NA | 11.5 | 16 |
| IVC (mm) | 30 | 44 | 22 | 22 | 18 |
| Mitral regurgitation grade | II | II | II | I | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orban, C.; Borjog, T.; Talpau, C.; Agapie, M.; Bratu, A.; Jafal, M.; Popescu, M. The Jack-in-the-Box: Pericardial Decompression Syndrome Managed by a Multidisciplinary Approach with Early Initiation of Veno-Arterial Extracorporeal Membrane Oxygenation: A Case Report. Medicina 2024, 60, 1747. https://doi.org/10.3390/medicina60111747
Orban C, Borjog T, Talpau C, Agapie M, Bratu A, Jafal M, Popescu M. The Jack-in-the-Box: Pericardial Decompression Syndrome Managed by a Multidisciplinary Approach with Early Initiation of Veno-Arterial Extracorporeal Membrane Oxygenation: A Case Report. Medicina. 2024; 60(11):1747. https://doi.org/10.3390/medicina60111747
Chicago/Turabian StyleOrban, Carmen, Tudor Borjog, Claudia Talpau, Mihaela Agapie, Angelica Bratu, Mugurel Jafal, and Mihai Popescu. 2024. "The Jack-in-the-Box: Pericardial Decompression Syndrome Managed by a Multidisciplinary Approach with Early Initiation of Veno-Arterial Extracorporeal Membrane Oxygenation: A Case Report" Medicina 60, no. 11: 1747. https://doi.org/10.3390/medicina60111747
APA StyleOrban, C., Borjog, T., Talpau, C., Agapie, M., Bratu, A., Jafal, M., & Popescu, M. (2024). The Jack-in-the-Box: Pericardial Decompression Syndrome Managed by a Multidisciplinary Approach with Early Initiation of Veno-Arterial Extracorporeal Membrane Oxygenation: A Case Report. Medicina, 60(11), 1747. https://doi.org/10.3390/medicina60111747






