Effect of Sarcopenia on Functional Recovery in Acute Stroke Patients Admitted for Standard Rehabilitation Program
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Procedure
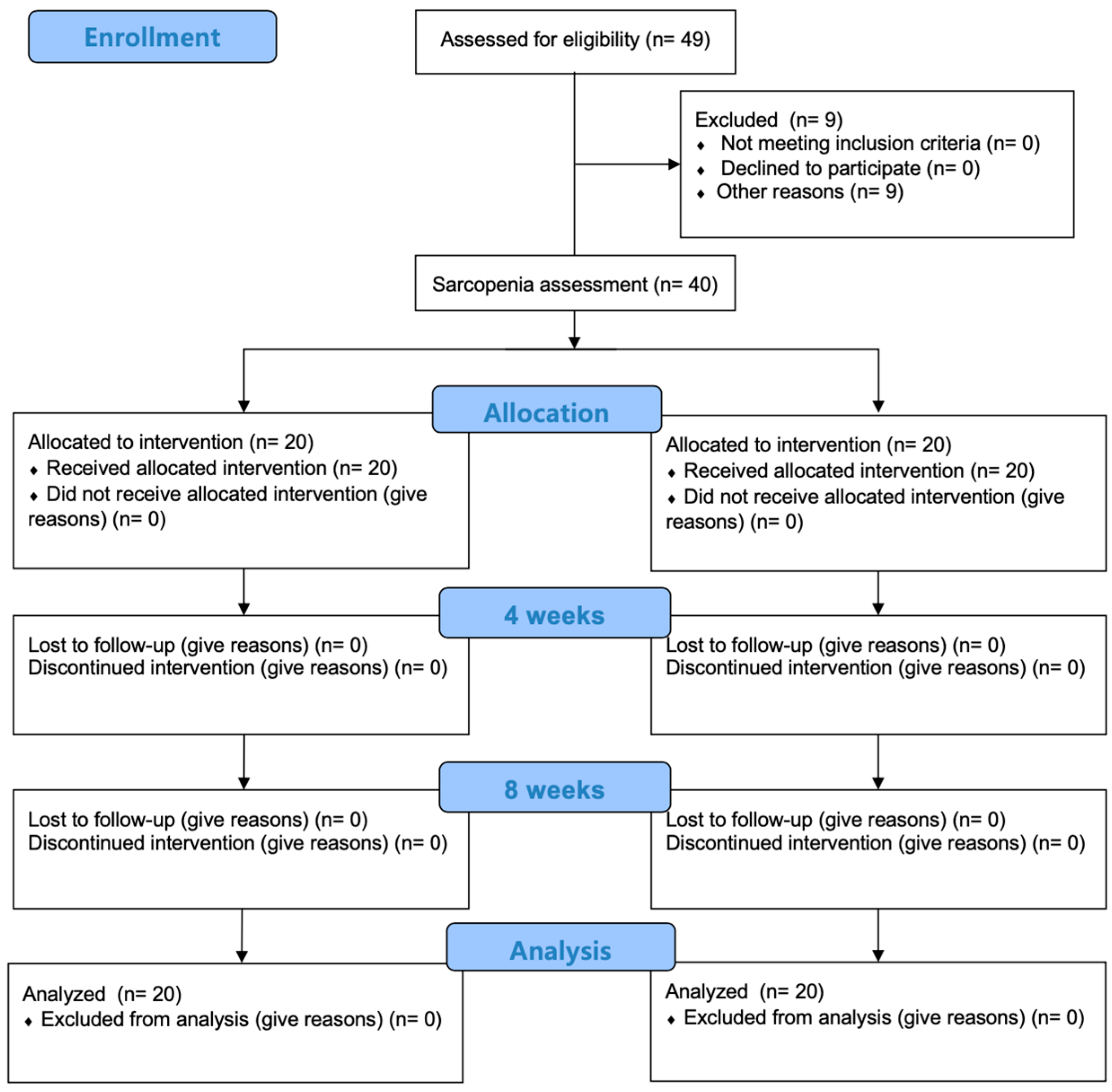
2.2. Participants
2.3. Diagnosis of Sarcopenia
2.4. Data Collection
2.4.1. Muscle Strength
2.4.2. Balance
2.4.3. Gait
2.4.4. Activities of Daily Living
2.5. Convalescent Rehabilitation Program
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knight-Greenfield, A.; Nario, J.J.Q.; Gupta, A. Causes of Acute Stroke: A Patterned Approach. Radiol. Clin. North Am. 2019, 57, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Sathian, K.; Buxbaum, L.J.; Cohen, L.G.; Krakauer, J.W.; Lang, C.E.; Corbetta, M.; Fitzpatrick, S.M. Neurological principles and rehabilitation of action disorders: Common clinical deficits. Neurorehabil. Neural. Repair. 2011, 25, 21S–32S. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.Y.; Kim, B.G.; Park, C.B.; Cho, W.S. Association of Lower-Limb Muscle Strength Asymmetry and Functional Factors in Patients with Stroke. J. Int. Acad. Phys. Ther. Res. 2023, 14, 2951–2956. [Google Scholar] [CrossRef]
- Hayes, S.; Donnellan, C.; Stokes, E. Executive dysfunction and balance function post-stroke: A cross-sectional study. Physiotherapy 2016, 102, 64–70. [Google Scholar] [CrossRef]
- Ikeji, R.; Nozoe, M.; Yamamoto, M.; Seike, H.; Kubo, H.; Shimada, S. Sarcopenia in patients following stroke: Prevalence and associated factors. Clin. Neurol. Neurosurg. 2023, 233, 107910. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, Y.A. Prevalence and risk factors of possible sarcopenia in patients with subacute stroke. PLoS ONE 2023, 18, e0291452. [Google Scholar] [CrossRef]
- Li, Y.; Hong, M.; Shi, H. Premorbid sarcopenia and functional outcome after acute stroke: A meta-analysis. Asia. Pac. J. Clin. Nutr. 2023, 32, 330–338. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, B.G.; Park, S.J. Association of Characteristics between Acute Stroke Patients and Sarcopenia: A Cross-Sectional Study. Exerc. Sci. 2024, 33, 216–222. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, F.; Yang, M.; Chen, Z. Sarcopenia and nervous system disorders. J. Neurol. 2022, 269, 5787–5797. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Broccatelli, M.; Savera, G.; D’Elia, M.; Pahor, M.; et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 35–42. [Google Scholar] [CrossRef]
- Galvão, A.C.J.; Dias, C.; Miranda, A.L.; Moura, D.; Palhares, C.V.T.; Oliveira Leopoldino, A.; Polese, J.C. Stroke related sarcopenia in individuals with different physical activity levels: A cross-sectional study. Physiother. Res. Int. 2024, 29, e2084. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Iwata, K.; Yoshimura, Y.; Shinoda, T.; Inagaki, Y.; Ohya, S.; Yamada, K.; Oyanagi, K.; Maekawa, Y.; Honda, A.; et al. Low Muscle Mass is Associated with Walking Function in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105259. [Google Scholar] [CrossRef]
- Sato, K.; Wakugami, K.; Iwata, T.; Tanaka, S.; Koike, M.; Ogawa, T. Low muscle mass in patients with stroke on admission reduces walking ability at discharge. Clin. Nutr. ESPEN 2024, 61, 333–337. [Google Scholar] [CrossRef]
- Mas, M.; González, J.; Frontera, W.R. Stroke and sarcopenia. Curr. Phys. Med. Rehabil. Rep. 2020, 8, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Bise, T.; Nagano, F.; Shimazu, S.; Shiraishi, A.; Yamaga, M.; Koga, H. Sarcopenia is associated with worse recovery of physical function and dysphagia and a lower rate of home discharge in Japanese hospitalized adults undergoing convalescent rehabilitation. Nutrition 2019, 61, 111–118. [Google Scholar] [CrossRef]
- Ohyama, K.; Watanabe, M.; Nosaki, Y.; Hara, T.; Iwai, K.; Mokuno, K. Correlation Between Skeletal Muscle Mass Deficit and Poor Functional Outcome in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 104623. [Google Scholar] [CrossRef] [PubMed]
- Jee, Y.S. Exercise rehabilitation strategy for the prevention of sarcopenia in cancer populations: 8th in a series of scientific evidence. J. Exerc. Rehabil. 2022, 18, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Z.; No, M.H.; Heo, J.W.; Park, D.H.; Kang, J.H.; Kim, S.H.; Kwak, H.B. Role of exercise in age-related sarcopenia. J. Exerc. Rehabil. 2018, 14, 551–558. [Google Scholar] [CrossRef]
- Park, J.G.; Lee, K.W.; Kim, S.B.; Lee, J.H.; Kim, Y.H. Effect of Decreased Skeletal Muscle Index and Hand Grip Strength on Functional Recovery in Subacute Ambulatory Stroke Patients. Ann. Rehabil. Med. 2019, 43, 535–543. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lim, J.H.; Park, C.B.; Kim, B.G. Immediate effects of a vibrating foam roller on dorsiflexion rom, balance, and gait in stroke patients: A randomized controlled trial. J. Exerc. Rehabil. 2024, 20, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in stroke rehabilitation: A systematic review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Usuda, S.; Araya, K.; Umehara, K.; Endo, M.; Shimizu, T.; Endo, F. Construct Validity of Functional Balance Scale in Stroke Inpatients. J. Phys. Ther. Sci. 1998, 10, 53–56. [Google Scholar] [CrossRef]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meissner, D.; Pohl, M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef]
- Ohura, T.; Hase, K.; Nakajima, Y.; Nakayama, T. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med. Res. Methodol. 2017, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshimura, Y.; Abe, T.; Nagano, F.; Matsumoto, A. Impact of trunk and appendicular skeletal muscle mass on improving swallowing function in acute stroke patients. J. Stroke Cerebrovasc. Dis. 2022, 31, 106636. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Wakabayashi, H.; Nagano, F.; Bise, T.; Shimazu, S.; Shiraishi, A.; Kido, Y.; Matsumoto, A. Chair-Stand Exercise Improves Sarcopenia in Rehabilitation Patients after Stroke. Nutrients 2022, 14, 461. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2019, 58, 1–6. [Google Scholar] [CrossRef]
| Variable | SWSG (n = 20) | SWOSG (n = 20) | p |
|---|---|---|---|
| Type of stroke (hemorrhage/infarct) | 10/10 | 7/13 | 0.337 |
| Vessel (large/small) | 20/0 | 19/1 | 0.311 |
| Territory (Carotid artery/ vertebro-basilar artery) | 15/5 | 14/6 | 0.723 |
| Hemiplegic side (Lt/Rt) | 9/11 | 11/9 | 0.527 |
| Sex (male/female) | 8/12 | 9/11 | 0.749 |
| Onset of stroke (day) | 32.25 ± 21.48 | 32.20 ± 20.95 | 0.646 |
| Age (years) | 72.00 ± 10.48 | 66.45 ± 11.79 | 0.096 |
| Height (cm) | 160.15 ± 7.65 | 162.33 ± 6.40 | 0.272 |
| Weight (kg) | 57.64 ± 9.54 | 62.47 ± 10.19 | 0.175 |
| MMSE (Score) | 20.35 ± 5.88 | 24.00 ± 4.60 | 0.065 |
| Skeletal muscle mass index (kg/m2) | 5.42 ± 0.91 | 6.90 ± 0.88 | <0.001 * |
| Grip strength (kg) | 10.43 ± 8.50 | 17.50 ± 10.81 | 0.025 * |
| Pre-MMT (score) | 106.35 ± 16.09 | 111.85 ± 12.80 | 0.357 |
| Pre-BBS (score) | 24.40 ± 17.53 | 26.80 ± 13.71 | 0.725 |
| Pre-FAC (score) | 1.55 ± 1.10 | 1.65 ± 0.99 | 0.692 |
| Pre-MBI (score) | 43.95 ± 22.41 | 48.75 ± 21.86 | 0.440 |
| Variable | Groups | 4 Weeks | 8 Weeks | χ2 (p) | 1–4 Weeks Z (p) | 1–8 Weeks Z (p) | 4–8 Weeks Z (p) |
|---|---|---|---|---|---|---|---|
| MMT (score) | SWSG (n = 20) | 107.95 ± 15.43 | 108.70 ± 15.42 | 7.538 (0.023 *) | −0.931 (0.352) | −1.980 (0.048) | −2.588 (0.100) |
| SWOSG (n = 20) | 116.20 ± 10.87 | 117.7 ± 11.73 | 24.000 (< 0.001 *) | −2.812 (0.005 †) | −3.299 (0.001 †) | −2.842 (0.004 †) | |
| BBS (score) | SWSG (n = 20) | 25.85 ± 16.62 | 28.00 ± 15.66 | 12.035 (0.002 *) | −2.119 (0.034) | −2.617 (0.009 †) | −2.613 (0.009 †) |
| SWOSG (n = 20) | 37.10 ± 13.95 | 39.70 ± 13.30 | 29.681 (<0.001 *) | −3.434 (0.001 †) | −3.622 (<0.001 †) | −3.507 (<0.001 †) | |
| FAC (score) | SWSG (n = 20) | 1.75 ± 1.16 | 2.00 ± 1.26 | 12.560 (0.002 *) | −2.000 (0.046) | −2.714 (0.007 †) | −2.236 (0.025) |
| SWOSG (n = 20) | 2.5 ± 1.19 | 3.10 ± 1.33 | 25.107 (<0.001 *) | −2.942 (0.003 †) | −3.482 (<0.001 †) | −3.464 (0.001 †) | |
| MBI (score) | SWSG (n = 20) | 47.10 ± 21.15 | 49.10 ± 20.96 | 15.500 (<0.001 *) | −2.439 (0.015 †) | −2.731 (0.006 †) | −2.099 (0.036) |
| SWOSG (n = 20) | 59.25 ± 16.77 | 64.10 ± 17.69 | 34.060 (<0.001 *) | −3.728 (<0.001 †) | −3.725 (<0.001 †) | −3.186 (0.001 †) |
| Difference Value | SWSG (n = 20) | SWOSG (n = 20) | p |
|---|---|---|---|
| MMT difference after 4 weeks | 1.60 ± 5.17 | 4.35 ± 6.63 | 0.027 * |
| MMT difference after 8 weeks | 2.35 ± 5.26 | 5.85 ± 7.13 | 0.038 * |
| BBS difference after 4 weeks | 1.45 ± 3.63 | 10.30 ± 12.22 | 0.004 * |
| BBS difference after 8 weeks | 3.60 ± 5.83 | 12.90 ± 12.86 | 0.006 * |
| FAC difference after 4 weeks | 0.20 ± 0.41 | 0.85 ± 1.04 | 0.014 * |
| FAC difference after 8 weeks | 0.45 ± 0.60 | 1.45 ± 1.19 | 0.003 * |
| MBI difference after 4 weeks | 3.15 ± 5.12 | 10.50 ± 11.69 | 0.009 * |
| MBI difference after 8 weeks | 5.15 ± 7.49 | 15.35 ± 14.49 | 0.007 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Cho, W.-S.; Park, C.-B.; Kim, B.-G. Effect of Sarcopenia on Functional Recovery in Acute Stroke Patients Admitted for Standard Rehabilitation Program. Medicina 2024, 60, 1716. https://doi.org/10.3390/medicina60101716
Kim S-Y, Cho W-S, Park C-B, Kim B-G. Effect of Sarcopenia on Functional Recovery in Acute Stroke Patients Admitted for Standard Rehabilitation Program. Medicina. 2024; 60(10):1716. https://doi.org/10.3390/medicina60101716
Chicago/Turabian StyleKim, So-Yeong, Woon-Su Cho, Chi-Bok Park, and Byeong-Geun Kim. 2024. "Effect of Sarcopenia on Functional Recovery in Acute Stroke Patients Admitted for Standard Rehabilitation Program" Medicina 60, no. 10: 1716. https://doi.org/10.3390/medicina60101716
APA StyleKim, S.-Y., Cho, W.-S., Park, C.-B., & Kim, B.-G. (2024). Effect of Sarcopenia on Functional Recovery in Acute Stroke Patients Admitted for Standard Rehabilitation Program. Medicina, 60(10), 1716. https://doi.org/10.3390/medicina60101716






