Adrenal Insufficiency in Patients with Beta Thalassemia: A Meta-Analysis
Abstract
1. Introduction
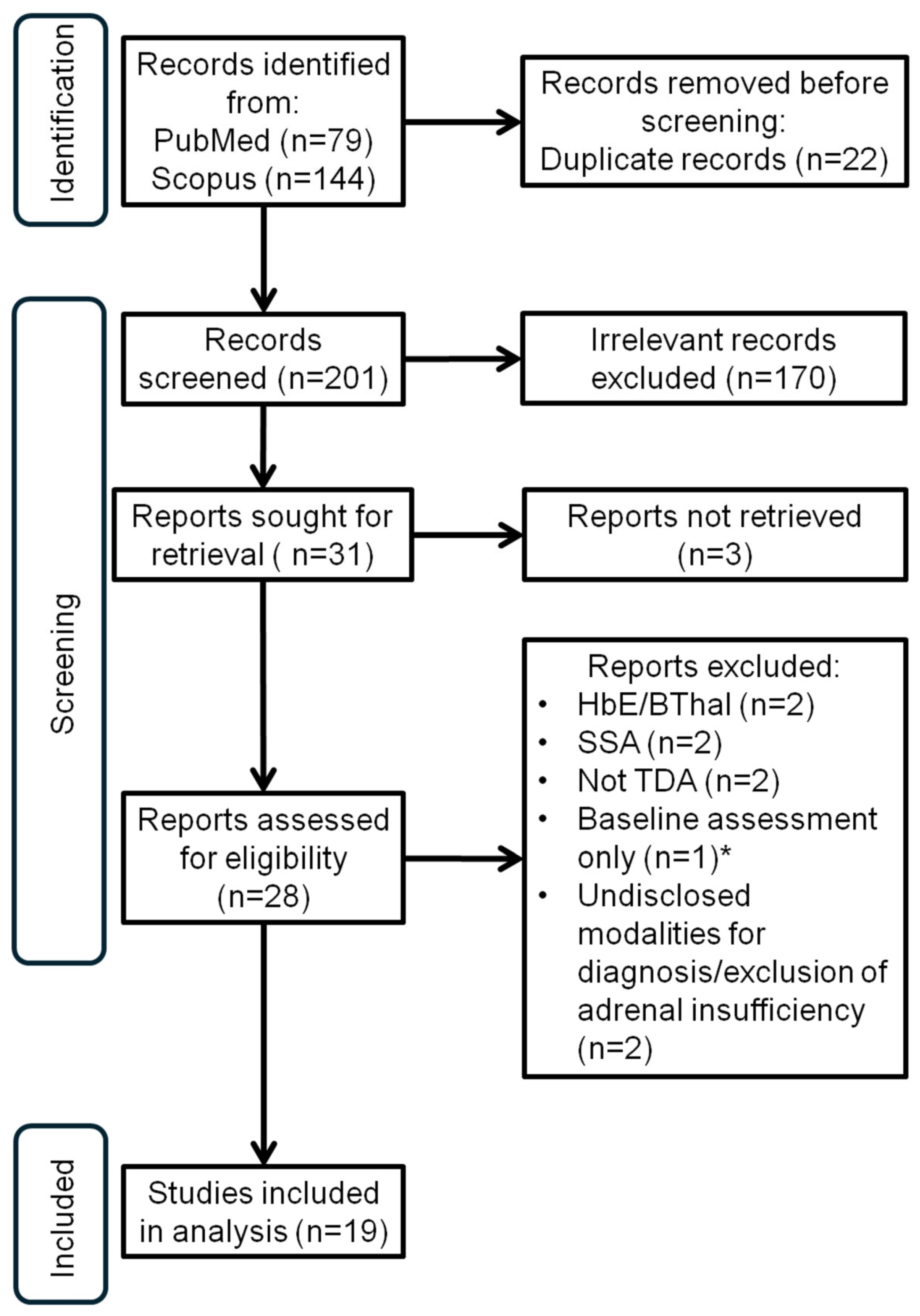
2. Materials and Methods
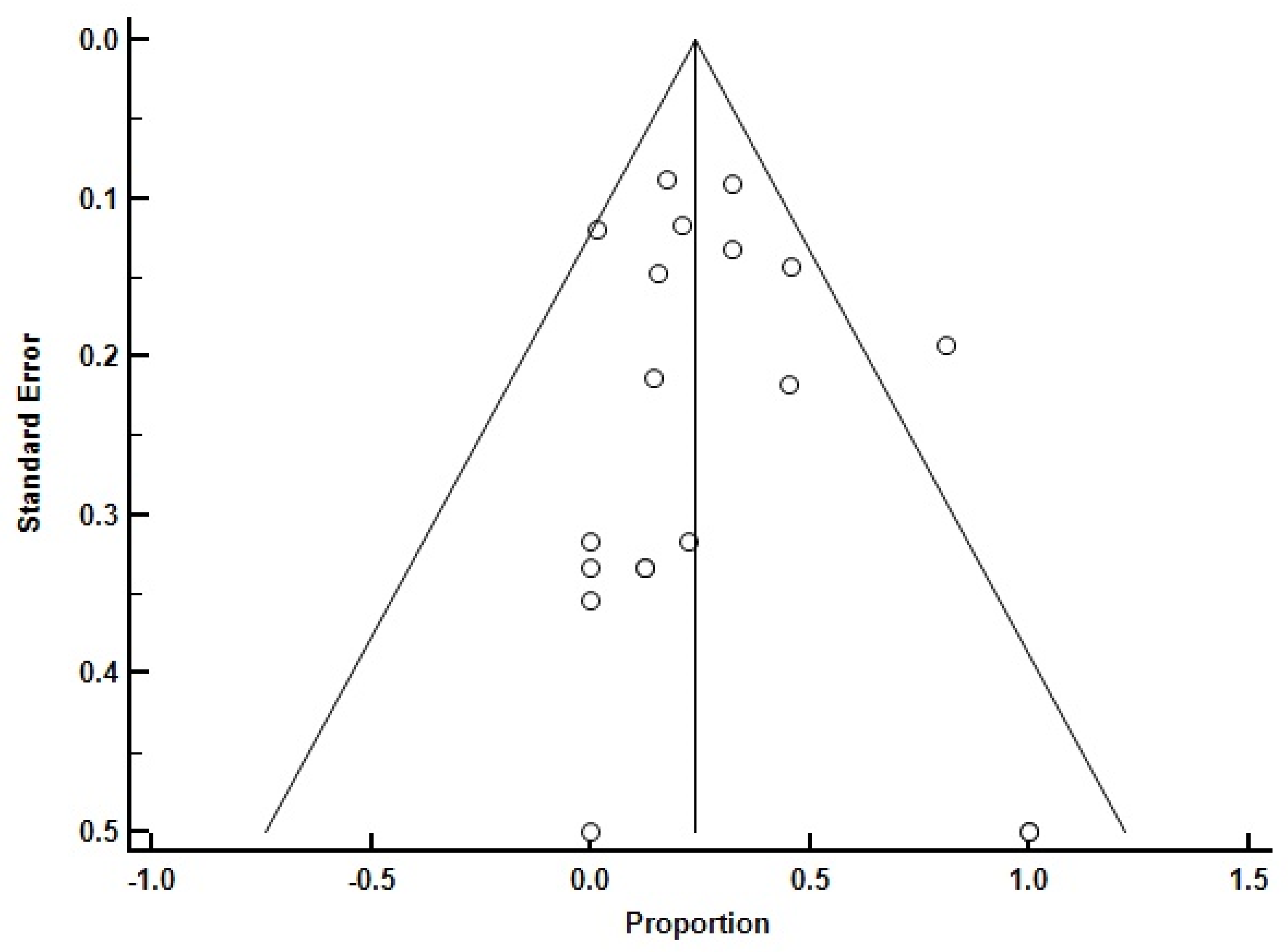
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Virgiliis, S.; Congia, M.; Frau, F.; Argiolu, F.; Diana, G.; Cucca, F.; Varsi, A.; Sanna, G.; Podda, G.; Fodde, M.; et al. Deferoxamine-induced growth retardation in patients with thalassemia major. J. Pediatr. 1988, 113, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chabre, O.; Goichot, B.; Zenaty, D.; Bertherat, J. Epidemiology of primary and secondary adrenal insufficiency: Prevalence and incidence, acute adrenal insufficiency, long-term morbidity and mortality. Ann. Endocrinol. 2017, 78, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Evangelidis, P.; Venou, T.M.; Fani, B.; Vlachaki, E.; Gavriilaki, E. Endocrinopathies in Hemoglobinopathies: What Is the Role of Iron? Int. J. Mol. Sci. 2023, 24, 16263. [Google Scholar] [CrossRef] [PubMed]
- Bou-Fakhredin, R.; Motta, I.; Cappellini, M.D.; Taher, A.T. Clinical Complications and Their Management. Hematol. Oncol. Clin. N. Am. 2023, 37, 365–378. [Google Scholar] [CrossRef]
- Baldini, M.; Mancarella, M.; Cassinerio, E.; Marcon, A.; Ambrogio, A.G.; Motta, I. Adrenal insufficiency: An emerging challenge in thalassemia? Am. J. Hematol. 2017, 92, E119–E121. [Google Scholar] [CrossRef] [PubMed]
- Uçar, A.; Öner, N.; Özek, G.; Çetinçakmak, M.G.; Abuhandan, M.; Yıldırım, A.; Kaya, C.; Ünverdi, S.; Emeksiz, H.C.; Yılmaz, Y.; et al. Evaluation of the glucocorticoid, mineralocorticoid, and adrenal androgen secretion dynamics in a large cohort of patients aged 6–18 years with transfusion-dependent β-thalassemia major, with an emphasis on the impact of cardiac iron load. Endocrine 2016, 53, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Jaruratanasirikul, S.; Tanchotikul, S.; Wongcharnchailert, M.; Laosombat, V.; Sangsupavanich, P.; Leetanaporn, K. A low dose adrenocorticotropin test (1 microg ACTH) for the evaluation of adrenal function in children with beta-thalassemia receiving hypertransfusion with suboptimal iron-chelating therapy. J. Pediatr. Endocrinol. Metab. 2007, 20, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Srivatsa, A.; Marwaha, R.K.; Muraldharan, R.; Trehan, A. Assessment of adrenal endocrine function in Asian thalassemics. Indian Pediatr. 2005, 42, 31–35. [Google Scholar] [PubMed]
- Karamifar, H.; Shahriari, M.; Sadjadian, N. Prevalence of endocrine complications in beta-thalassaemia major in the Islamic Republic of Iran. East Mediterr. Health J. 2003, 9, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Elsedfy, H.H.; El Kholy, M.; Hamza, R.T.; Hamed, A.; Elalfy, M. Adrenal function in thalassemia major adolescents. Pediatr. Endocrinol. Rev. 2011, 8 (Suppl. 2), 295–299. [Google Scholar] [PubMed]
- McIntosh, N. Pituitary-adrenal function in thalassaemia major. Arch. Dis. Child. 1973, 48, 653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- McIntosh, N. Endocrinopathy in thalassaemia major. Arch. Dis. Child. 1976, 51, 195–201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- McIntosh, N. Threshold adrenocortical function in children with thalassaemia. J. Endocrinol. 1976, 68, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Vannasaeng, S.; Ploybutr, S.; Visutkul, P.; Tandhanand, S.; Suwanik, R.; Wasi, P. Endocrine function in thalassaemia. Clin. Endocrinol. 1981, 14, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lassman, M.N.; O’Brien, R.T.; Pearson, H.A.; Wise, J.K.; Donabedian, R.K.; Felig, P.; Genel, M. Endocrine evaluation in thalassemia major. Ann. N. Y. Acad Sci. 1974, 232, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Ambrogio, A.G.; Danesi, L.; Baldini, M.; Radin, R.; Cassinerio, E.; Graziadei, G.; Mirra, N.; D’Angelo, E.; Marcon, A.; Mancarella, M.; et al. Low-dose Synachten test with measurement of salivary cortisol in adult patients with β-thalassemia major. Endocrine 2018, 60, 348–354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scacchi, M.; Danesi, L.; Cattaneo, A.; Valassi, E.; Pecori Giraldi, F.; Radaelli, P.; Ambrogio, A.; D’Angelo, E.; Mirra, N.; Zanaboni, L.; et al. The pituitary-adrenal axis in adult thalassaemic patients. Eur. J. Endocrinol. 2010, 162, 43–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Sanctis, V.; Elsedfy, H.; Soliman, A.T.; Elhakim, I.Z.; Soliman, N.A.; Karimi, M.; Elalaily, R. The diagnostic approach to central adrenocortical insufficiency (CAI) in thalassemia. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016026. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.E.; Mittelman, S.D.; Coates, T.D.; Geffner, M.E.; Wood, J.C. A significant proportion of thalassemia major patients have adrenal insufficiency detectable on provocative testing. J. Pediatr. Hematol. Oncol. 2015, 37, 54–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poomthavorn, P.; Isaradisaikul, B.; Chuansumrit, A.; Khlairit, P.; Sriphrapradang, A.; Mahachoklertwattana, P. High prevalence of “biochemical” adrenal insufficiency in thalassemics: Is it a matter of different testings or decreased cortisol binding globulin? J. Clin. Endocrinol. Metab. 2010, 95, 4609–4615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwan, E.Y.; Lee, A.C.; Li, A.M.; Tam, S.C.; Chan, C.F.; Lau, Y.L.; Low, L.C. A cross-sectional study of growth, puberty and endocrine function in patients with thalassaemia major in Hong Kong. J. Paediatr. Child Health 1995, 31, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Sklar, C.A.; Lew, L.Q.; Yoon, D.J.; David, R. Adrenal function in thalassemia major following long-term treatment with multiple transfusions and chelation therapy. Evidence for dissociation of cortisol and adrenal androgen secretion. Am. J. Dis. Child. 1987, 141, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Canale, V.C.; Steinherz, P.; New, M.; Erlandson, M. Endocrine function in thalassemia major. Ann. N. Y. Acad. Sci. 1974, 232, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.A.; Bonduel, M.; Sciuccati, G.; del Pozo, A.; Roldán, A.; Ciaccio, M.; Orazi, V.; Fano, V.; Ozuna, B.; Lejarraga, H.; et al. Talasemia Mayor en la Argentina. Medicina 2002, 62, 124–134. [Google Scholar] [PubMed]
- Rafsanjani, K.A.; Mafi, N.; Tafreshi, R.I. Complications of β-thalassemia intermedia in Iran during 1996–2010 (single-center study). Pediatr. Hematol. Oncol. 2011, 28, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Hamidieh, A.A.; Mohseni, F.; Behfar, M.; Hamidi, Z.; Alimoghaddam, K.; Pajouhi, M.; Larijani, B.; Mohajeri-Tehrani, M.R.; Ghavamzadeh, A. Short-term Assessment of HSCT Effects on the Hypothalamus-Pituitary Axis in Pediatric Thalassemic Patients. Arch. Iran. Med. 2018, 21, 56–60. [Google Scholar]
- Shaikh, S.; Nagendra, L.; Shaikh, S.; Pappachan, J.M. Adrenal Failure: An Evidence-Based Diagnostic Approach. Diagnostics 2023, 13, 1812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Sanctis, V.; Soliman, A.T.; Elsedfy, H.; Skordis, N.; Kattamis, C.; Angastiniotis, M.; Karimi, M.; Yassin, M.A.; El Awwa, A.; Stoeva, I.; et al. Growth and endocrine disorders in thalassemia: The international network on endocrine complications in thalassemia (I-CET) position statement and guidelines. Indian J. Endocrinol. Metab. 2013, 17, 8–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lazarus, K.; Hayes, A.; Narula, K.; Papadopolou, D.; Tan, T.M.; Meeran, K.; Choudhury, S. Redefining ITT cortisol thresholds on Abbott platforms to prevent misdiagnosis of adrenal insufficiency. Clin. Endocrinol. 2024, 101, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Charoensri, S.; Auchus, R.J. A Contemporary Approach to the Diagnosis and Management of Adrenal Insufficiency. Endocrinol. Metab. 2024, 39, 73–82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bangar, V.; Clayton, R.N. How reliable is the short synacthen test for the investigation of the hypothalamic-pituitary-adrenal axis? Eur. J. Endocrinol. 1998, 139, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Yalovitsky, G.; Shaki, D.; Hershkovitz, E.; Friger, M.; Haim, A. Comparison of glucagon stimulation test and low dose ACTH test in assessing hypothalamic-pituitary-adrenal (HPA) axis in children. Clin. Endocrinol. 2023, 98, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Ach, T.; Yosra, H.; Jihen, M.; Abdelkarim Asma, B.; Maha, K.; Molka, C.; Rouatbi, S.; Monia, Z.; Ach, K. Cortisol cut-points for the glucagon stimulation test in the evaluation of hypothalamic pituitary adrenal axis. Endocr. J. 2018, 65, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Eleftheriou, A.; Bain, B. Management of Transfusion-Dependent Thalassaemia (TDT). A Short Guide; Thalassaemia Intertational Federation: Nicosia, Cyprus, 2022. [Google Scholar]
- Lal, A.; Wong, T.; Keel, S.; Pagano, M.; Chung, J.; Kamdar, A.; Rao, L.; Ikeda, A.; Puthenveetil, G.; Shah, S.; et al. The transfusion management of beta thalassemia in the United States. Transfusion 2021, 61, 3027–3039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olivieri, N.F.; Nathan, D.G.; MacMillan, J.H.; Wayne, A.S.; Liu, P.P.; McGee, A.; Martin, M.; Koren, G.; Cohen, A.R. Survival in medically treated patients with homozygous beta-thalassemia. N. Engl. J. Med. 1994, 331, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, J.L.; Kim, H.Y.; Thompson, A.A.; Quinn, C.T.; Mueller, B.U.; Odame, I.; Giardina, P.J.; Vichinsky, E.P.; Boudreaux, J.M.; Cohen, A.R.; et al. Chelation use and iron burden in North American and British thalassemia patients: A report from the Thalassemia Longitudinal Cohort. Blood 2012, 119, 2746–2753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farmakis, D.; Porter, J.; Taher, A.; Domenica Cappellini, M.; Angastiniotis, M.; Eleftheriou, A. 2021 Thalassaemia International Federation Guidelines for the Management of Transfusion-dependent Thalassemia. Hemasphere 2022, 6, e732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patterson, S.; Singleton, A.; Branscomb, J.; Nsonwu, V.; Spratling, R. Transfusion Complications in Thalassemia: Patient Knowledge and Perspectives. Front. Med. 2022, 9, 772886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cappellini, M.D.; Farmakis, D.; Porter, J.; Taher, A. Guidelines for the Management of Transfusion Dependent Thalassemia (TDT); Thalassaemia International Federation: Nicosia, Cyprus, 2021. [Google Scholar]
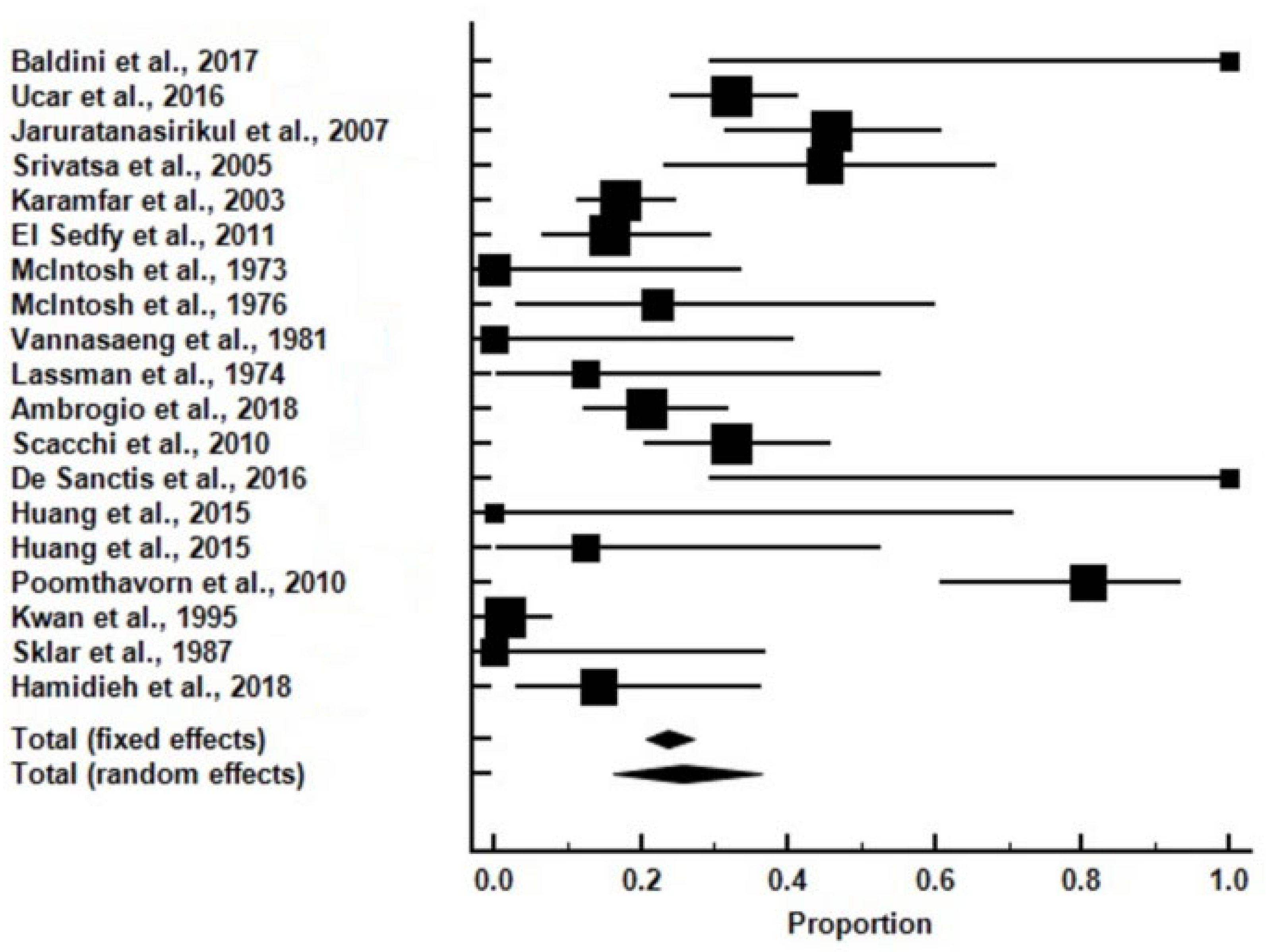
| Study | Subjects (n) | Subjects with Adrenal Insufficiency (n) | Testing Modality | Subjects |
|---|---|---|---|---|
| Hamidieh et al., 2018 [27] | 21 | 3 | Tetracosactrin | Young persons |
| Ucar et al., 2016 [7] | 121 | 39 | Tetracosactrin | Young persons |
| Jaruratanasirikul et al., 2007 [8] | 48 | 22 | Tetracosactrin | Young persons |
| Srivatsa et al., 2005 [9] | 20 | 9 | Tetracosactrin | Young persons |
| Karamfar et al., 2003 [10] | 128 | 22 | Tetracosactrin | Young persons |
| El Sedfyet al., 2011 [11] | 45 | 7 | Tetracosactrin | Young persons |
| McIntosh et al., 1973 [12] | 9 | 0 | Tetracosactrin | Young persons |
| McIntosh et al., 1976 [13] | 9 | 2 | Tetracosactrin | Young persons |
| Vannasaeng et al., 1981 [15] | 7 | 0 | Tetracosactrin | Young persons |
| Lassman et al., 1974 [16] | 8 | 1 | Tetracosactrin | All ages |
| Ambrogio et al., 2018 [17] | 72 | 15 | Tetracosactrin | Adults |
| Scacchi et al., 2010 [18] | 56 | 18 | Tetracosactrin | Adults |
| Baldini et al., 2017 [6] | 3 | 3 | Tetracosactrin | Adults |
| De Sanctis et al., 2016 [19] | 3 | 3 | Tetracosactrin | Adults |
| Huang et al., 2015 [20] | 3 | 0 | GST and ITT | Adults |
| Huang et al., 2015 [20] | 8 | 1 | GST and ITT | Young persons |
| Poomthavorn et al., 2010 [21] | 26 | 21 | ITT | Young persons |
| Kwan et al., 1995 [22] | 68 | 1 | ITT | Young persons |
| Sklar et al., 1987 [23] | 8 | 0 | ITT | Young persons |
| Total | 663 | 167 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savvidis, C.; Ragia, D.; Delicou, S.; Xydaki, A.; Rizzo, M.; Ilias, I. Adrenal Insufficiency in Patients with Beta Thalassemia: A Meta-Analysis. Medicina 2024, 60, 1571. https://doi.org/10.3390/medicina60101571
Savvidis C, Ragia D, Delicou S, Xydaki A, Rizzo M, Ilias I. Adrenal Insufficiency in Patients with Beta Thalassemia: A Meta-Analysis. Medicina. 2024; 60(10):1571. https://doi.org/10.3390/medicina60101571
Chicago/Turabian StyleSavvidis, Christos, Dimitra Ragia, Sophia Delicou, Aikaterini Xydaki, Manfredi Rizzo, and Ioannis Ilias. 2024. "Adrenal Insufficiency in Patients with Beta Thalassemia: A Meta-Analysis" Medicina 60, no. 10: 1571. https://doi.org/10.3390/medicina60101571
APA StyleSavvidis, C., Ragia, D., Delicou, S., Xydaki, A., Rizzo, M., & Ilias, I. (2024). Adrenal Insufficiency in Patients with Beta Thalassemia: A Meta-Analysis. Medicina, 60(10), 1571. https://doi.org/10.3390/medicina60101571









