Cardiac Masses and Pseudomasses: An Overview about Diagnostic Imaging and Clinical Background
Abstract
:1. Introduction
2. Pseudomasses
2.1. Normal Intracardiac Structures
2.2. Lipomatous Hypertrophy of the Inter-Atrial Septum (LHIS or LHAS)
3. Non-Neoplastic Masses
3.1. Thrombus
3.2. Vegetations
3.3. Mitral Annular Calcification and Its Caseous Degeneration
3.4. Pericardial Cyst
3.5. Coronary Artery Aneurism (CAA)
3.6. Cardiac Chambers Aneurysm and Pseudoaneurysm
3.7. IgG4-Related Disease (IgG4-RD)
3.8. Sarcoidosis
3.9. Foreign Body
3.10. Hematoma
3.11. Echinococcus Cyst
4. Benign Tumors
4.1. Myxoma
4.2. Papillary Fibroelastoma (PFE)
4.3. Lipoma
4.4. Rhabdomyoma
4.5. Fibroma
4.6. Hemangioma
4.7. Lymphangioma
4.8. Erdheim-Chester Disease (ECD)
4.9. Solitary Fibrous Tumor (SFT)
4.10. Teratoma
4.11. Schwannoma
4.12. Paraganglioma
5. Malignant Tumors
5.1. Secondary Tumors-Metastasis
5.2. Primary Cardiac Sarcomas
5.3. Primary Cardiac Lymphoma
5.4. Primary Pericardial Mesothelioma
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maleszewski, J.J.; Basso, C.; Bois, M.C.; Glass, C.; Klarich, K.W.; Leduc, C.; Padera, R.F.; Tavora, F. The 2021 WHO Classification of Tumors of the Heart. J. Thorac. Oncol. 2022, 17, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Bergquist, P.J.; Srichai, M.B. Multimodality Imaging in the Evaluation of Intracardiac Masses. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 55. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Gore, A.; Goyal, N. Evaluation of Incidental Cardiac Masses on Computed Tomography Imaging: An Algorithmic Approach. J. Thorac. Imaging. 2019, 34, W1–W9. [Google Scholar] [CrossRef] [PubMed]
- Aghayev, A.; Cheezum, M.K.; Steigner, M.L.; Mousavi, N.; Padera, R.; Barac, A.; Kwong, R.Y.; Di Carli, M.F.; Blankstein, R. Multimodality imaging to distinguish between benign and malignant cardiac masses. J. Nucl. Cardiol. 2022, 29, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Casal, D.; Datino, T.; Soto, N.; González-Panizo, J.; Sánchez-Quintana, D.; Macias, Y.; Cabrera, J.Á. Anatomy of the left atrial appendage for the interventional cardiologist. Herzschrittmacherther. Elektrophysiol. 2022, 33, 195–202. [Google Scholar] [CrossRef]
- Lak, H.M.; Kerndt, C.C.; Unai, S.; Maroo, A. Cardiac papillary fibroelastoma originating from the coumadin ridge and review of literature. BMJ Case Rep. 2020, 13, e235361. [Google Scholar] [CrossRef]
- Moustafa, S.; Patton, D.J.; Connelly, M.S.; Alvarez, N.; Prieur, T.; Mookadam, F. An atypical case of left atrial myxoma. Rev. Port. Cardiol. 2015, 34, 75–77. [Google Scholar] [CrossRef]
- Lakhani, D.A.; Balar, A.B.; Kim, C. Prominent crista terminalis mimicking a right atrial mass: A case report and brief review of the literature. Radiol. Case Rep. 2021, 17, 434–438. [Google Scholar] [CrossRef]
- Kucybała, I.; Ciuk, K.; Klimek-Piotrowska, W. Clinical anatomy of human heart atria and interatrial septum—Anatomical basis for interventional cardiologists and electrocardiologists. Part 1: Right atrium and interatrial septum. Kardiol. Pol. 2018, 76, 499–509. [Google Scholar] [CrossRef]
- Islam, A.K.; Sayami, L.A.; Zaman, S. Chiari network: A case report and brief overview. J. Saudi Heart Assoc. 2013, 25, 225–229. [Google Scholar] [CrossRef]
- Meetham, K.; Taerujjirakul, T.; Garitjirapath, N.; Navic, P.; Shinlapawittayatorn, K.; Mahakkanukrauh, P. The morphometric study of the moderator band in Thais. Anat. Sci. Int. 2022, 97, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; MacNamara, J.; Chaturvedi, A.; Ashwath, R.; Fulton, N.L.; Goerne, H. Bands in the Heart: Multimodality Imaging Review. Radiographics 2019, 39, 1238–1263. [Google Scholar] [CrossRef] [PubMed]
- Hamer, O.W. Lipomatous Hypertrophy of the Atrial Septum. Dtsch. Arztebl. Int. 2022, 119, 187. [Google Scholar] [CrossRef] [PubMed]
- Yavar, Z.; Gilge, J.L.; Patel, P.J.; Patel, A.C.; Garcia-Cortes, R.S.; Fetters, J.K.; Fouts, A.M.; Salerno, C.T. Lipomatous Hypertrophy of the Interatrial Septum Manifesting as Third Degree Atrioventricular Block. JACC Case Rep. 2020, 2, 2235–2239. [Google Scholar] [CrossRef] [PubMed]
- Siminiak, N.; Rajewska-Tabor, J.; Pyda, M.; Czepczyński, R.; Ruchała, M. Changing appearance of lipomatous hypertrophy of the interatrial septum on positron emission tomography scan. Kardiol. Pol. 2021, 79, 1032–1033. [Google Scholar] [CrossRef]
- Adeniyi, A.; Abadir, S.; Parikh, K.; Khanna, R.; Yusuf, S.; Anais Hichard, M. Atypical Intracavitary Cardiac Mass: Tumor or Thrombus? Cureus 2022, 14, e21937. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, C.H.; Im, D.J.; Lee, K.H.; Kim, T.H.; Han, K.; Hur, J. CT-based radiomics signature for differentiation between cardiac tumors and a thrombi: A retrospective, multicenter study. Sci. Rep. 2022, 12, 8173. [Google Scholar] [CrossRef]
- Johnson, E.M.; Gage, K.L.; Feuerlein, S.; Jeong, D. Cardiac Magnetic Resonance for the Evaluation of Suspected Cardiac Thrombus: Conventional and Emerging Techniques. J. Vis. Exp. 2019, 148, e58808. [Google Scholar]
- Gatti, M.; D’Angelo, T.; Muscogiuri, G.; Dell’aversana, S.; Andreis, A.; Carisio, A.; Darvizeh, F.; Tore, D.; Pontone, G.; Faletti, R. Cardiovascular magnetic resonance of cardiac tumors and masses. World J. Cardiol. 2021, 13, 628–649. [Google Scholar] [CrossRef]
- Shah, B.N.; Babu-Narayan, S.; Li, W.; Rubens, M.; Wong, T. Severe mitral annular calcification: Insights from multimodality imaging. Tex. Heart Inst. J. 2014, 41, 245–247. [Google Scholar] [CrossRef]
- Motwani, M.; Kidambi, A.; Herzog, B.A.; Uddin, A.; Greenwood, J.P.; Plein, S. MR imaging of cardiac tumors and masses: A review of methods and clinical applications. Radiology 2013, 268, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Tower-Rader, A.; Kwon, D. Pericardial Masses, Cysts and Diverticula: A Comprehensive Review Using Multimodality Imaging. Prog. Cardiovasc. Dis. 2017, 59, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Glockner, J.F. Magnetic Resonance Imaging and Computed Tomography of Cardiac Masses and Pseudomasses in the Atrioventricular Groove. Can. Assoc. Radiol. J. 2018, 69, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.C.; Mattman, A.; Seidman, M.A.; Carruthers, M.N. IgG4-related disease: What a hematologist needs to know. Haematologica 2019, 104, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Fathala, A. Multimodalities Imaging of Immunoglobulin 4-related Cardiovascular Disorders. Curr. Cardiol. Rev. 2019, 15, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, R.; Asano, T.; Maezawa, H.; Shimojima, H.; Tsujiuchi, M.; Hori, Y.; Ebato, M.; Suzuki, H. Cardiac Sarcoidosis Mimicking Septal Tumor with Intermittent Complete Atrioventricular Block. Int. Heart J. 2018, 59, 1473–1479. [Google Scholar] [CrossRef]
- Yodogawa, K.; Fujimoto, Y.; Hagiwara, K.; Oka, E.; Hayashi, H.; Murata, H.; Yamamoto, T.; Iwasaki, Y.K.; Shimizu, W. Possibility of steroid therapy without pacemaker implantation in patients with sarcoidosis presenting atrioventricular block. Heart Vessel. 2022, 37, 1892–1898. [Google Scholar] [CrossRef]
- Pyo, W.K.; Kim, W.K.; Kim, J.B. A Huge Pericardial Gossypiboma Causing Severe Cardiac Dysfunction. Ann. Thorac. Surg. 2020, 109, e167–e169. [Google Scholar] [CrossRef]
- Caushi, F.; Çoku, L.; Skenduli, I.; Xhemalaj, D.; Mezini, A.; Hysa, E.; Rulli, F. Intrapericardial gossypiboma found 14 years after coronary artery bypass grafting. J. Cardiothorac. Surg. 2019, 14, 69. [Google Scholar] [CrossRef]
- Stewart, J.; Baltabaeva, A.; Beeton, I.; Wignall, O. Case report of a mysterious myocardial mass: An aetiological conundrum. Eur. Heart J. Case Rep. 2018, 2, yty018. [Google Scholar] [CrossRef]
- Choi, C.H.; Elahi, M.M.; Konda, S. Iatrogenic retained foreign body in the right atrium. Lessons to Learn. Int. J. Surg. Case Rep. 2013, 4, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Chomei, S.; Gan, K. A rare case of idiopathic chronic expanding pericardial hematoma presenting as pericardial tamponade: A case report and literature review. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Hamanaka, Y.; Mitsui, N.; Isaka, M.; Kobayashi, T. Chronic expanding hematoma in the pericardial cavity after cardiac surgery. Ann. Thorac. Surg. 2003, 75, 1629–1631. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Terashima, M.; Takamura, C.; Sakurai, H.; Ooishi, K.; Yoshizaki, T.; Yamaguchi, J.; Hijikata, S.; Iwai, T.; Sagawa, Y.; et al. Cardiac Magnetic Resonance Imaging of Very Late Intrapericardial Hematoma 8 Years after Coronary Artery Bypass Grafting. Intern. Med. 2018, 57, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Kumar, R.; Halder, V.; Raju, M.; Negi, S.L.; Naganur, S. Multimodality imaging of an interventricular septum hydatid cyst. Egypt. Heart J. 2021, 73, 23. [Google Scholar] [CrossRef] [PubMed]
- Khalilian, M.R.; Norouzi, A.R.; Zamani, H.; Nia, S.K.F.; Teymoordash, S.N. A Rare Case of Cardiac Hydatid Disease without Liver and Lungs Involvement. Iran. J. Public Health. 2021, 50, 2332–2336. [Google Scholar] [CrossRef] [PubMed]
- Ameen, A.; Hilal, K.; Shaikh, A.; Khan, F.; Fatimi, S. Cardiac hydatid cyst presenting as ventricular arrhythmia: A case report. Egypt. Heart J. 2021, 73, 105. [Google Scholar] [CrossRef] [PubMed]
- Shetty Roy, A.N.; Radin, M.; Sarabi, D.; Shaoulian, E. Familial recurrent atrial myxoma: Carney’s complex. Clin. Cardiol. 2011, 34, 83–86. [Google Scholar] [CrossRef]
- Nasser, S.B.; Doeblin, P.; Doltra, A.; Schnackenburg, B.; Wassilew, K.; Berger, A.; Gebker, R.; Bigvava, T.; Hennig, F.; Pieske, B.; et al. Cardiac Myxomas Show Elevated Native T1, T2 Relaxation Time and ECV on Parametric CMR. Front. Cardiovasc. Med. 2020, 7, 602137. [Google Scholar] [CrossRef]
- Islam, A.K.M.M. Cardiac myxomas: A narrative review. World J. Cardiol. 2022, 14, 206–219. [Google Scholar] [CrossRef]
- Liddy, S.; McQuade, C.; Walsh, K.P.; Loo, B.; Buckley, O. The Assessment of Cardiac Masses by Cardiac CT and CMR Including Pre-op 3D Reconstruction and Planning. Curr. Cardiol. Rep. 2019, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Hoey, E.T.; Shahid, M.; Ganeshan, A.; Baijal, S.; Simpson, H.; Watkin, R.W. MRI assessment of cardiac tumours: Part 1, multiparametric imaging protocols and spectrum of appearances of histologically benign lesions. Quant. Imaging Med. Surg. 2014, 4, 478–488. [Google Scholar] [PubMed]
- Mankad, R.; Herrmann, J. Cardiac tumors: Echo assessment. Echo Res. Pract. 2016, 3, R65–R77. [Google Scholar] [CrossRef] [PubMed]
- Cannavale, G.; Francone, M.; Galea, N.; Vullo, F.; Molisso, A.; Carbone, I.; Catalano, C. Fatty Images of the Heart: Spectrum of Normal and Pathological Findings by Computed Tomography and Cardiac Magnetic Resonance Imaging. Biomed. Res. Int. 2018, 2018, 5610347. [Google Scholar] [CrossRef] [PubMed]
- Maleszewski, J.J.; Anavekar, N.S.; Moynihan, T.J.; Klarich, K.W. Pathology, imaging, and treatment of cardiac tumours. Nat. Rev. Cardiol. 2017, 14, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Grgat, D.; Dilber, D.; Hrabak Paar, M. Common benign primary pediatric cardiac tumors: A primer for radiologists. Jpn. J. Radiol. 2023, 41, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.; Lundberg, J.; Richardson, R.R.; Prasad, D. Utility of cardiac imaging in diagnosis of atypical presentation of cardiac fibroma. BMJ Case Rep. 2019, 12, e230333. [Google Scholar] [CrossRef]
- Lichtenberger, J.P., 3rd; Dulberger, A.R.; Gonzales, P.E.; Bueno, J.; Carter, B.W. MR Imaging of Cardiac Masses. Top. Magn. Reson. Imaging 2018, 27, 103–111. [Google Scholar] [CrossRef]
- Junqueira, N.; Ferreira, R.; Gonçalves, J.; Nobre, Â. Lymphangioma of the heart as a rare tumor: A case report. Int. J. Surg. Case Rep. 2018, 53, 246–249. [Google Scholar] [CrossRef]
- Diao, W.J.; Shi, C.; Liu, G.; Liu, X.G.; Li, H.H.; Meng, J.J.; Shi, Y.; Chang, M.M.; Liu, Y.Y. The diagnosis and treatment of cardiac lymphangioma: A case report and literature review. Medicine 2019, 98, e14000. [Google Scholar] [CrossRef]
- Pichler Sekulic, S.; Sekulic, M. Primary cardiac and pericardial lymphangiomas: Clinical, radiologic, and pathologic characterization derived from an institutional series and review of the literature. Virchows Arch. 2022, 480, 1211–1221. [Google Scholar] [CrossRef]
- Emile, J.F.; Abla, O.; Fraitag, S.; Horne, A.; Haroche, J.; Donadieu, J.; Requena-Caballero, L.; Jordan, M.B.; Abdel-Wahab, O.; Allen, C.E.; et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 2016, 127, 2672–2681. [Google Scholar] [CrossRef] [PubMed]
- Diamond, E.L.; Dagna, L.; Hyman, D.M.; Cavalli, G.; Janku, F.; Estrada-Veras, J.; Ferrarini, M.; Abdel-Wahab, O.; Heaney, M.L.; Scheel, P.J.; et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood 2014, 124, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Haroche, J.; Cluzel, P.; Toledano, D.; Montalescot, G.; Touitou, D.; Grenier, P.A.; Piette, J.C.; Amoura, Z. Images in cardiovascular medicine. Cardiac involvement in Erdheim-Chester disease: Magnetic resonance and computed tomographic scan imaging in a monocentric series of 37 patients. Circulation 2009, 119, e597–e598. [Google Scholar] [CrossRef] [PubMed]
- Goyal, G.; Heaney, M.L.; Collin, M.; Cohen-Aubart, F.; Vaglio, A.; Durham, B.H.; Hershkovitz-Rokah, O.; Girschikofsky, M.; Jacobsen, E.D.; Toyama, K.; et al. Erdheim-Chester disease: Consensus recommendations for evaluation, diagnosis, and treatment in the molecular era. Blood 2020, 135, 1929–1945. [Google Scholar] [CrossRef] [PubMed]
- Czimbalmos, C.; Csecs, I.; Polos, M.; Bartha, E.; Szucs, N.; Toth, A.; Maurovich-Horvat, P.; Becker, D.; Sapi, Z.; Szabolcs, Z.; et al. Uncommon presentation of a rare tumour-incidental finding in an asymptomatic patient: Case report and comprehensive review of the literature on intrapericardial solitary fibrous tumours. BMC Cancer 2017, 17, 612. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kakkar, S.; Arora, A.; Garg, A.; Harjai, M.M. A rare case of intra-pericardial teratoma presenting as a mediastinal mass in an infant. Med. J. Armed Forces India 2015, 71, S49–S51. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J.; Khalek, N.; Gaynor, J.W.; Johnson, M.P.; Adzick, N.S.; Flake, A.W.; Hedrick, H.L. Fetal intrapericardial teratoma: Natural history and management including successful in utero surgery. Am. J. Obstet. Gynecol. 2016, 215, 780.e1–780.e7. [Google Scholar] [CrossRef]
- Yokoyama, K.; Yoshizaki, T.; Tasaki, D. Left atrial schwannoma in schwannomatosis: A case report. Surg. Case Rep. 2021, 7, 75. [Google Scholar] [CrossRef]
- Li, S.; Kusmirek, J.E.; Buehler, D.; Kelly, A.; Schilling, R.; François, C.; Rahko, P.; Deaño, R. A Rare Case of Primary Pericardial Schwannoma. Radiol. Cardiothorac. Imaging 2021, 3, e200176. [Google Scholar] [CrossRef]
- Laurin, C.; Claveau, J.; Trahan, S.; Gagnon, L.P.; Kalavrouziotis, D.; Perron, J. Asymptomatic Left Ventricular Malignant Psammomatous Melanotic Schwannoma. CJC Open. 2021, 3, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, P.; Zhang, Y.; Sun, X.; Hua, S. A case with primary cardiac paraganglioma: Imaging findings. Radiol. Case Rep. 2022, 17, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Pradella, S.; Grazzini, G.; Letteriello, M.; De Amicis, C.; Grassi, R.; Maggialetti, N.; Carbone, M.; Palumbo, P.; Carotti, M.; Di Cesare, E.; et al. Masses in right side of the heart: Spectrum of imaging findings. Acta Biomed. 2020, 91, 60–70. [Google Scholar] [PubMed]
- Garcia Brás, P.; Branco, L.M.; Galrinho, A.; Timóteo, A.T.; Branco Mano, T.; Ferreira, V.; Cardoso, I.; Castelo, A.; Pinto, E.; Coelho, P.; et al. Malignant Primary and Metastatic Cardiac Tumors: A Single-Center 27-Year Case Review. Oncology 2023, 101, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Hoey, E.T.; Shahid, M.; Ganeshan, A.; Baijal, S.; Simpson, H.; Watkin, R.W. MRI assessment of cardiac tumours: Part 2, spectrum of appearances of histologically malignant lesions and tumour mimics. Quant. Imaging Med. Surg. 2014, 4, 489–497. [Google Scholar] [PubMed]
- Aggeli, C.; Dimitroglou, Y.; Raftopoulos, L.; Sarri, G.; Mavrogeni, S.; Wong, J.; Tsiamis, E.; Tsioufis, C. Cardiac Masses: The Role of Cardiovascular Imaging in the Differential Diagnosis. Diagnostics 2020, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Liu, J.; Xu, L.; Li, Y.; Liu, D.; Sun, Z.; Wen, Z. Cardiac magnetic resonance imaging of primary cardiac tumors. Quant. Imaging Med. Surg. 2020, 10, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, S.C.; Chung, M.T.; Fang, R.; Hsiung, M.C.; Young, M.S.; Lu, H.F. Primary cardiac lymphoma. J. Chin. Med. Assoc. 2006, 69, 169–174. [Google Scholar] [CrossRef]
- Maleszewski, J.J.; Anavekar, N.S. Neoplastic Pericardial Disease. Cardiol. Clin. 2017, 35, 589–600. [Google Scholar] [CrossRef]
- Parato, V.M.; Nocco, S.; Alunni, G.; Becherini, F.; Conti, S.; Cucchini, U.; Di Giannuario, G.; Di Nora, C.; Fabiani, D.; La Carrubba, S.; et al. Imaging of Cardiac Masses: An Updated Overview. J. Cardiovasc. Echogr. 2022, 32, 65–75. [Google Scholar]
- Pontone, G.; Di Cesare, E.; Castelletti, S.; De Cobelli, F.; De Lazzari, M.; Esposito, A.; Focardi, M.; Di Renzi, P.; Indolfi, C.; Lanzillo, C.; et al. Appropriate use criteria for cardiovascular magnetic resonance imaging (CMR): SIC-SIRM position paper part 1 (ischemic and congenital heart diseases, cardio-oncology, cardiac masses and heart transplant). Radiol. Med. 2021, 126, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, M.S.; Achenbach, S.; Marwan, M.; Seltmann, M.; Muschiol, G.; Ropers, D.; Daniel, W.G.; Pflederer, T. Left ventricular thrombus attenuation characterization in cardiac computed tomography angiography. J. Cardiovasc. Comput. Tomogr. 2012, 6, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; McEvoy, J.W.; Fang, J.C.; Ibeh, C.; McCarthy, C.P.; Misra, A.; Shah, Z.I.; Shenoy, C.; Spinler, S.A.; Vallurupalli, S.; et al. Management of Patients at Risk for and With Left Ventricular Thrombus: A Scientific Statement from the American Heart Association. Circulation 2022, 146, e205–e223. [Google Scholar] [CrossRef] [PubMed]
- Lazoura, O.; Ismail, T.F.; Pavitt, C.; Lindsay, A.; Sriharan, M.; Rubens, M.; Padley, S.; Duncan, A.; Wong, T.; Nicol, E. A low-dose, dual-phase cardiovascular CT protocol to assess left atrial appendage anatomy and exclude thrombus prior to left atrial intervention. Int. J. Cardiovasc. Imaging 2016, 32, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Husain, S.A.; Kelesidis, I.; Sanz, J.; Medina, H.M.; Garcia, M.J. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: A meta-analysis. Circ. Cardiovasc. Imaging. 2013, 6, 185–194. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, Y.; Tong, J.; Liu, Z. Multidetector computed tomography for detecting left atrial/left atrial appendage thrombus: A meta-analysis. Intern. Med. J. 2015, 45, 1044–1053. [Google Scholar] [CrossRef]
- Spagnolo, P.; Giglio, M.; Di Marco, D.; Cannaò, P.M.; Agricola, E.; Della Bella, P.E.; Monti, C.B.; Sardanelli, F. Diagnosis of left atrial appendage thrombus in patients with atrial fibrillation: Delayed contrast-enhanced cardiac CT. Eur. Radiol. 2021, 31, 1236–1244. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, ehad193. [Google Scholar] [CrossRef]
- Saad, E.A.; Mukherjee, T.; Gandour, G.; Fatayerji, N.; Rammal, A.; Samuel, P.; Abdallah, N.; Ashok, T. Cardiac myxomas: Causes, presentations, diagnosis, and management. Ir. J. Med. Sci. 2023. Online ahead of print. Available online: https://pubmed.ncbi.nlm.nih.gov/37737916 (accessed on 8 October 2023). [CrossRef]
- Manche, N.; Mercer, M.K.; Baruah, D. Diagnosis and Treatment of Cardiac Myxoma. Cureus 2023, 15, e39148. [Google Scholar] [CrossRef]
- Díaz Angulo, C.; Méndez Díaz, C.; Rodríguez García, E.; Soler Fernández, R.; Rois Siso, A.; Marini Díaz, M. Imaging findings in cardiac masses (Part I): Study protocol and benign tumors. Radiologia 2015, 57, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, E.; Carbone, I.; Carriero, A.; Centonze, M.; De Cobelli, F.; De Rosa, R.; Di Renzi, P.; Esposito, A.; Faletti, R.; Fattori, R.; et al. Clinical indications for cardiac computed tomography. From the Working Group of the Cardiac Radiology Section of the Italian Society of Medical Radiology (SIRM). Radiol. Med. 2012, 117, 901–938. [Google Scholar] [CrossRef] [PubMed]
- Schicchi, N.; Fogante, M.; Palumbo, P.; Agliata, G.; Esposto Pirani, P.; Di Cesare, E.; Giovagnoni, A. The sub-millisievert era in CTCA: The technical basis of the new radiation dose approach. Radiol. Med. 2020, 125, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Kassop, D.; Donovan, M.S.; Cheezum, M.K.; Nguyen, B.T.; Gambill, N.B.; Blankstein, R.; Villines, T.C. Cardiac Masses on Cardiac CT: A Review. Curr. Cardiovasc. Imaging Rep. 2014, 7, 9281. [Google Scholar] [CrossRef] [PubMed]
- Araoz, P.A.; Mulvagh, S.L.; Tazelaar, H.D.; Julsrud, P.R.; Breen, J.F. CT and MR imaging of benign primary cardiac neoplasms with echocardiographic correlation. Radiographics 2000, 20, 1303–1319. [Google Scholar] [CrossRef] [PubMed]
- Grazzini, G.; Pradella, S.; Rossi, A.; Basile, R.P.; Ruggieri, M.; Galli, D.; Palmisano, A.; Palumbo, P.; Esposito, A.; Miele, V. Practical Guide to Interpreting Cardiac Magnetic Resonance in Patients with Cardiac Masses. J. Cardiovasc. Dev. Dis. 2023, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Fogante, M.; Ventura, F.; Schicchi, N.; Regnicolo, L.; Potente, C.; Argalia, G.; Polonara, G. Cardiac rhabdomyomas and cerebral lesions in 4 pediatric patients with tuberous sclerosis. Radiol. Case Rep. 2023, 18, 2645–2648. [Google Scholar] [CrossRef]
- Burt, J.; Rop, B.; Derrick, E.; Armaly, J.; Siddiqui, U. Myocardial Fatty Foci in Tuberous Sclerosis Complex: Imaging Findings. Cureus 2016, 8, e693. [Google Scholar] [CrossRef]
- Carvalho, J.G.; Gho, J.M.I.H.; Budde, R.P.J.; Hofland, J.; Hirsch, A. Multimodality Imaging of Cardiac Paragangliomas. Radiol. Cardiothorac. Imaging 2023, 5, e230049. [Google Scholar] [CrossRef]
- Restrepo, C.S.; Largoza, A.; Lemos, D.F.; Diethelm, L.; Koshy, P.; Castillo, P.; Gomez, R.; Moncada, R.; Pandit, M. CT and MR imaging findings of benign cardiac tumors. Curr. Probl. Diagn. Radiol. 2005, 34, 12–21. [Google Scholar] [CrossRef]
- Martineau, P.; Dilsizian, V.; Pelletier-Galarneau, M. Incremental Value of FDG-PET in the Evaluation of Cardiac Masses. Curr. Cardiol. Rep. 2021, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Dhingra, V.; Girdhani, B. Scope of PET imaging in the evaluation of cardiac tumors. Cancer Treat. Res. Commun. 2023, 37, 100754. [Google Scholar] [CrossRef]
- Bussani, R.; Castrichini, M.; Restivo, L.; Fabris, E.; Porcari, A.; Ferro, F.; Pivetta, A.; Korcova, R.; Cappelletto, C.; Manca, P.; et al. Cardiac Tumors: Diagnosis, Prognosis, and Treatment. Curr. Cardiol. Rep. 2020, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Tyebally, S.; Chen, D.; Bhattacharyya, S.; Mughrabi, A.; Hussain, Z.; Manisty, C.; Westwood, M.; Ghosh, A.K.; Guha, A. Cardiac Tumors: JACC CardioOncology State-of-the-Art Review. JACC CardioOncol. 2020, 2, 293–311. [Google Scholar] [CrossRef] [PubMed]

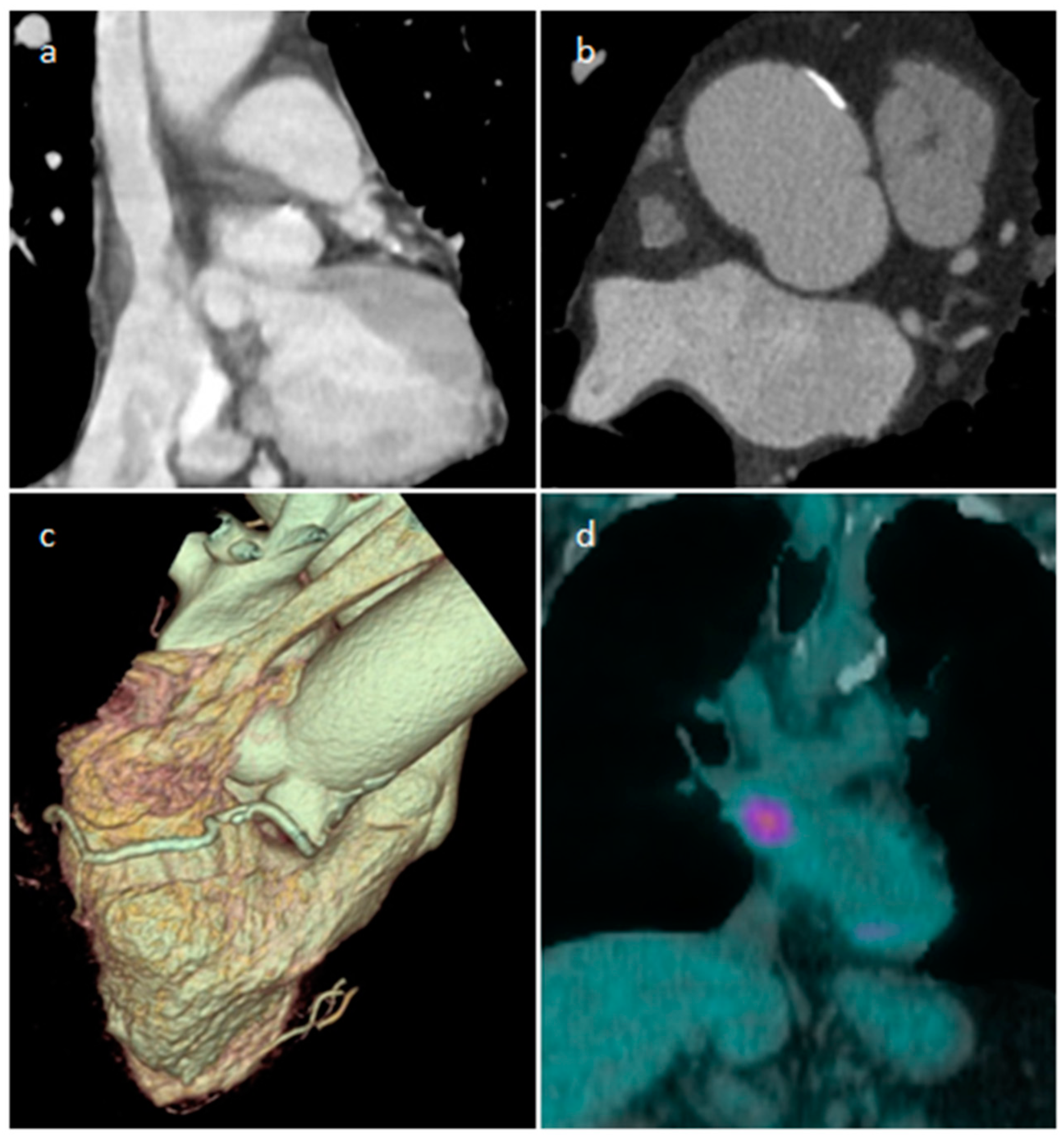
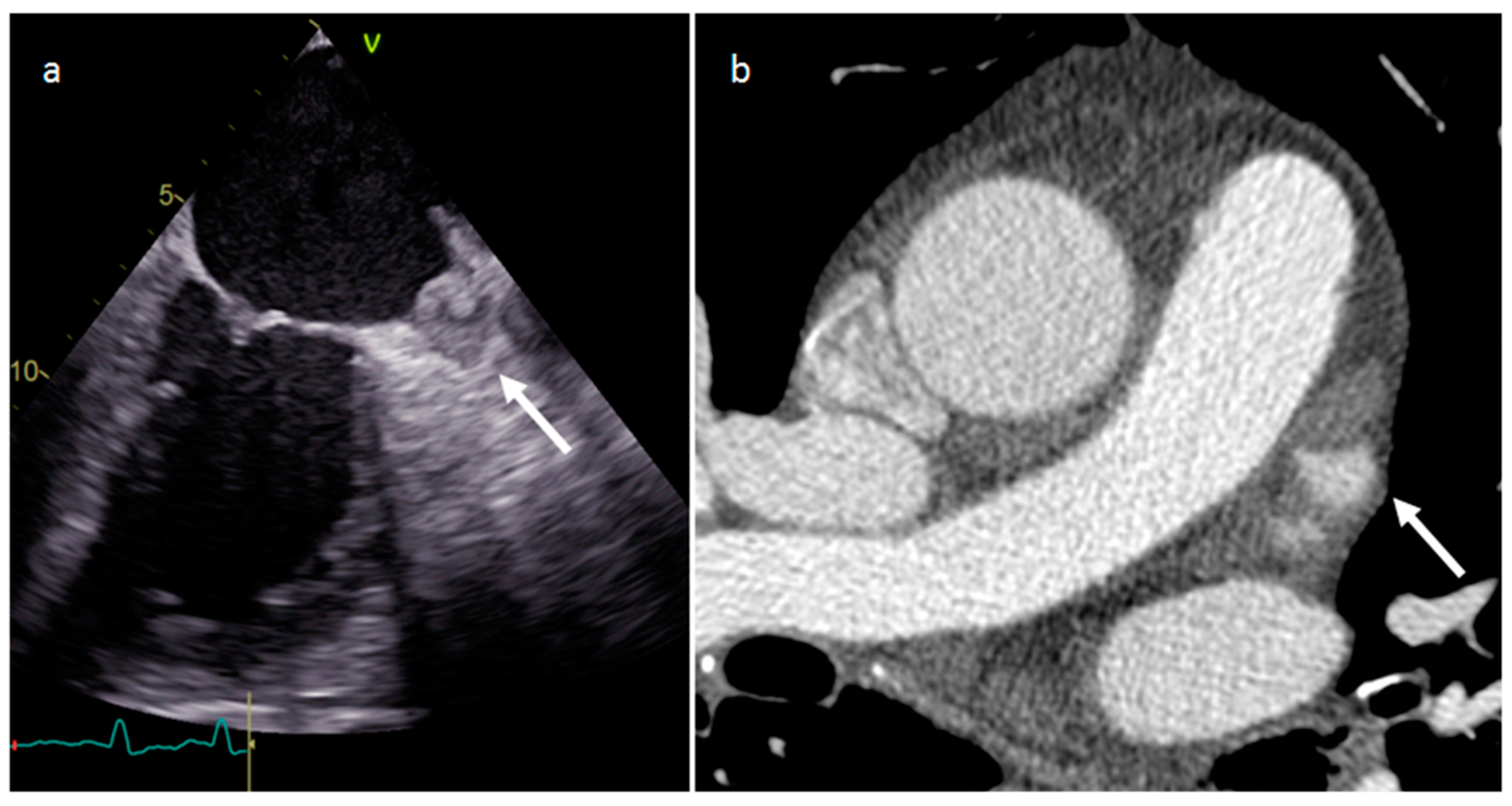

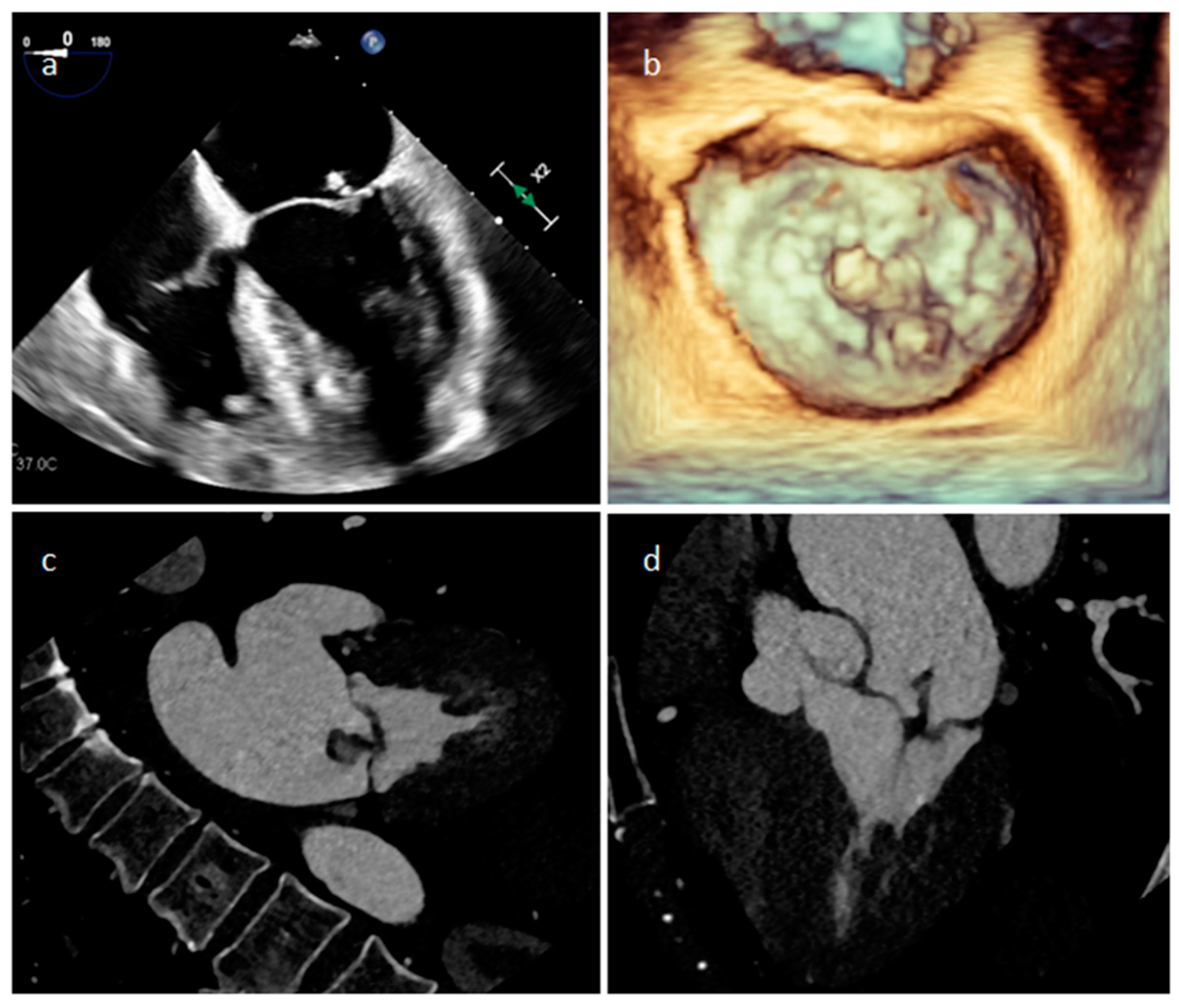
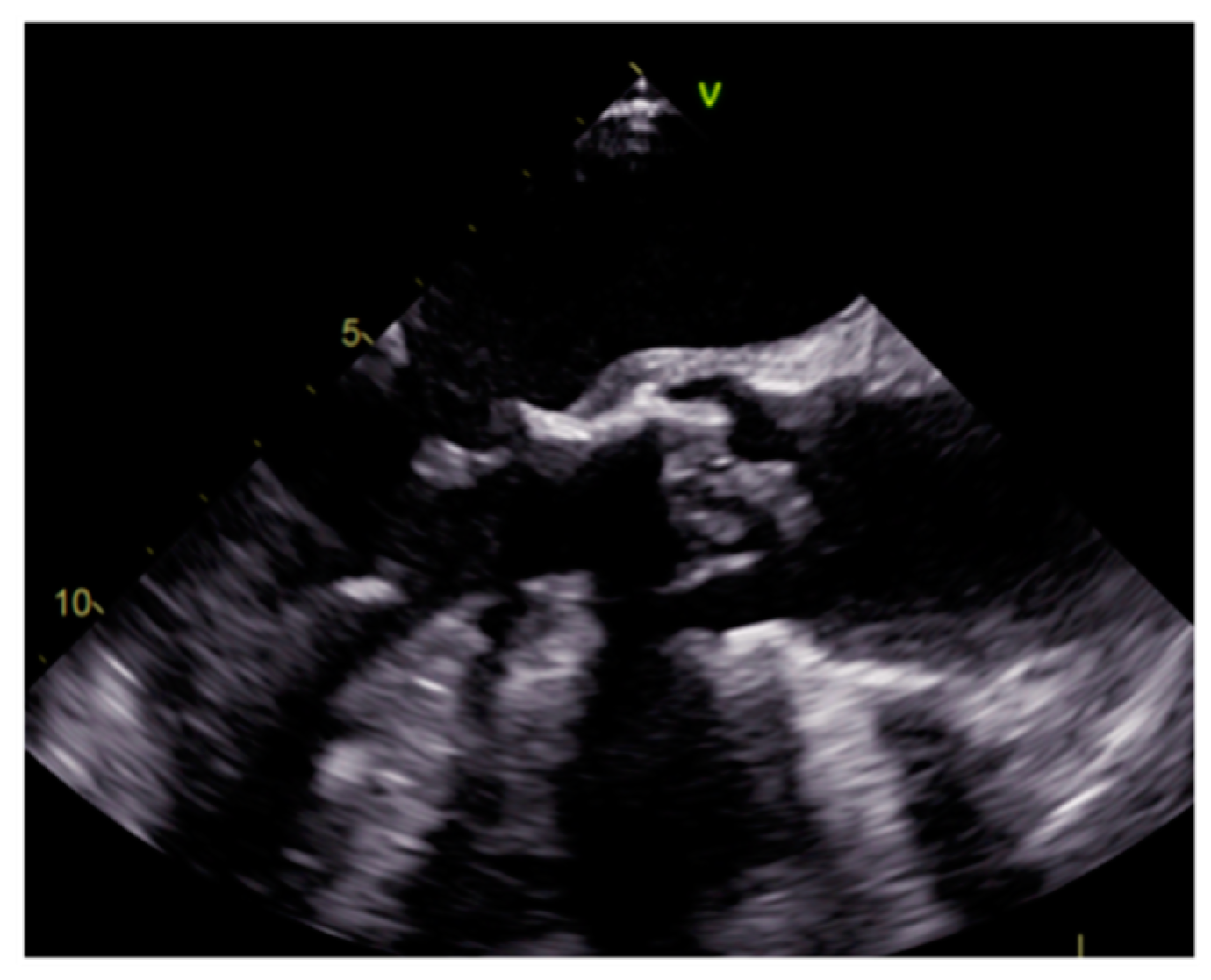
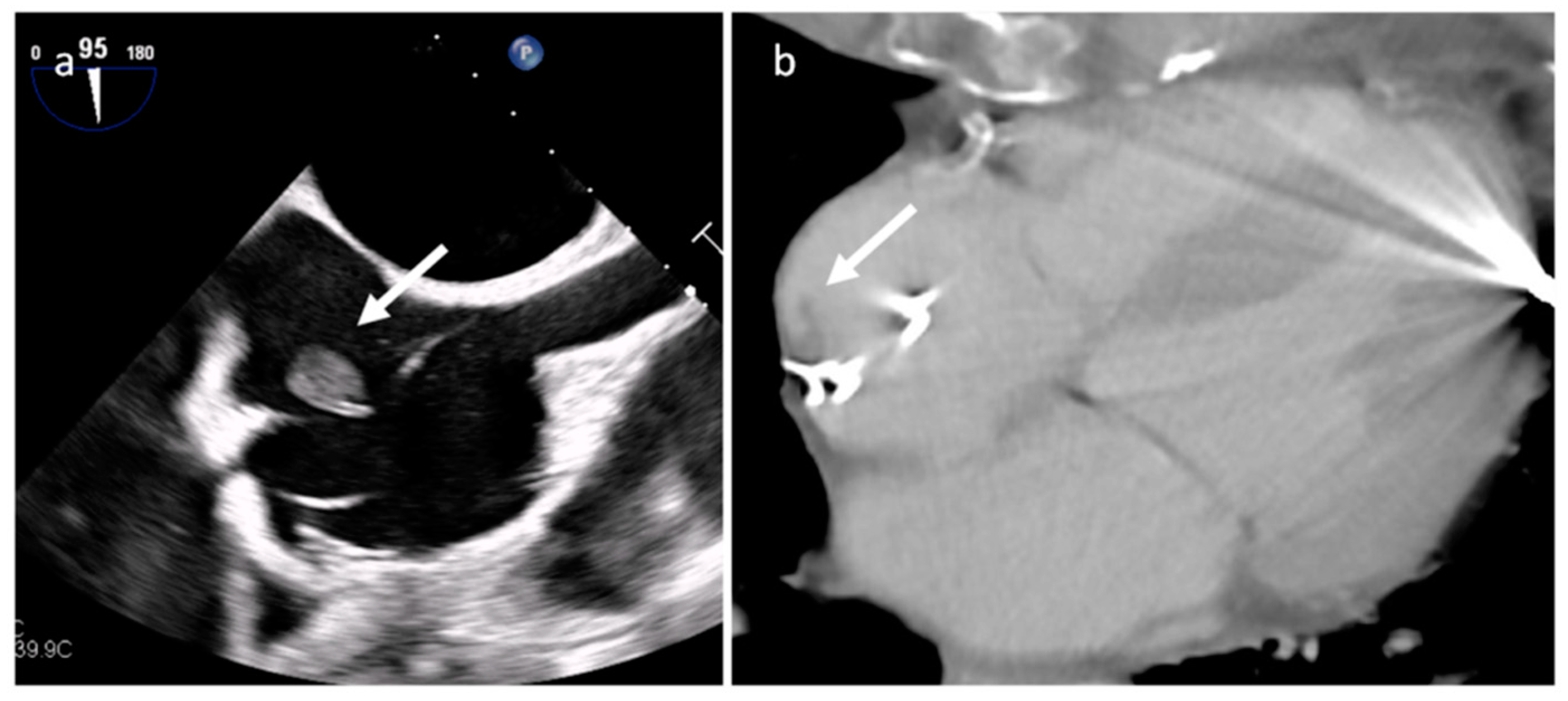


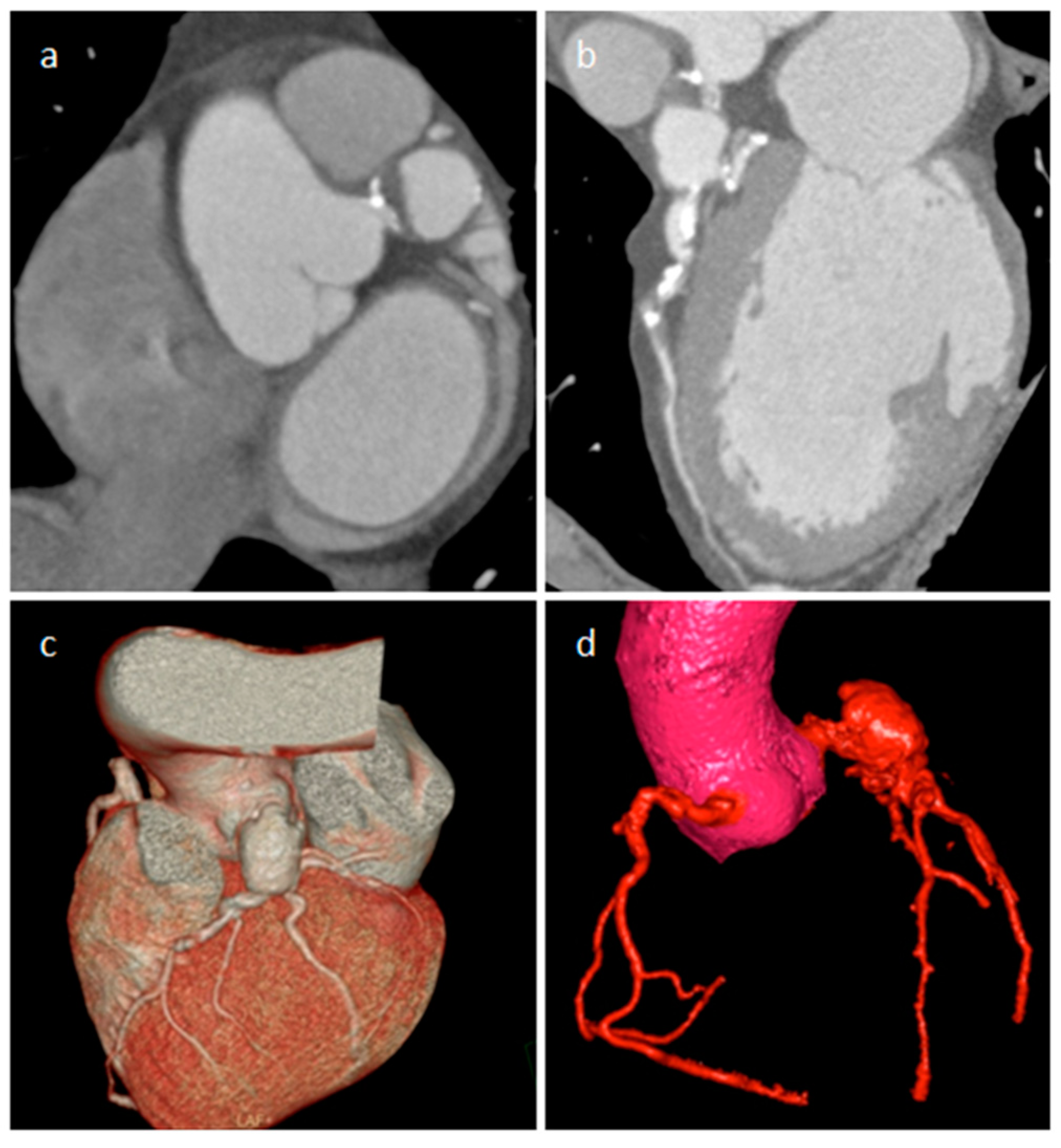
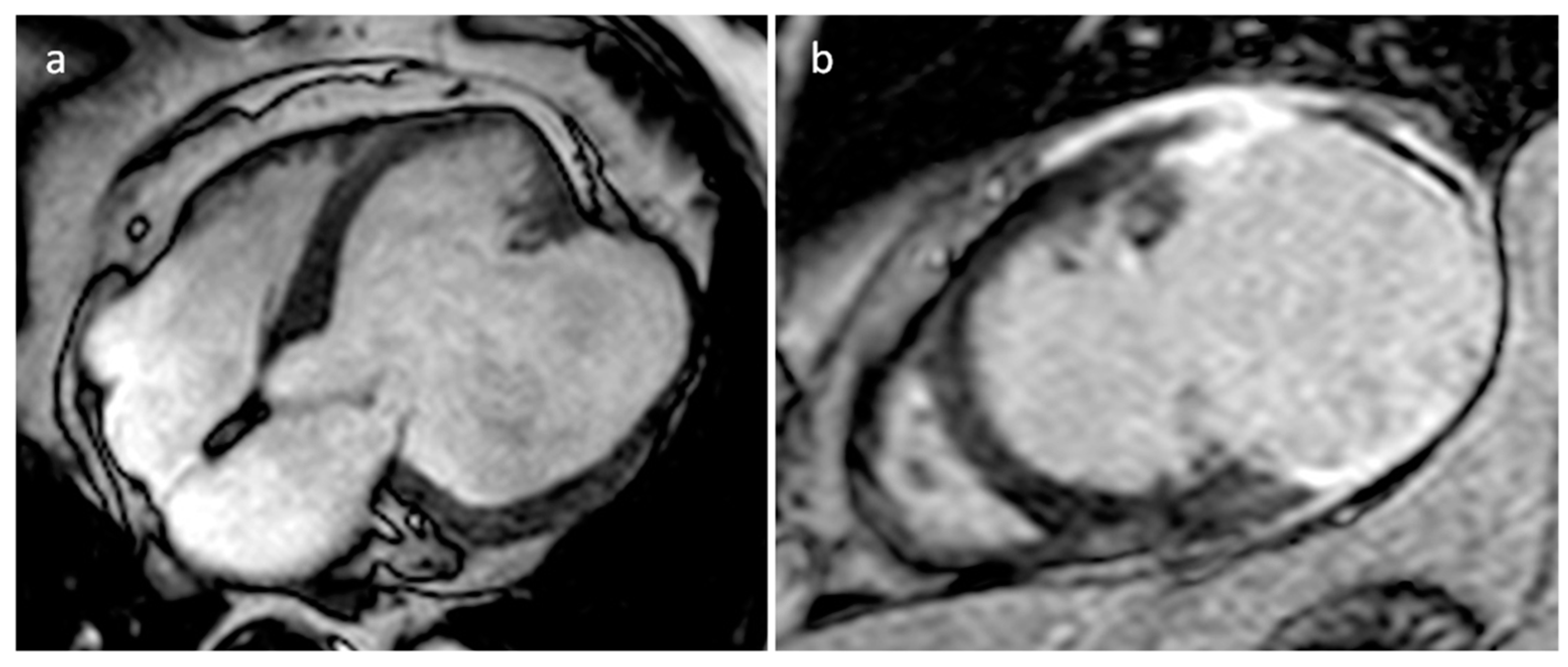

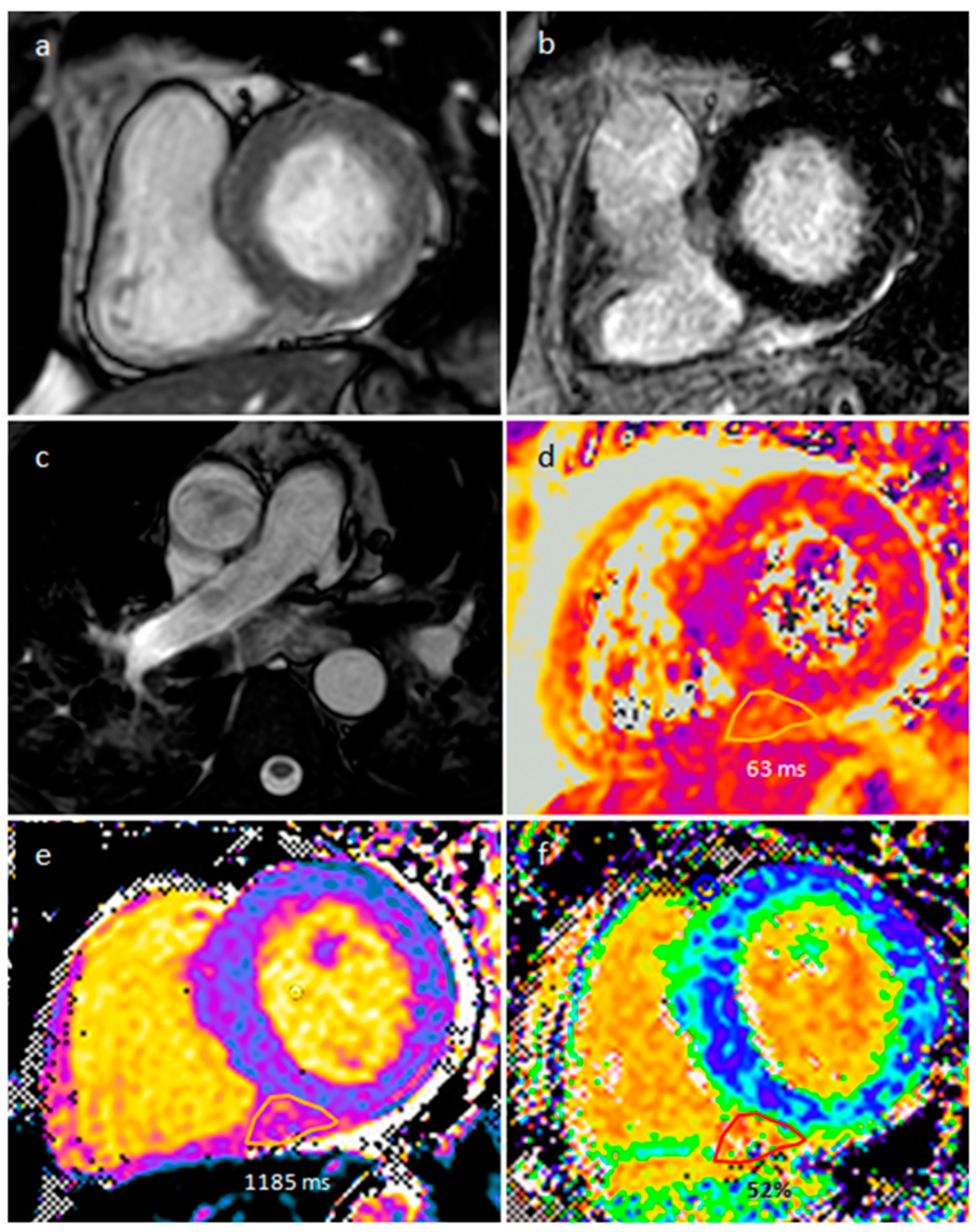


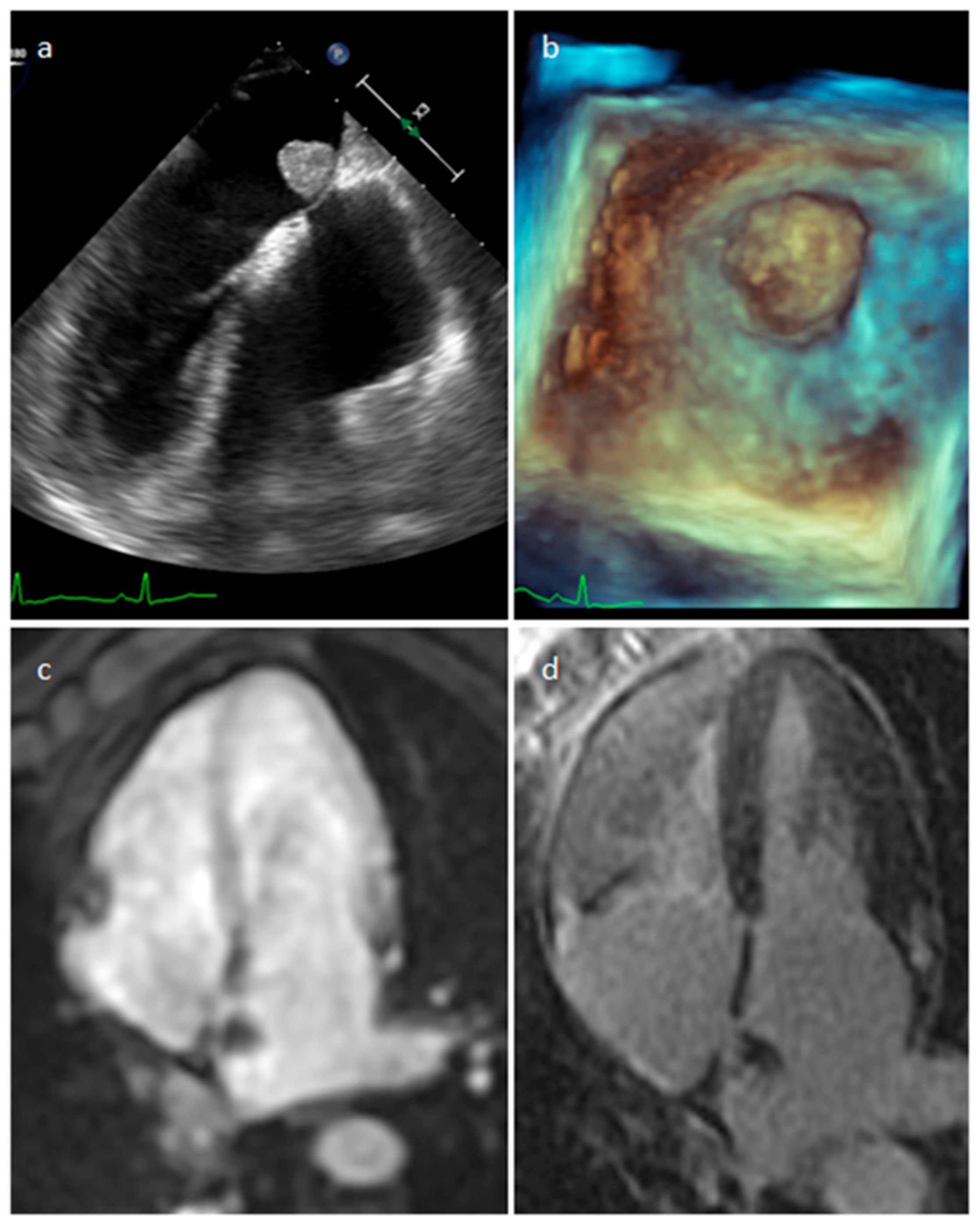
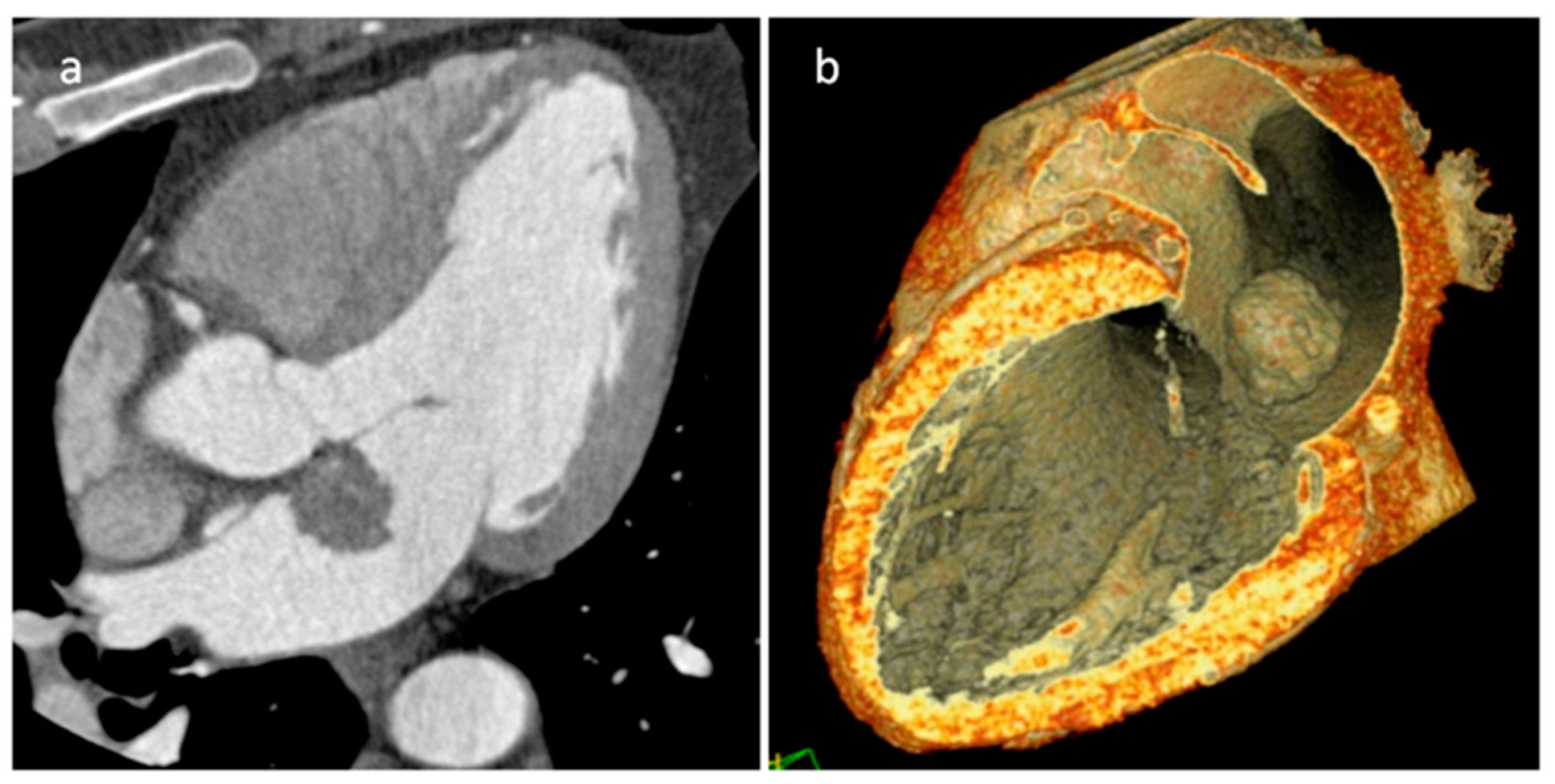
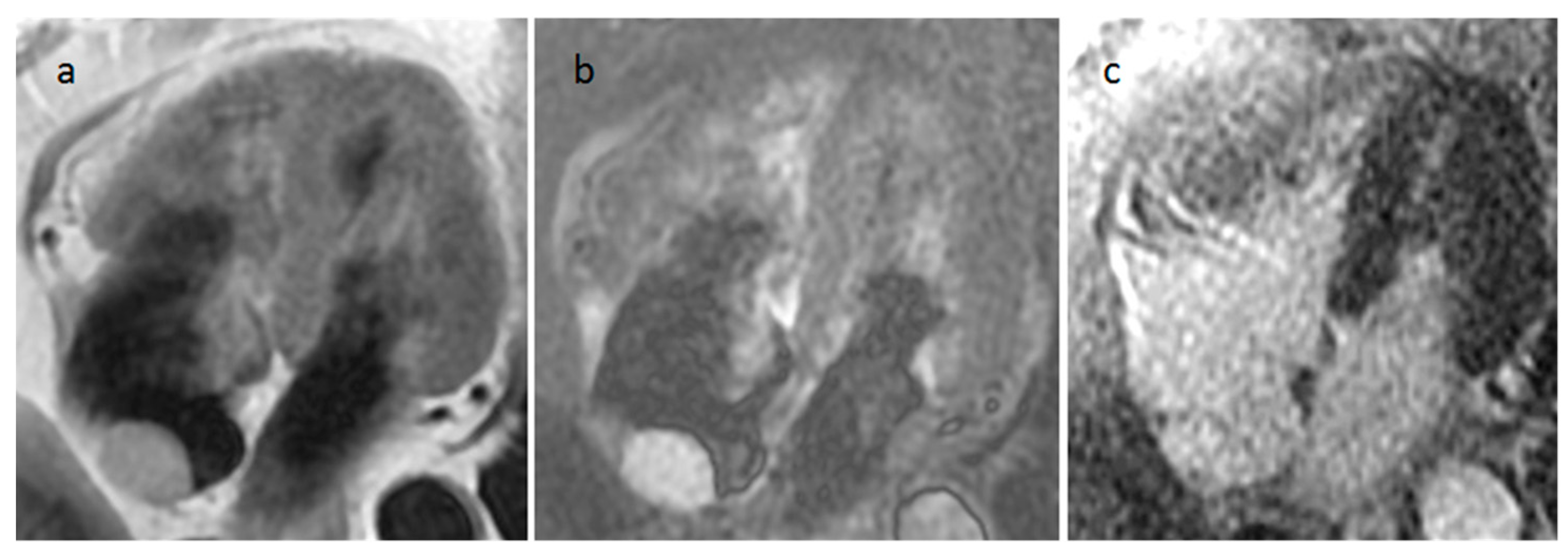
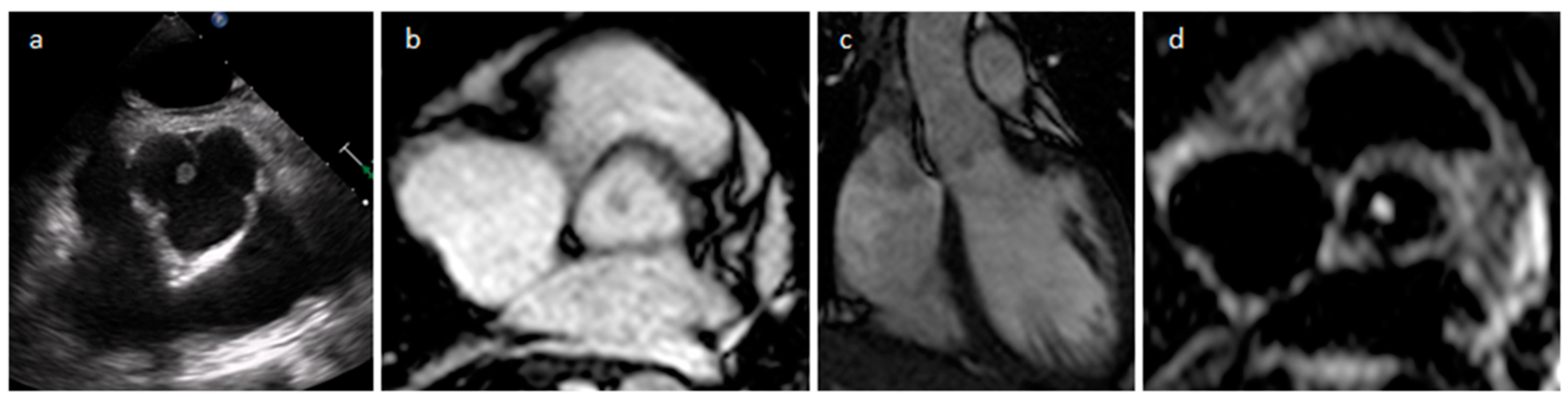
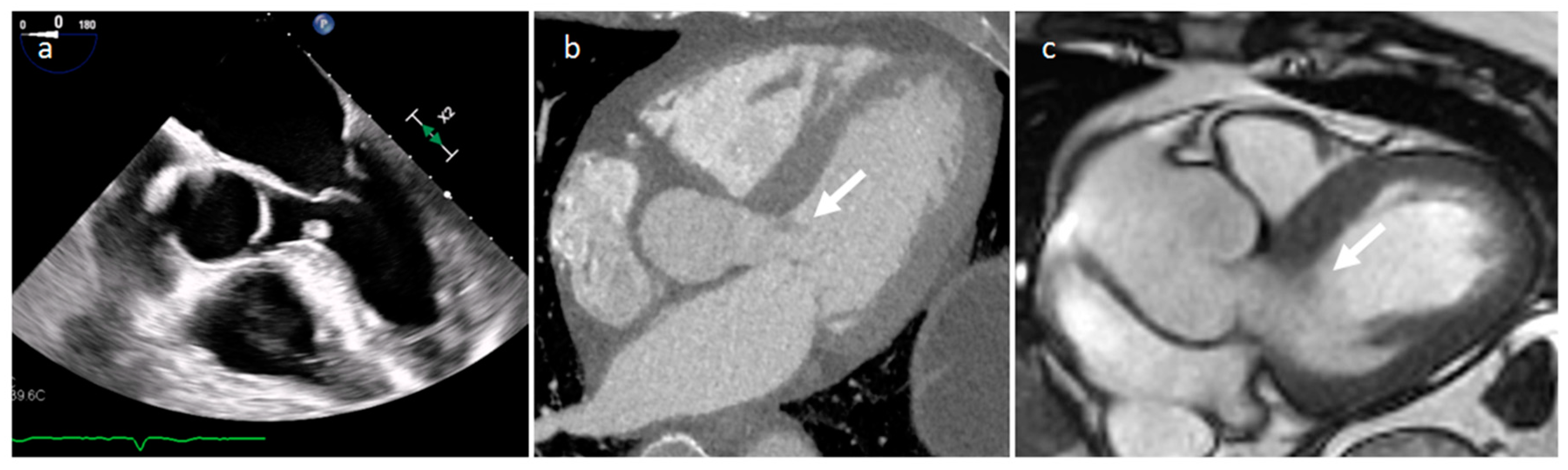

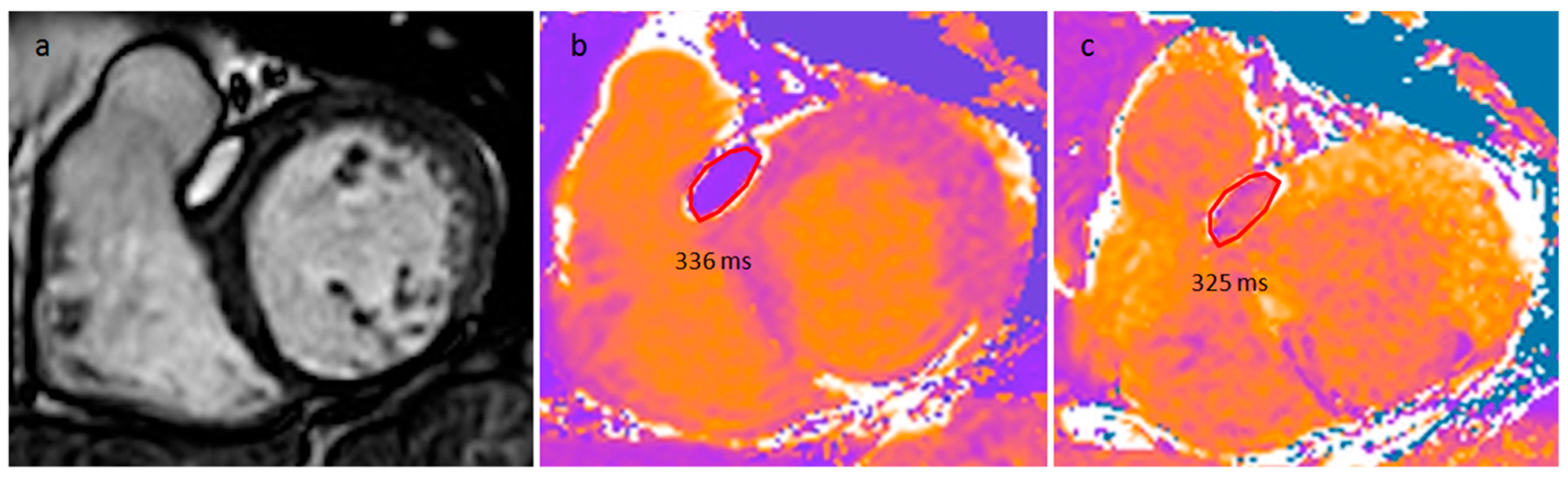

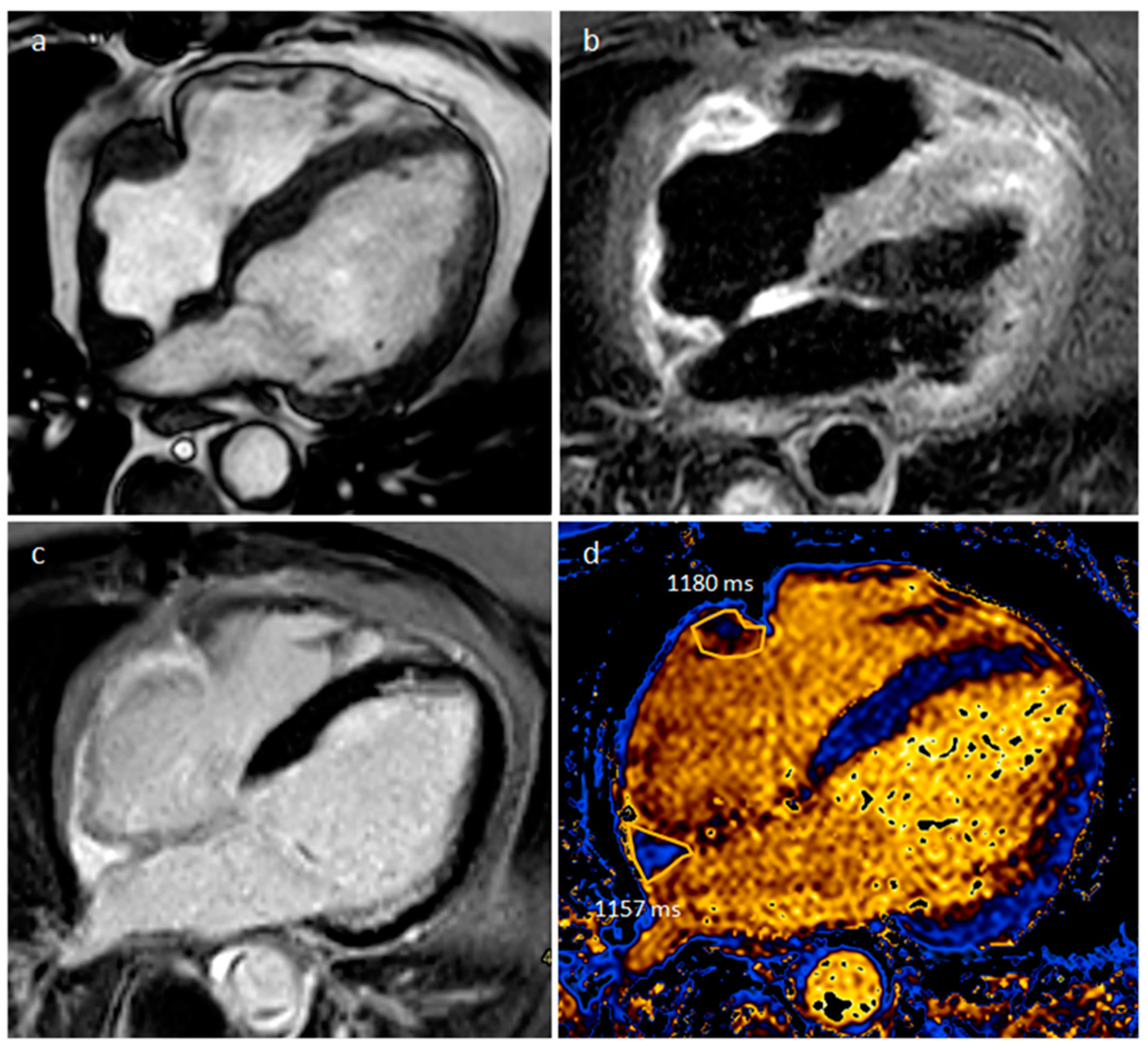

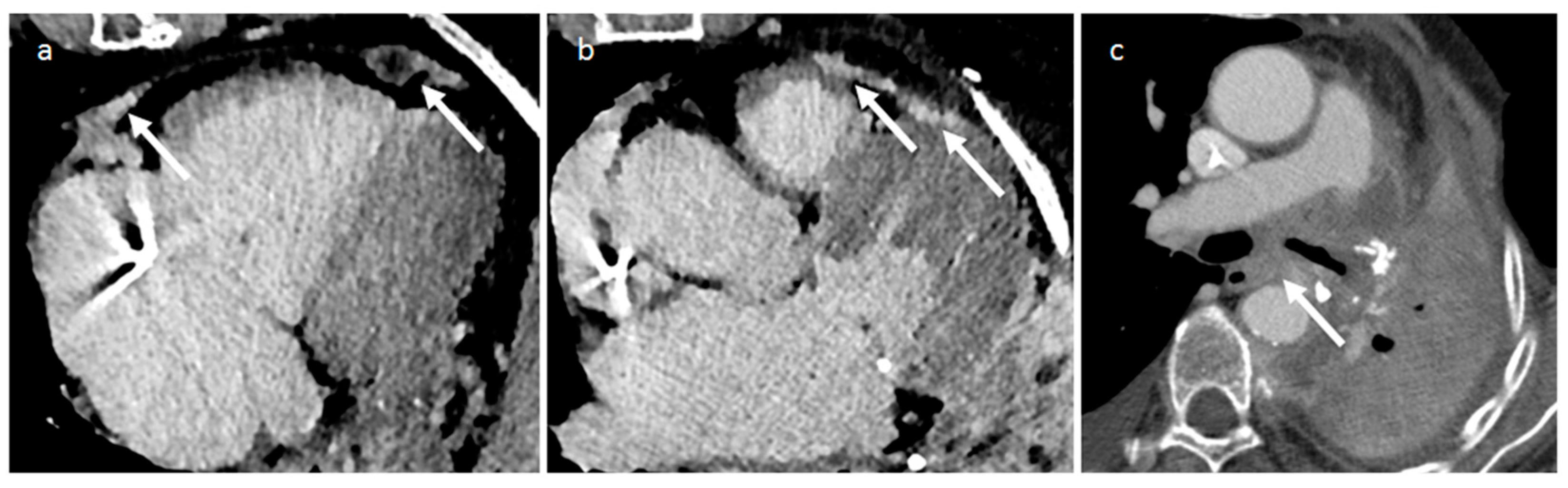
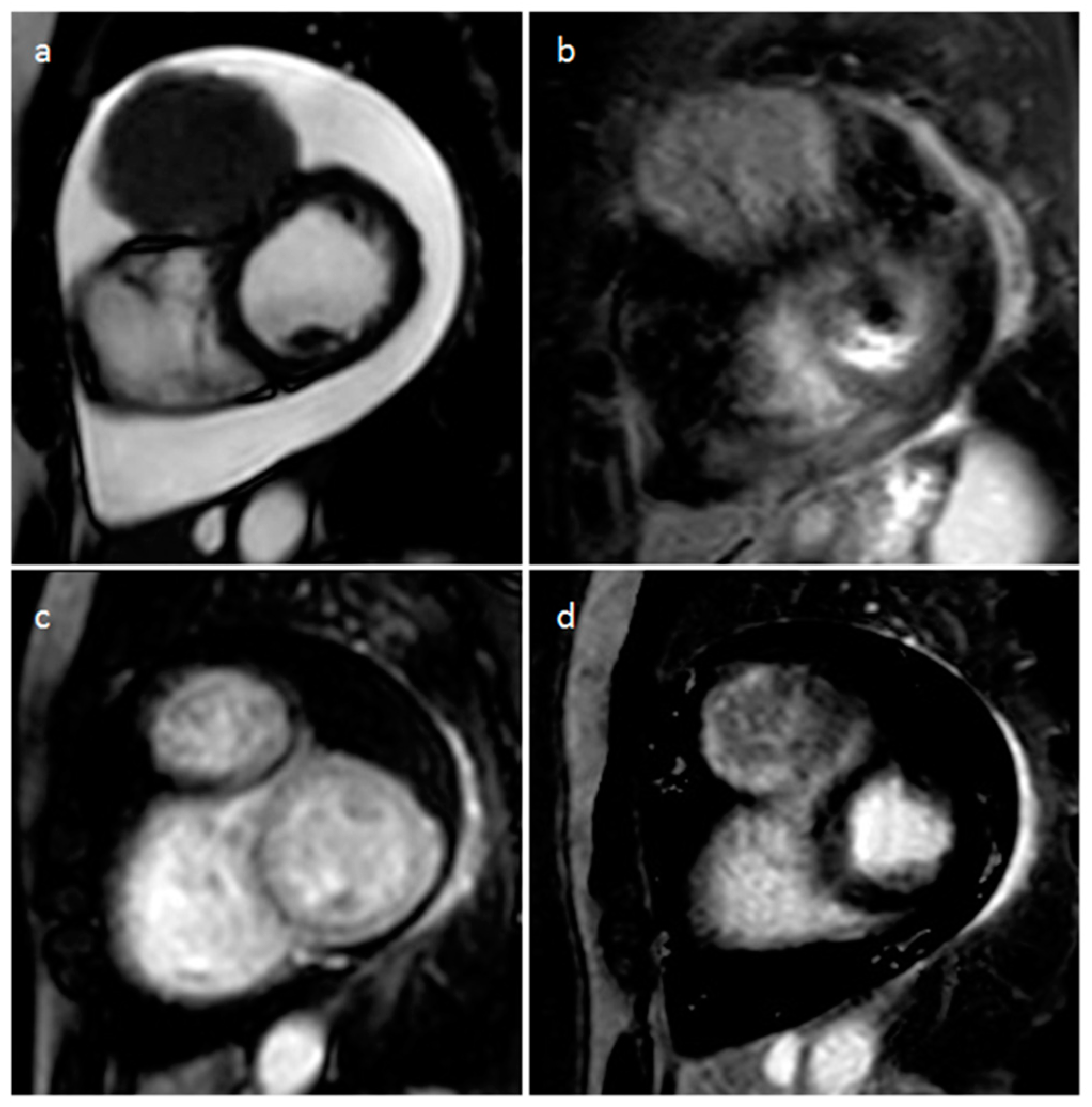
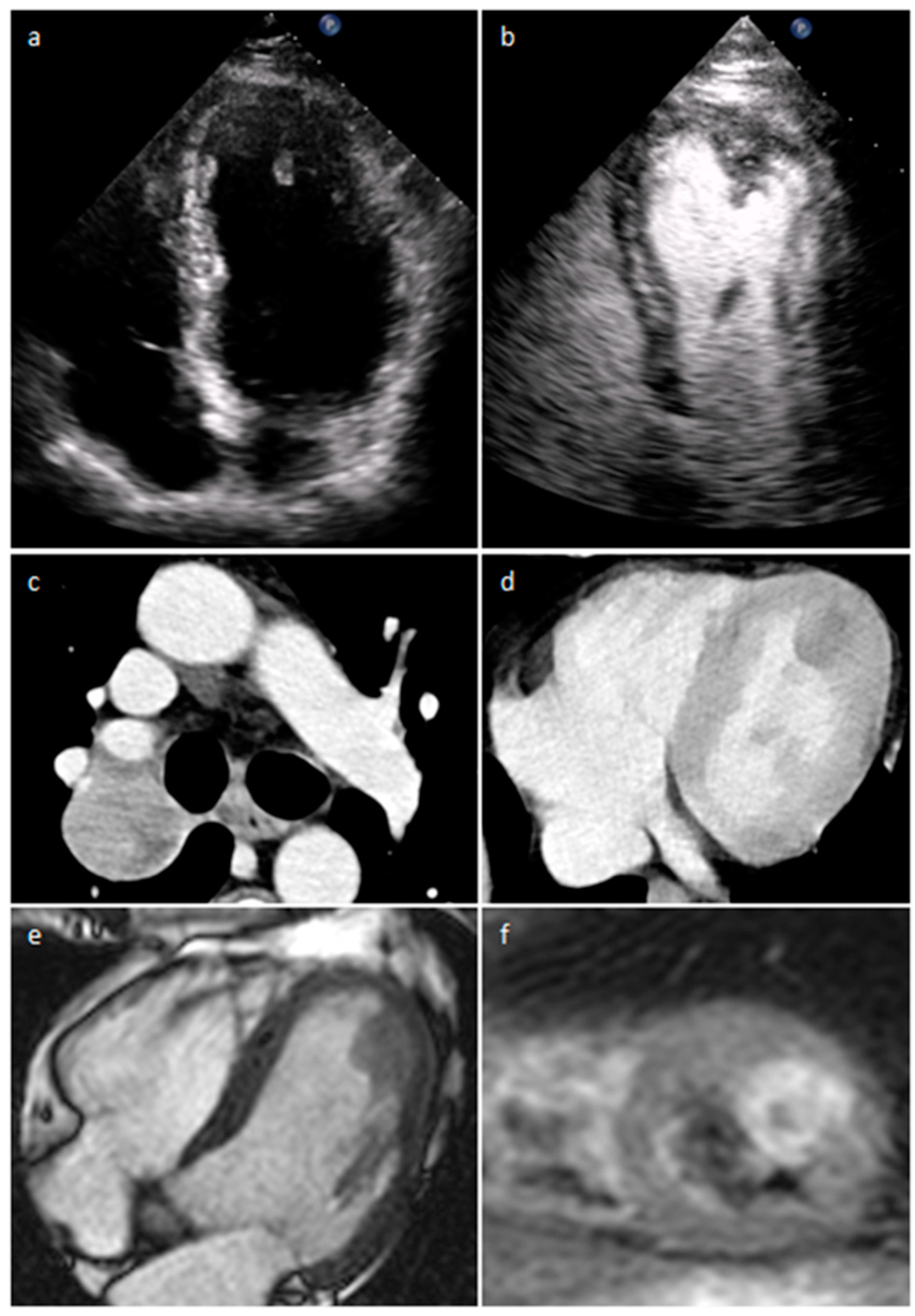
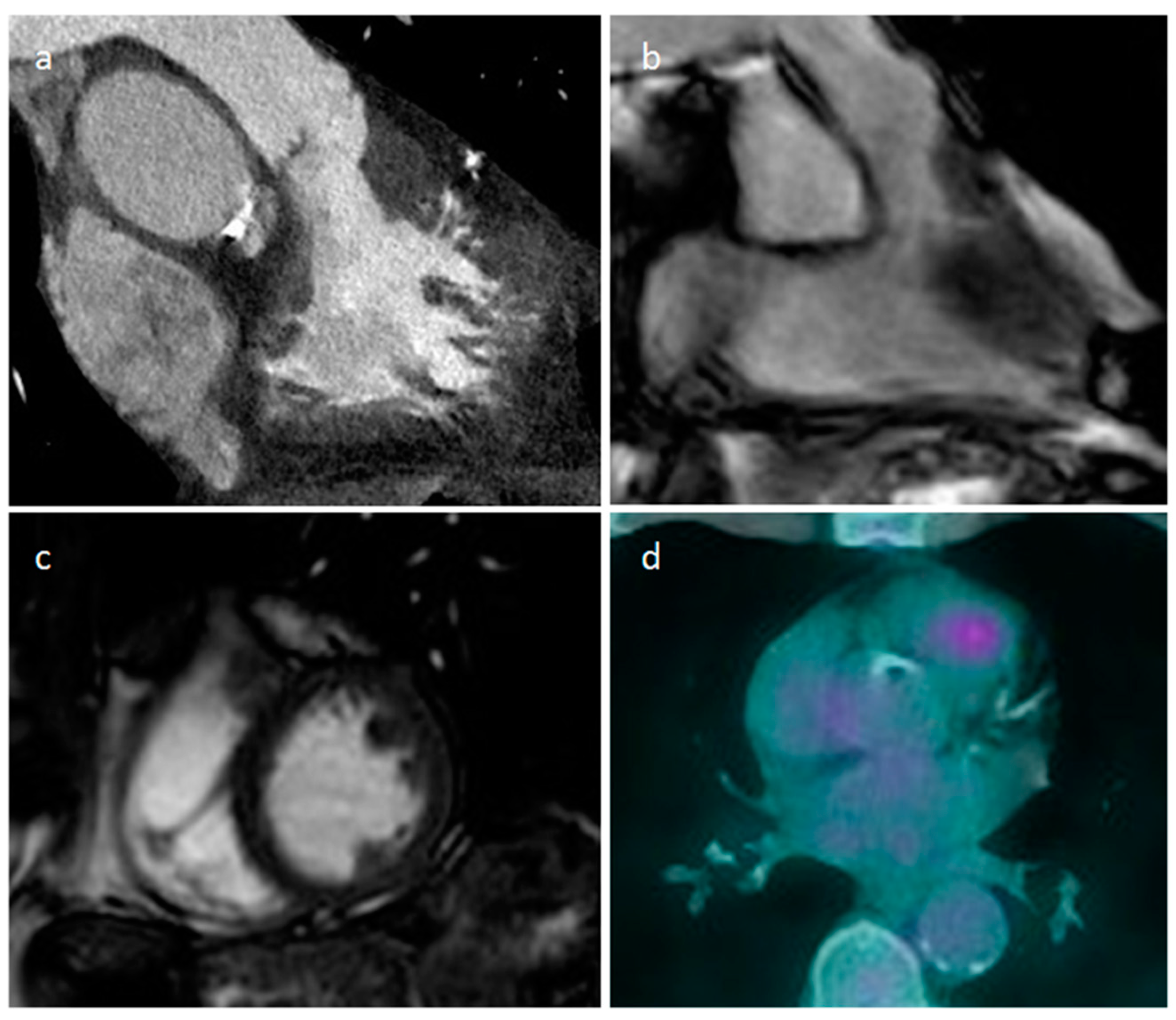
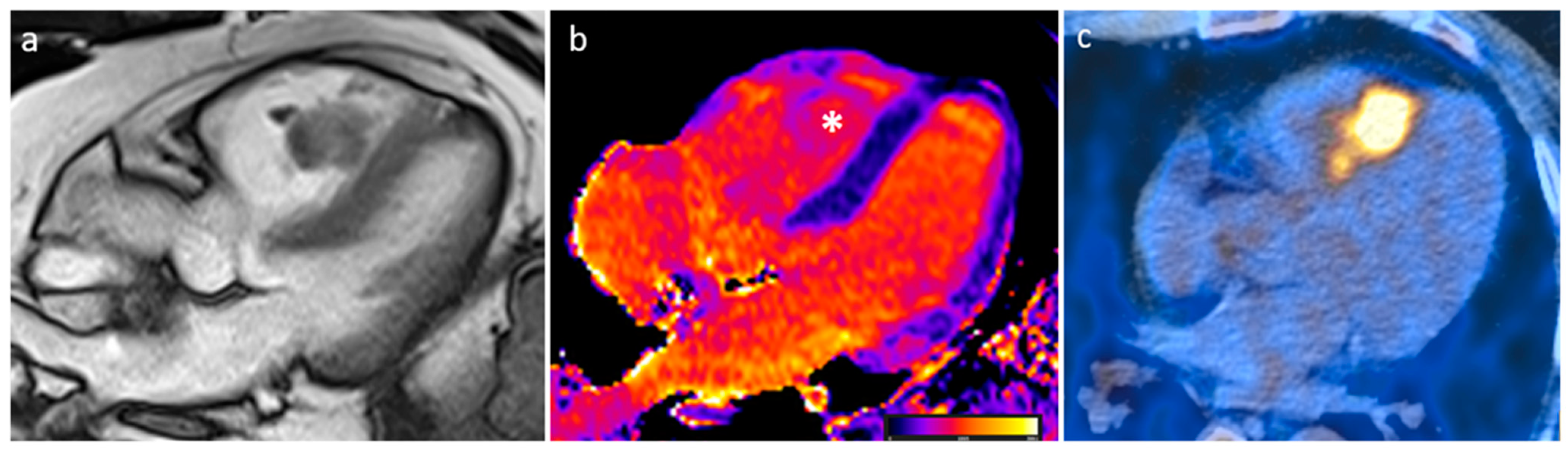
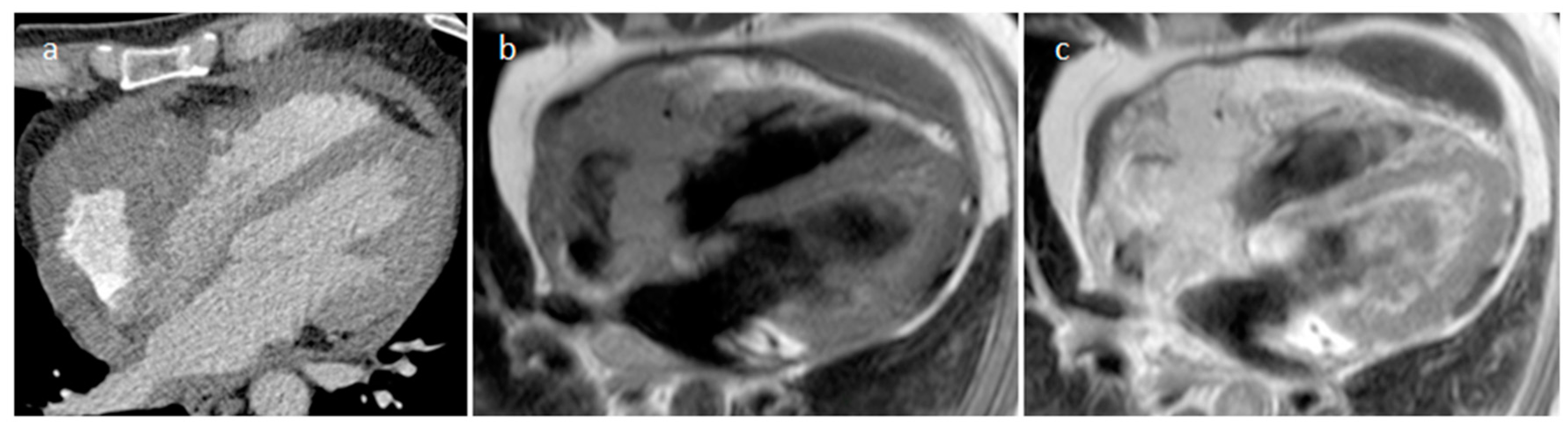
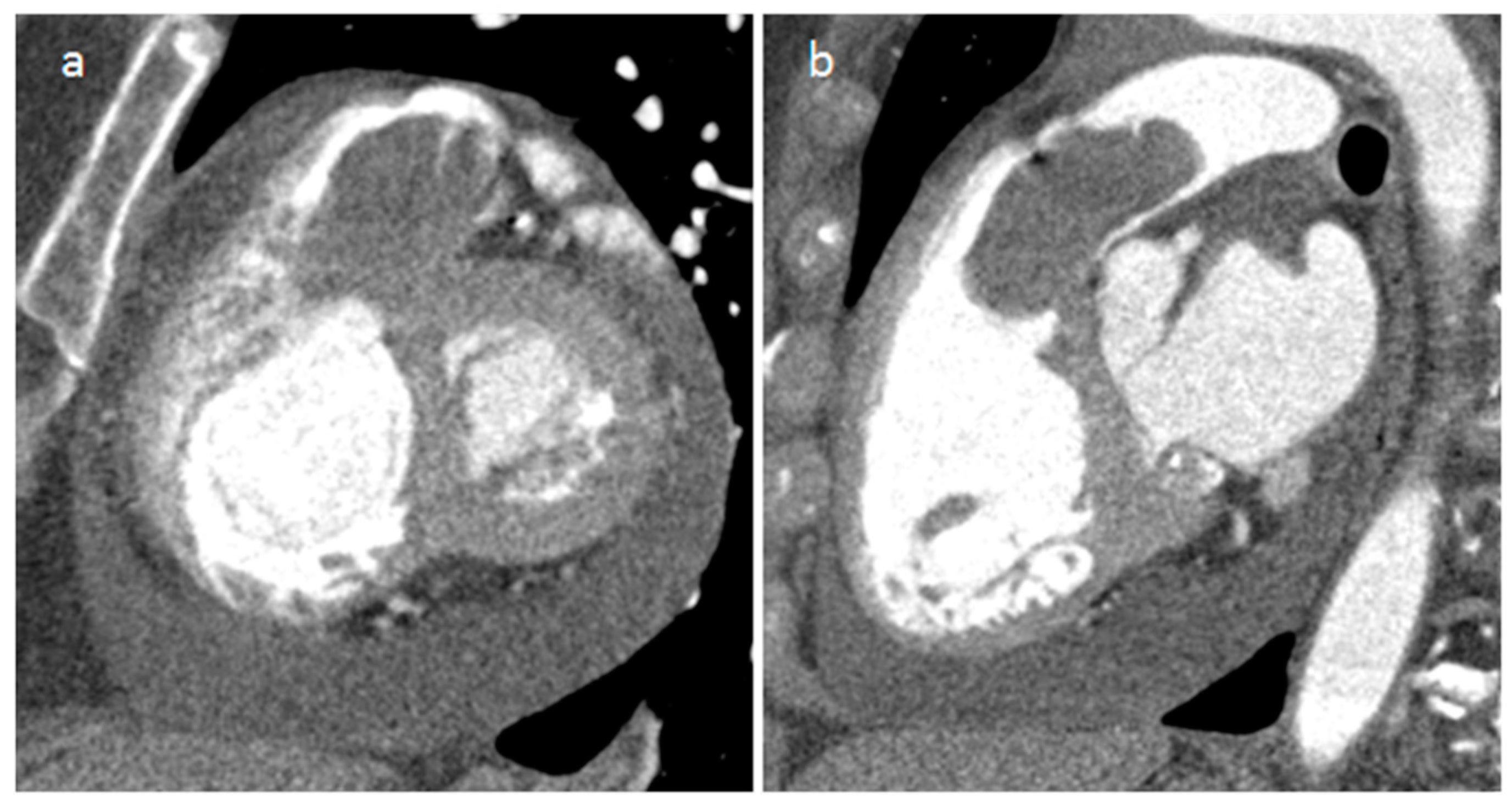
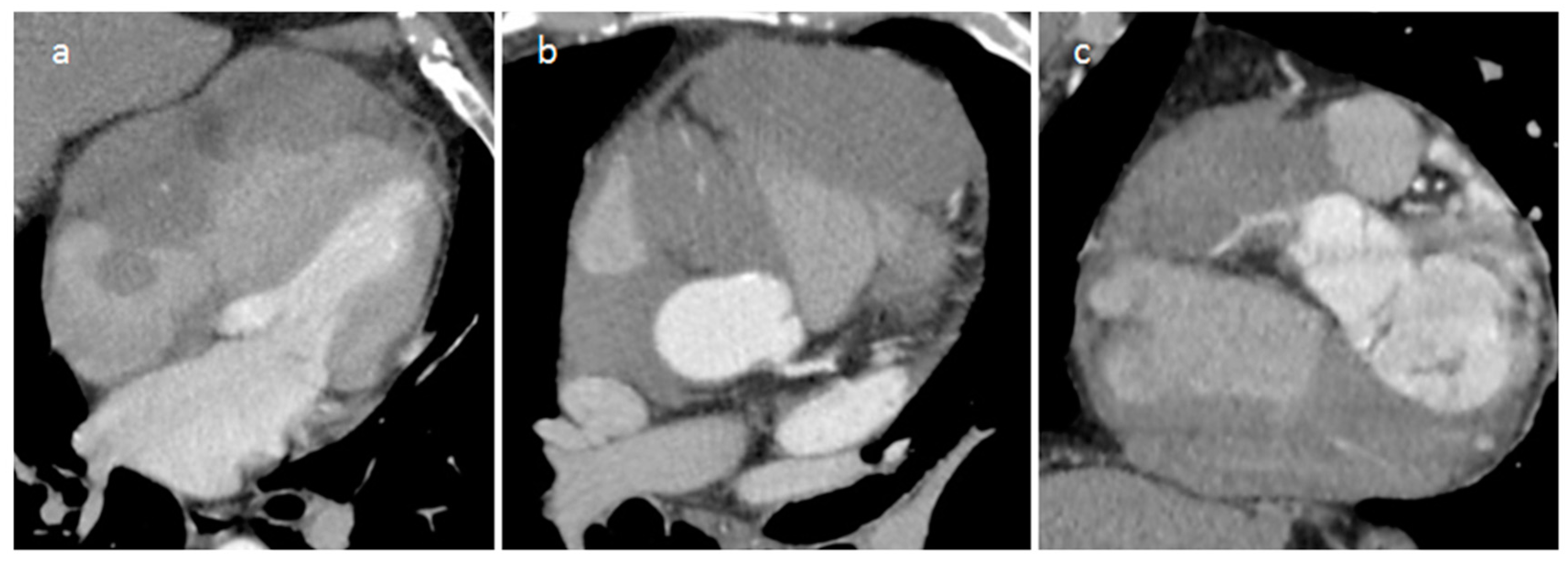

| Cardiac Lesion | Epidemiology | Location | Clinical Manifestations | Key Characteristics | Echocardiography | CT | MR |
|---|---|---|---|---|---|---|---|
| Coumadin ridge | Any age. Everybody | LA | Asymptomatic | Fold of the LA wall with a rounded edge between the left superior pulmonary vein and the left atrial appendage | nodular, pedunculated, or linear structure | Elongated structure similar to the atrial wall | Elongated structure similar to the atrial wall |
| Crista terminalis | Any age. Everybody | RA | Asymptomatic | Ridge in the posterolateral wall of the RA | Linear echogenic ridge | Linear ridge similar to the atrial wall | Linear ridge similar to the atrial wall |
| Taenia sagittalis | Any age. 80% of the population | RA, RAA | Asymptomatic | Ridge in the anterolateral wall of the RA | usually not visualized | Linear structure similar to the atrial wall | Linear structure similar to the atrial wall |
| Chiari network | Any age. 2–14% of the population | RA | Asymptomatic | lacelike structure in the RA over the IVC ostium or a linear band from the eustachian and/or thebesian valves to the crista terminalis | Reported in 2% of the population, mobile echogenic lacelike structure or a linear band | lacelike structure in the RA or a linear band | lacelike structure in the RA or a linear band |
| Moderator band | Any age, up to 92% of patients | RV | Asymptomatic | prominent muscular ridge in the RV | RV stripe | Muscular ridge similar to the RV wall | Muscular ridge similar to the RV wall |
| Papillary muscles | Any age. Everybody | LV, RV | Asymptomatic | from the inner wall of the ventricles in their middle or apical portions, with an elongated and tapered trunk | Echogenic elongated structures | Elongated structures similar to the LV and RV walls | Elongated structures similar to the LV and RV walls |
| LHIS | Late adulthood. Obesity | IAS | Usually, asymptomatic. Atrial arrhythmias | mass like deposition of brown fat in the IAS which spares the fossa ovalis | Homogeneous hyperechoic dumbbell appearance of atrial septum | mass with fat-attenuation which spares the fossa ovalis | Hyper T1w and T2w, no LGE, hypo on STIR and other fat-suppression sequences |
| Cardiac Lesion | Epidemiology | Location | Clinical Manifestations | Key Characteristics | Echocardiography | CT | MR |
|---|---|---|---|---|---|---|---|
| Thrombus | Adulthood | LA, LAA (AF) LV (MI) | Asymptomatic, embolic events | Non-enhancing Intracardiac lesion | Acute: Low echodensity, rounded with smooth contours Chronic: High echodensity, linear or crescentic lesions along the endocardial surface | Low attenuation, no contrast enhancement, chronic thrombus may be calcified Usefulness of delayed CT imaging, as in the LAA stasis of blood can simulate a thrombus on early arterial images | Acute: hyper T1w and T2w Subacute: hyper T1w and hypo T2w Chronic: low T1w and T2w No enhancement. |
| Vegetations | Adulthood | Valves | Valve dysfunction, emboli, heart failure | Highly mobile, non-enhancing | Highly mobile, oscillating, protruding, valve dysfunction | Low attenuation, may recognize, perivalvular extension, fistulas, abscess | Highly mobile |
| Mitral annular calcification | Old patients | annular fibrous ring of the mitral valve | Asymptomatic | Calcifications, mitral valve | Hyperechoic | Calcific mass | Hypo T1w, hypo T2w, peripheral rim enhancement |
| Caseous degeneration of mitral annular calcification | Old patients | annular fibrous ring of the left atrio-ventricular valve | Asymptomatic | Calcifications, mitral valve | hyperechoic | Calcifications within and around the mass | Mildly hyper T1w, mildly hyper T2w, peripheral rim enhancement sometimes with central enhancement |
| Pericardial cyst | Adulthood | Right pericardiophrenic angle | Asymptomatic | Fluid-filled, thin-walled, homogeneous, no internal enhancement | Low echodensity | Hypodense well-defined lesion with a wall | Hypo T1w, hyper T2w, no internal enhancement |
| Coronary artery aneurisms | Adulthood | AV groove | Asymptomatic | Vascular mass | AV groove mass | Dilatation, thrombus, fistula | Vascular enhancement |
| Cardiac aneurysm and pseudoaneurysm | Adulthood | Cardiac chamber | heart failure | Blood-filled | Dilatation | Dilatation, thrombus | Dilatation, thrombus |
| IgG4-related disease | Adulthood Male to female ratio is 2:1. | AV groove and right atrial infiltration, pericardium, coronary arteries | pericardial tamponade, constrictive pericarditis, valvula stenosis or regurgitation | Coronary periaortitis, AV groove and right atrial infiltration, associated pancreatic, biliary, renal involvement | Pericardial effusion with highly echogenic thickening of the pericardium, valvular stenosis or regurgitation, pulmonary arterial hypertension | aortitis, periarteritis, coronary aneurysms, perivascular infiltration with coronary stenosis | Cardiac masses, pericardial thickening, valvular disease |
| Sarcoidosis | Adulthood | Basal septum, AV groove | AV block, LVEF reduction | Basal septal thickening, delayed myocardial enhancement, associated mediastinal/hilar adenopathy | Septal mass lesion | Basal septum thickening, rarely soft tissue infiltration into AV groove encasing the coronary artery | LGE, Active disease (oedema): hyper T2w |
| Foreign body | Adulthood | Intracardiac, pericardium | Usually asymptomatic; rarely life-threatening and highly symptomatic | Intracardiac or pericardial mass. Previous surgical or intravascular procedures | Hyperechoic, sometimes with posterior acoustic shadowing | High-density. Gossypiboma: heterogeneous, cystic | Gossypiboma: hypo T1w, hyper T2w with internal low signal intensity stripes Others: usually hypo T1w and T2w with possible surrounding artifacts |
| Hematoma | Usually adulthood, previous surgery, traumatic heart injury, coagulopaty, anticoagulants | Pericardium | Usually, asymptomatic | Previous cardiac surgery, mass near surgical site or clips, usually well-defined borders. Usually absent contrast-enhancement | Acute phase: echo-lucent Chronic phase: mass like and echogenic | Heterogeneous, clips, hyperdense in acute phase, density decrease in chronic phase, calcific components in chronic hematoma | Acute: hyper T1w and T2w Subacute: heterogeneous with hyper T1 and T2w areas Chronic: hypo T1w and T2w with dark peripheral rim. No internal enhancement, possible rim enhancement |
| Echinococcus cyst | Adulthood | Myocardium, pericardium | Usually, asymptomatic | Typical hydatic cyst imaging characteristics | Well defined cystic mass with or without septations | Hypodense lesion with a wall, daughter cysts, peripheral calcifications | Hypo T1w, hyper T2w |
| Cardiac Lesion | Epidemiology | Location | Clinical Manifestations | Key Characteristics | Echocardiography | CT | MR |
|---|---|---|---|---|---|---|---|
| Myxoma | Adulthood. Carney complex. | LA | Usually, asymptomatic. Rarely, intracardiac obstruction, embolic events and constitutional symptoms | Mobile mass arising from the IAS | Globular or spherical, with a friable surface and heterogeneous internal echogenicity | Heterogeneous, low attenuation, may be calcified | Isointense T1w, High T2w, heterogeneous LGE |
| Papillary fibroelastoma | Adulthood | Valves | Usually, asymptomatic. Rarely embolic events | Atrial side of the mitral valve or the aortic surface of the aortic valve leaflet | Stippling and vibration or shimmer of the peripheral edge. | Hypodense, smooth, peduncolated, attached to the valve leaflet by a short pedicle | Iso T1w, Hyper T2w, hypo cine with surrounding turbolent flow, poor LGE |
| Lipoma | Adulthood Tuberous sclerosis | LV, any other site | Asymptomatic. If large, sometimes arrhythmias or obstructive symptoms | Circumscribed homogeneous fat-containing mass | Homogeneous, hyperechoic in the cavity but hypoechoic in the pericardium | Smooth, homogeneous mass with fat-attenuation | Hyper T1w and T2w, no LGE, hypo on STIR and other fat-suppression sequences |
| Rhabdomyoma | Fetal life and childhood. Tuberous sclerosis | LV, IVS | Asymptomatic. Rarely flow obstruction, heart failure, arrhythmias | Intramyocardial masses, frequently multiple | Homogenous, slightly echogenic | Attenuation similar to myocardium | Iso T1w, iso-hyper T2w, no or minimal enhancement |
| Fibroma | Childhood. Gorlin Sd | LV, IVS | Asymptomatic. Rarely arrhythmias | Intramyocardial mass, solitary | Heterogeneous, echogenic, non-contractible, can mimic HCM | Soft tissue attenuation, low contrast enhancement, may show central calcification | Iso-hypo T1w, hypo T2w, no or minimal enhancement in perfusion imaging. High LGE |
| Hemangioma | Adulthood | RA, RV, LV, any other site including pericardium | Usually, asymptomatic. | Inhomogeneous. Evident post-contrast enhancement. Well-demarcated without invasion of adjacent tissues and structures | hyperechogenic mass | water attenuation. Rarely, calcifications | Iso T1w, hyper T2w, strong enhancement in perfusion imaging, high LGE |
| Lymphangioma | Any age | Pericardium, any other site | respiratory distress/dyspnea, arrhythmia, chest pain, heart failure | Mass with cystic and septal components | Echogenic mass with cystic and septal components | Cysts, absence of calcifications or macroscopic fat. | Hypo T1w, hyper T2w, enhancement of cystic walls and septa, no internal enhancement |
| Erdheim-Chester disease | Adulthood Male to female ratio is 3:1. | RA, AV groove, pericardium | Heart failure, myocardial infarction, tamponade | symmetric osteosclerosis of the metadiaphysis of the lower-extremity bones, RA masses, AV groove infiltration, periarterial infiltration | Pericardial effusion | Right atrium or AV sulcus mass, coronary periaortitis, pericardial thickening and effusion | Strong enhancement |
| Solitary fibrous tumor | Adulthood | Pericardium | Asymptomatic, dyspnea, palpitation | Slow-growing pericardial mass | inhomogeneous pericardial mass, pericardial effusion | Low attenuation, scarce enhancement | Iso T1w, heterogeneous or homogeneous hyper T2w, hyper on SPIR, septated or patchy LGE |
| Teratoma | Fetal life and childhood. | pericardium | compression on the heart and cardiac tamponade | Mass with cysts, gross fat, calcification, and regions of soft tissue. | Heterogeneous | well-defined mass with a variable extent of cystic degeneration, calcification, and areas of fatty tissue | gross fat: hyper T1w and T2w, with signal decrease on a fat-suppressed T2w |
| Schwannoma | Adulthood. Schwannomatosis | RA, right atrioventricular groove | Usually, asymptomatic. Rarely, chest tightness, dyspnea, cough, fatigue, and facial edema | circumscribed, slow enhancement | Heterogeneous, echogenic mass | Low attenuation, scarce enhancement, scattered calcifications | Iso-hypo T1w, target appearance on T2w images with central hypointensity and peripheral hyperintensity. Slow internal enhancement |
| Paraganglioma | Adulthood | On the roof of left atrium, right atrioventricular groove | elevated blood pressure and severe headaches | “salt and pepper” appearance, highly vascular, parasitizes blood supply from coronary arteries, elevated serum metanephrines Malignant up to 25% | Solid heterogeneous echogenic mass with clear boundaries, and detectable blood flow signals inside it in the color Doppler | A mass of soft-tissue density with homogeneous (smaller lesions) or peripheral (larger lesions with hemorrhage, necrosis and cystic degeneration) enhancement | Iso-hypo T1w, hyper T2w, peripheral LGE. Perfusion imaging shows strong enhancement |
| Cardiac Lesion | Epidemiology | Location | Clinical Manifestations | Key Characteristics | Echocardiography | CT | MR |
|---|---|---|---|---|---|---|---|
| Metastasis | Adulthood Melanoma, lung, breast and esophageal cancers common. Transvenous: HCC and RCC | Myocardium, pericardium, right atrium in transvenous spread | Flow obstruction/heart failure, arrhythmia, pericardial effusion | Multiple myocardial or pericardial masses. Right atrium lesions in transvenous spread. More often circumscribed lesions | Heterogeneous, highly echogenic with contrast infusion | Similar to soft tissue attenuation | Hypo-iso T1w, Iso-hyper T2w, heterogeneous LGE. Melanoma: hyper T1w |
| Angiosarcoma | Early and middle adulthood, Li-Fraumeni Sd | RA near AV sulcus, pericardium | Constitutional symptoms, heart failure, pericardial effusion | Vascular nature; hemorrhage and necrosis. Lung, liver and brain metastasis. | Iso-hyperechogenic and irregular mass, often as a nonmobile, broad-based, endocardial neoplasm with myocardial extension. Pericardial effusion | low-attenuation, irregular, intracavitary mass is often shown, pericardial thickening with effusion, heterogeneous enhancement | Heterogeneous Iso-hyper T1w, heterogeneous-hyper T2w, marked and heterogeneous enhancement and LGE. Perfusion imaging in the arterial phase shows immediate and strong enhancement |
| Undifferentiated pleomorphic sarcoma (UPS) | Early and middle adulthood, Li-Fraumeni Sd | LA | Flow obstruction/heart failure, pericardial effusion, metastatic | Endocardial growths that protrude into the chamber and invade the adjacent myocardium. Distant metastasis | Heterogeneous, normally-highly echogenic | Heterogeneous, large, irregular low attenuation | Heterogeneous iso T1w, hyper T2w and LGE. Heterogeneous enhancement at first pass |
| Leiomyosarcoma | Adulthood, Li-Fraumeni Sd | LA | Flow obstruction/heart failure, pericardial effusion | Distant metastasis and local recurrence | Echogenic and irregular mass | irregular low attenuation | Iso-hypo T1w, hyper T2w, heterogeneous LGE. Heterogeneous enhancement at first pass |
| Rhabdomyosarcoma | Childhood, early adulthood | LV, RV | Heart failure, arrhythmia, eosinophilia | Multiple myocardial masses | Normally-highly Echogenic | Irregular, low attenuation | Iso T1w, T2w, hyper STIR, homogeneous LGE. Enhancement at first pass |
| Lymphoma | Adulthood, Immunocompromised | RA, RV, pericardium | Pericardial effusion, flow obstruction/heart failure | Uniform, infiltrative, extend along the epicardial surfaces of the heart, encase adjacent vascular structures, hypermetabolic on PET | Pericardial effusion. Tumor might be detected with homogeneous echogenicity. | Pericardial effusion. Normal to low attenuation, heterogeneous and mild contrast enhancement | Iso-hypo T1w, Iso-hyper T2w, none-minimal homogeneous LGE. Evident diffusion restriction |
| Mesothelioma | Adulthood | Pericardium | constrictive pericarditis, tamponade | Diffuse pericardial involvement with complete encasement of the heart | Pleural effusion with echogenic lesions | Diffuse irregular pericardial thickening with heterogeneous enhancement | Iso T1w, heterogeneous T2w, heterogeneous LGE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagliati, C.; Fogante, M.; Palmisano, A.; Catapano, F.; Lisi, C.; Monti, L.; Lanni, G.; Cerimele, F.; Bernardini, A.; Procaccini, L.; et al. Cardiac Masses and Pseudomasses: An Overview about Diagnostic Imaging and Clinical Background. Medicina 2024, 60, 70. https://doi.org/10.3390/medicina60010070
Tagliati C, Fogante M, Palmisano A, Catapano F, Lisi C, Monti L, Lanni G, Cerimele F, Bernardini A, Procaccini L, et al. Cardiac Masses and Pseudomasses: An Overview about Diagnostic Imaging and Clinical Background. Medicina. 2024; 60(1):70. https://doi.org/10.3390/medicina60010070
Chicago/Turabian StyleTagliati, Corrado, Marco Fogante, Anna Palmisano, Federica Catapano, Costanza Lisi, Lorenzo Monti, Giuseppe Lanni, Federico Cerimele, Antonio Bernardini, Luca Procaccini, and et al. 2024. "Cardiac Masses and Pseudomasses: An Overview about Diagnostic Imaging and Clinical Background" Medicina 60, no. 1: 70. https://doi.org/10.3390/medicina60010070
APA StyleTagliati, C., Fogante, M., Palmisano, A., Catapano, F., Lisi, C., Monti, L., Lanni, G., Cerimele, F., Bernardini, A., Procaccini, L., Argalia, G., Esposto Pirani, P., Marcucci, M., Rebonato, A., Cerimele, C., Luciano, A., Cesarotto, M., Belgrano, M., Pagnan, L., ... Schicchi, N. (2024). Cardiac Masses and Pseudomasses: An Overview about Diagnostic Imaging and Clinical Background. Medicina, 60(1), 70. https://doi.org/10.3390/medicina60010070








