Abstract
In 1968, Jean Berger first introduced the medical world to IgA nephropathy (IgAN). Fifty-five years later, its pathogenesis is still unclear, but treatments such as renin–angiotensin–aldosterone system inhibitors (RAAS-Is), tonsillectomies, and glucocorticoids are currently used worldwide. There have been great strides in the past 20 years since the discoveries of the specific dysregulation of mucosal immunity, galactose-deficient IgA1 (Gd-IgA1), and Gd-IgA1 immune complexes in patients with IgAN. According to these findings, a multi-hit hypothesis was developed, and this multi-hit hypothesis has provided several putative therapeutic targets. A number of novel agents, including molecularly targeted drugs for targets such as APRIL, plasma cells, complement systems, and endothelin, are undergoing clinical trials. Some candidate drugs have been found to be effective, with minimal side effects. Over half a century after the discovery of IgAN, these therapies will soon be available for clinical use.
1. Introduction
IgAN, a prevalent form of primary glomerulonephritis (GN), is distinguished by the accumulation of IgA antibodies within the glomeruli. Within 10-to-20 years of being diagnosed, approximately 20–40% of patients progress to end-stage renal disease (ESRD) [1,2,3]. The discovery of galactose-deficient IgA1 (Gd-IgA1) in patients led to the proposal of a multi-hit hypothesis for the etiology of IgAN [4,5,6]. In this review, we discuss Gd-IgA1, the multi-hit hypothesis, and treatments for IgAN based on those currently used in emerging strategies.
2. Materials and Methods
A narrative review was undertaken. We accessed the National Library of Medicine, National Center for Biotechnology Information (PubMed.gov), on 15 June 2023. A literature search was performed using the keywords “IgA nephropathy”, “treatment”, and “options”. The literature search was completed on 13 July 2023. The selected studies came in the form of RCTs, clinical trials, intervention studies, and observational studies. Some good-quality reviews were also selected for new drugs in development for which information is scarce. The selected studies were limited to English-language studies. Among the studies obtained, 8 articles focused on renin–angiotensin–aldosterone system inhibitors (RAAS-Is) (Table 1), 4 articles focused on tonsillectomy (Table 2), 2 articles focused on sodium glucose transporter 2 (SGLT2) inhibitors (Table 3), 4 articles focused on glucocorticoid (Table 4), and 8 articles focused on upcoming therapeutic options (Table 5). Supportive references for review were also selected from PubMed.

Table 1.
RAAS-Is.

Table 2.
Tonsillectomy.

Table 3.
SGLT2 inhibitors.

Table 4.
Glucocorticoids.

Table 5.
Upcoming therapeutic options for IgAN.
3. Gd-IgA1 and the Multi-Hit Hypothesis
The multi-hit hypothesis posits that the production of Gd-IgA1 is the initial hit, which is then followed by the creation of IgG autoantibodies that recognize Gd-IgA1, constituting the second hit. The formation of immune complexes serves as the third hit, and their subsequent deposition in the glomeruli is the fourth hit. This sequence results in hematuria, proteinuria, and a decline in renal function [37,38] (Figure 1).
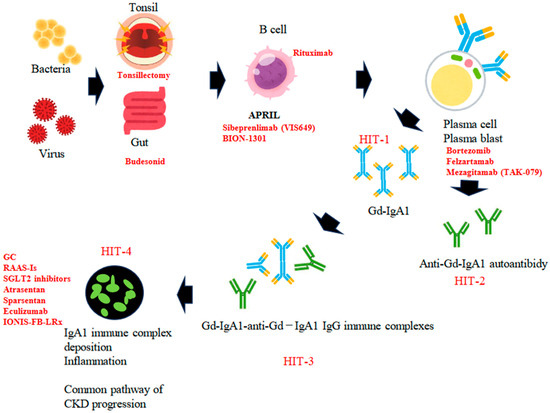
Figure 1.
The putative pathogenesis of IgAN, based on the four-hit hypothesis. Hit 1: Initial production of Gd-IgA1 in secondary lymphoid tissues (tonsil or Peyer’s patch in the gut) and transfer of Gd-IgA1-producing cells into bone marrow. Hit 2: Production and circulation of anti-Gd-IgA1 IgG. Hit 3: Formation of Gd-IgA1-anti-Gd-IgA1 IgG immune complexes. Hit 4: Deposition of Gd-IgA1 immune complexes in glomeruli induces focal inflammation. Key molecules or cells and drugs in each step are shown.
This comprehension of IgAN’s autoimmune pathogenesis has paved the way for the discovery of various pharmacological therapeutic targets, encompassing the immune response, mucosal immunity, renal inflammation, and complement activation. Therapies based on these mechanisms have been investigated in both preclinical and clinical studies [39].
4. Current Therapeutic Options for IgAN
Renal disorders (the pathogenesis of which is described above) can be treated with various therapies, and these therapies have been the subject of clinical studies that have led to the accumulation of evidence of their efficacy. The currently available treatments include renin–angiotensin–aldosterone system inhibitors (RAAS-Is), tonsillectomies, sodium glucose co-transporter 2 (SGLT2) inhibitors, and glucocorticoids.
4.1. RAAS-Is
The RAAS is a key player in maintaining blood pressure and volume balance, and its inhibition with ACE inhibitors (ACE-Is) has proven to be an effective treatment strategy in conditions such as hypertension and congestive heart failure. The prevention of angiotensin II formation may also be advantageous in decelerating the progression of renal disease by decreasing glomerular hydrostatic pressure. This presents a hopeful therapeutic avenue for patients with IgAN, potentially enhancing outcomes and decelerating disease progression [40].
Valsartan, an angiotensin receptor blocker (ARB), has been found to significantly reduce proteinuria and slow down renal deterioration in IgAN patients [7], while enalapril, an ACE-I, has demonstrated significant improvements in renal function in proteinuric IgAN [8]. A randomized controlled trial (RCT) that tracked IgAN patients for 5 years to study the long-term renal outcomes of ACE-I/ARB therapy discovered that the treated group had lower serum creatinine, reduced proteinuria, and slower progression to ESRD compared to the control group [9]. One study on the impact of ACE-Is on tubulointerstitial fibrosis (TIF) in IgAN patients observed a slower rate of decline in creatinine clearance in the ACE-I group [10]. A controlled trial examining the response of IgAN patients to ACE-I/ARB therapy based on reduced proteinuria and its effect on the selectivity index suggested that this treatment could be beneficial for IgAN patients with renal impairment and non-selective proteinuria [11].
A combined treatment approach using prednisolone and losartan has been shown to be more effective than using prednisolone alone in decreasing proteinuria and preserving renal function in IgAN patients [12]. The combination of trandolapril and candesartan cilexetil has also been found to be more effective than verapamil in reducing the count of urinary podocytes, which could be an indicator of disease activity in adult IgAN patients [13]. Low-dose losartan significantly diminished proteinuria and urinary N-acetyl-beta-D-glucosaminidase (NAG) excretion without affecting systemic blood pressure in normotensive IgAN patients [14]. These results suggest that these medications could have a positive impact on the management of IgAN.
4.2. Tonsillectomies
IgAN patients frequently exhibit macroscopic hematuria following an acute tonsillar infection, leading to the proposition of a tonsillectomy as a potential treatment for IgAN. In Japan, tonsillectomies are employed in conjunction with steroid pulse therapy, and this combination has demonstrated promising outcomes. One RCT involving IgAN patients with proteinuria and low serum creatinine revealed that those who underwent a tonsillectomy and received glucocorticoid pulse therapy had significantly reduced urinary protein excretion compared to those who were administered only a glucocorticoid pulse [15]. Logistic regression analysis identified tonsillectomy and glucocorticoid pulse therapy to be significant independent factors contributing to the elimination of proteinuria. One patient in the tonsillectomy group (n = 40) and three patients in the no-tonsillectomy group (n = 40) developed diabetes during observation. No other serious complications were observed [15].
A retrospective cohort study conducted in Japan, which included 1065 IgAN patients enrolled between 2002 and 2004, implemented 1: 1 propensity score matching (to mitigate intergroup differences) for patients who underwent a tonsillectomy and those who did not. Among 153 matched pairs, the study revealed a reduced risk of the first instance of a 1.5-fold increase in serum creatinine from baseline or the initiation of dialysis in patients who were treated via a tonsillectomy. These patients also needed fewer additional treatments for one year following renal biopsy without an elevated risk for adverse events, barring temporary complications related to tonsillectomy. These findings imply that tonsillectomy could be a viable option for preventing ESRD in IgAN patients. In the entire study population (n = 1065), 59 patients had complications such as pneumonia and diabetes. No deaths occurred in the tonsillectomy group, but four deaths from malignancy, one death from obstructive lung disease, and one death from aortic dissection were observed in the no tonsillectomy group [16].
Tonsillectomies have mainly been performed in Japan, and they have been shown to be effective. In Europe, the efficacy of tonsillectomies has been questioned by many. A Hungarian study of 246 patients showed a significant prolongation of renal survival in the group of patients who underwent a tonsillectomy compared to the group of patients who did not have a tonsillectomy. This study also showed that tonsillectomy was a significant factor for prolonged renal survival [17]. On the other hand, a sub-analysis of the VALIGA study (described below) conducted in Europe to evaluate the efficacy of steroids showed no effect of tonsillectomies on the preservation of renal function [18]. In light of these circumstances, the KDIGO (2021) practice guidelines state that tonsillectomies may be considered in the treatment of Japanese patients [41].
4.3. SGLT2 Inhibitors
DAPA-CKD trial: The DAPA-CKD trial demonstrated a reduction in the risk of transitioning to ESRD and mortality in patients with CKD, including those with IgAN. The primary endpoint was a persistent decrease in eGFR of ≥50%, ESRD, or death due to kidney disease-related or cardiovascular causes. Among the 270 IgAN subjects, 137 were administered dapagliflozin, and 133 were given a placebo. The primary outcome was observed in 4% of the dapagliflozin group and 15% of the placebo group. Compared to the placebo group, dapagliflozin reduced UACR by 26%. No significant difference was observed between the two groups in terms of medication discontinuation due to adverse drug reactions. These findings suggest that dapagliflozin can safely slow down the progression of CKD in IgAN patients. A total of six complications in the dapagliflozin group (n = 137) forced the discontinuation of the study, while in the sham group (n = 133), seven complications forced the discontinuation of the study (no significant difference). Severe complications, including death, occurred in 22 patients (16.1%) in the dapagliflozin group and 34 (25.6%) in the sham group [19].
The EMPA-KIDNEY trial expanded the range of CKD-causing diseases in which SGLT2 inhibitors could be expected to provide benefit. The primary endpoint of the EMPA-KIDNEY trial was a sustained ≥40% reduction in eGFR from basal values occurring after allocation, the introduction of renal replacement therapy (eGFR < 10 mL/min/1.73 m2), and death from cardiovascular events. Serious AEs included six cases of ketoacidosis in the empagliflozin group (0.09 patient years) and one in the sham group (0.02 patient years), as well as leg amputations in 28 patients (0.43 patient years) in the empagliflozin group and 19 patients (0.29 patient years) in the sham group. The trial was stopped early due to confirmed efficacy, limiting the analysis to all prespecified subgroups [20]. Therefore, compared to the DAPA-CKD study, the effect of dapagliflozin on IgA nephropathy was not clearly demonstrated. However, the number of patients with CKD due to glomerular disease was larger than in the DAPA-CKD study, 1669/25% (DPA-CKD study: 695/16.9%), and a large number of IgA nephropathy patients would be expected to be among these patients, indirectly suggesting an effect on IgA nephropathy patients [42].
A meta-analysis revealed that SGLT2 inhibitors diminish the risk of renal and cardiovascular events in patients with conditions such as heart failure, CKD, type II diabetes, and atherosclerotic cardiovascular risk. When compared to a placebo, SGLT2 inhibitors decreased the risk of advancing to renal disease by 37% in patients with diabetes (relative risk (RR), 0.63; 95% CI, 0.58–0.69), exhibiting a similar risk reduction effect in non-diabetic patients. Furthermore, SGLT2 inhibitors reduced the risk of acute kidney injury by 23% (0.77, 0.70–0.84) and the risk of death or hospitalization due to heart failure by 23% (0.77, 0.74–0.81) [43].
4.4. Glucocorticoids
Early studies have shown that glucocorticoids have long-term renoprotective effects. One study found that patients treated with intravenous methylprednisolone (mPSL) and oral prednisolone had a significantly lower rate of reaching endpoints of a 1.5- or 2-fold increase in baseline serum creatinine compared to controls. No serious AEs, such as hyperkalemia, were recorded [21]. Another study showed that oral prednisolone was renoprotective compared to controls over an 8-year observation period. No serious AEs were observed in either the combination treatment group or the ramipril monotherapy group [22].
(1) VALIGA study: The VALIGA observational cohort study analyzed data from 1147 IgAN patients from 13 European countries. The effects of various treatments, including immunosuppressive agents, on renal outcomes were assessed in a median follow-up period of 4.7 years. The results showed that immunosuppressive agents were associated with a lower risk of renal function decline (> 50% decrease in eGFR or ESRD) than no treatment or antihypertensive drugs, especially in patients with severe histological lesions or high-risk clinical features [23].
(2) STOP-IgAN trial: The STOP-IgAN trial, a randomized controlled trial (RCT), examined the effects of supportive care (SC) alone (including RAAS-Is) versus SC plus immunosuppression (IS) (corticosteroids or cyclophosphamide/azathioprine) in 162 IgAN patients with persistent proteinuria (>0.75 g/day) despite 6 months of optimized RAAS blockade. The trial did not find a significant difference between the two groups in terms of the primary endpoint of full clinical remission (proteinuria < 0.2 g/day and stable renal function) at 3 years. However, the combination of SC and IS was linked to a higher rate of partial remission (proteinuria < 0.5 g/day and stable renal function) and a slower rate of renal function decline (>15 mL/min/1.73 m2 decrease in eGFR) compared to SC alone. The immunosuppressed group had significantly more cases of infection and weight gain than the supportive care group, and one patient died of sepsis [24]
(3) TESTING and TESTING 2.0 trial: The TESTING trial was carried out on Chinese patients with IgAN who had urinary protein excretion exceeding 1 g/day and eGFR ranging from 20 to 120 mL/min/1.73 m2. The group receiving SC plus IS was administered oral mPSL for a duration of 2 months, and this treatment was gradually tapered off over a period of 6–10 months. The incidence of the primary composite endpoint was significantly lower in the SC plus-IS group compared to the SC-alone group (p = 0.02). The SC plus-IS group also exhibited lower urinary protein excretion at the endpoint (p < 0.01) and more favorable secondary endpoints (p < 0.01, p = 0.01, p = 0.01). However, the occurrence of adverse effects and severe infection was significantly higher in the SC plus-IS group (p < 0.01), leading to a shortened observation period of 2.1 years instead of 3 years [25]. Therefore, this trial found that mPSL treatment had limited benefits. However, mPSL was associated with a greater reduction in proteinuria than SC alone.
The TESTING trial demonstrated the renoprotective effect of the glucocorticoids in IgAN, but this conclusion was considered uncertain due to the shortened observation period and adverse effects of mPSL. Consequently, the TESTING 2.0 trial, with its international, multicenter, and double-blind design, was conducted on patients receiving full-dose (n = 136) and reduced-dose (n = 126) mPSL. The control patients were given a placebo. A total of 503 patients were randomized and treated with mPSL or the placebo for 2 months and weaned for 6–9 months. Over an average of 4.2 years, the composite primary endpoint occurred in 28.8% of the mPSL patients and 43.1% of the placebo patients [HR 0.53 (95% CI 0.39–0.72); p < 0.01]. The effect of full-dose mPSL was an HR of 0.58 (95% CI, 0.41–0.81), and that of reduced-dose mPSL was an HR of 0.27 (95% CI, 0.11–0.65). Nine out of eleven secondary endpoints favored mPSL treatment, including kidney failure (HR, 0.59 (95% CI, 0.11–0.65)). Adverse events were higher in cases treated with mPSL, especially in the full-dose group [26]. Therefore, oral mPSL reduced the risk of kidney function decline, kidney failure, or death due to kidney disease in high-risk IgAN cases but increased the incidence of serious adverse events, mainly with high-dose mPSL.
(4) NEFIGAN and NefIgArd trial: Budesonide (Nefecon®) is an oral glucocorticoid engineered to selectively release in the distal ileum, an area densely populated with Peyer’s patches. The NEFIGAN phase IIb double-blind placebo-controlled trial demonstrated that budesonide use resulted in a 24.4% reduction from baseline in the urinary protein-to-creatinine ratio (UPCR) compared to a placebo. Two out of thirteen serious adverse events were potentially linked to budesonide [27]. In November 2021, the US FDA granted Tarpeyo (budesonide), a targeted-release formulation capsule, accelerated approval to decrease proteinuria in adults with primary IgAN who are at risk of rapid disease progression. As part of the conditions for the accelerated approval, a study on Tarpeyo is currently underway to confirm that the drug slows down kidney function decline in patients with IgAN [44].
The NefIgArd trial was an international, phase 3, randomized, double-blind, placebo-controlled multicenter study that aimed to examine the efficacy and safety of 16 mg of Tarpeyo (budesonide) once daily versus a placebo in adult patients with primary IgAN as an adjunct to optimized RAAS-Is therapy. In this study, the initial endpoint of 16 mg of Nefecon for 9 months achieved a significant reduction in UPCR compared to the placebo and also showed a sustained reduction in UPCR after 12 months of treatment. This study also demonstrated a second endpoint of 9 months of Nefecon treatment, a significant retention of eGFR compared to the placebo. The trial also showed that Nefecon was generally well tolerated. No serious AEs due to Nefecon were observed; all cases were minor, and all subjects recovered [28].
After being taken orally and absorbed, budesonide is metabolized at a rate of 90% during its first pass through the liver. This process results in the formation of 6β-hydroxybudesonide and 16α-hydroxyprednisolone. However, these metabolites possess less than 1% of the corticosteroid activity compared to the original compound, budesonide [45]. Given the characteristic pharmacokinetics of budesonide, the following questions require consideration in light of the interim results: (1) Is the intestinal tract the site of pathophysiologically relevant Gd-IgA1 production? (2) Does budesonide change systemic immunity by modifying the intestinal microbiota? (3) Do small amounts of absorbed budesonide have a systemic effect?
5. Upcoming Therapeutic Options for IgAN
5.1. Therapeutic Target: APRIL
(1) Sibeprenlimab (VIS649): Sibeprenlimab, previously known as VIS649, is a monoclonal antibody that neutralizes A Proliferation-Inducing Ligand (APRIL) and is currently being developed by Visterra Inc. for the treatment of IgAN. A worldwide phase 2 RCT of Sibeprenlimab is in progress to evaluate the efficacy, safety, and tolerability of multiple doses of this drug in patients with IgAN, as well as to assess the dose response to varying doses of Sibeprenlimab by measuring proteinuria. The results from this trial will lay the groundwork for the future clinical development of Sibeprenlimab. Neither the VIS649 group nor the sham group had any serious AEs that resulted in the discontinuation of the study. AEs requiring treatment occurred in 11 patients (39.3%) in the VIS649 group (n = 28) and 4 patients (50%) in the sham group (n = 8) [29].
(2) BION-1301: BION-1301 is a monoclonal antibody against APRIL. In basic studies, BION-1301 was found to bind to a specific epitope on APRIL and to be able to completely inhibit APRIL-mediated receptor activity. BION-1301 is being investigated as a potential therapeutic option for IgAN. It works by eliminating Gd-IgA1, a pathogenic variant of IgA, and decreasing proteinuria. Currently, BION-1301 is undergoing a phase 1/2 clinical trial with IgAN patients. Early results derived from studying the initial group of IgAN patients indicate that BION-1301 has demonstrated good tolerability and has not resulted in any serious adverse events or the discontinuation of treatment due to side effects [30].
5.2. Therapeutic Target: Plasma Cells
(1) Bortezomib: Bortezomib, a proteasome inhibitor, is being examined for its potential to decrease proteinuria in IgAN. A pilot trial (NCT01103778) assessed the impact of four doses of bortezomib on eight consecutive IgAN patients with significant proteinuria. At the one-year follow-up mark, complete remission with urinary protein excretion of less than 300 mg/day, which was the primary outcome, was achieved by three subjects (38%). One of the participants in the study had a T score of 0 according to the Oxford classification prior to entering the study. Among the remaining five subjects, one withdrew within one month of entering the study, and four (50%) either showed no response or experienced disease progression. These findings imply that proteasome inhibition with bortezomib can reduce proteinuria in some IgAN patients, particularly those with a T score of 0 according to the Oxford classification [31].
(2) Felzartamab: Felzartamab is an anti-human CD38 monoclonal antibody found in the MorphoSys HuCAL antibody library. Felzartamab is expected to improve renal function by targeting CD38 and thereby eliminating CD38-positive plasma cells. A phase 2 IGNAZ clinical trial of felzartamab for IgAN has commenced, with the first patient having received his/her dose. This trial, which is a multicenter, randomized, double-blind, parallel-group, placebo-controlled study, aims to enroll approximately 48 patients. Its objective is to evaluate the efficacy, safety, pharmacokinetics, and pharmacodynamics of felzartamab in patients with IgAN [32].
(3) Mezagitamab (TAK-079): Mezagitamab (TAK-079) is a fully human monoclonal antibody (IgG1) that targets the CD38 transmembrane glycoprotein found on the surface of tumor cells, leading to the depletion of these cells through antibody- and complement-dependent cytotoxicity. Initially developed for the treatment of multiple myeloma, this drug is currently being tested in a clinical trial involving adults with primary IgAN who are on stable background therapy. The primary objective of this trial is to evaluate the short-term and long-term side effects of Mezagitamab [33].
5.3. Therapeutic Target: Complement Systems
The alternative pathway is initiated by the cleavage of Factor B by Factor D, leading to the activation of additional C3. The lectin pathway is activated by the complexation of pattern-recognition molecules with MASP-3. Both pathways are suggested to be activated in disease progression, as indicated by changes in plasma MASP-3 and the glomerular deposition of Factor H and Factor H-related proteins. These pathways are being explored as potential targets for new treatments for IgAN [46].
(1) Eculizumab: Eculizumab (Soliris®) is a humanized recombinant monoclonal IgG antibody against human complement C5. Eculizumab inhibits responses at the end of the complement activation pathway. It was initially developed for the treatment of complement-mediated paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) [47]. Eculizumab, which selectively binds to C5, prevents the formation of C5a and MAC by inhibiting C5 cleavage. On the other hand, it does not prevent reactions mediated by upstream C3a and C3b. A case report in the literature detailed the case of a patient who experienced the rapid progression of IgAN, leading to renal failure despite undergoing immunosuppressive treatment. In an effort to salvage renal function, the patient was administered eculizumab (anti-C5) for a duration of 3 months. Eculizumab, a C5-neutralizing antibody, inhibits the formation of MAC and thus increases the risk of bacterial infections. The primary bacterial infection is meningitis caused by Neisseria meningitidis, but reports of meningitis caused by Pseudomonas aeruginosa also exist [48,49]. Eculizumab is already in clinical use in many countries, but its high cost is a problem. Until this problem is resolved, it is unlikely to be administered as a novel treatment for patients with IgAN [50].
(2) IONIS-FB-LRx: IONIS-FB-LRx is an antisense oligonucleotide that reduces the expression of factor B. It is currently being examined in two phase 2 clinical studies involving 25 patients with IgAN. One study involves the drug being administered to 10 patients for 29 weeks, and the other study’s subjects are undergoing dose modification. The results for the first dose cohort of this clinical study were reported at the 2022 American Society of Nephrology (KIDNEY WEEK). The primary endpoint of the study, urinary protein excretion in 24-h urine storage, was reduced by 44%. All subjects completed the protocol, although one subject had a reversible ALT elevation [34].
5.4. Therapeutic Target: Endothelin
Endothelin (ET) is an important endogenous vasoconstrictor, a polypeptide composed of 21 amino acids. ET1 is the most potent of the three isoforms and is the only one expressed in the kidney. Prolonged vasoconstriction by ET1 induces glomerular hyperfiltration, which is seen in early diabetic nephropathy and obesity-related nephropathy, and subsequently damages glomerular epithelial cells, causing proteinuria and a reduction in glomerular filtration rate [51].
(1) Atrasentan: Atrasentan, a potent and selective antagonist of the endothelin A receptor, could potentially be beneficial in IgAN and other proteinuric glomerular diseases by decreasing proteinuria. The AFFINITY study is a phase 2 international study aiming to determine the efficacy and safety of administering atrasentan to IgA patients with proteinuria, Alport syndrome, focal glomerulosclerosis, and diabetic kidney disease (DKD). The interim results through week 24 of treatment for the 20 patients in the IgAN cohort of the AFFINITY study showed that 79% achieved a >40% reduction in proteinuria [52]. The ALIGN study, a phase 3, double-blind, placebo-controlled trial, is currently investigating the efficacy and safety of atrasentan in patients with IgAN who are at risk of progressive renal function loss. A total of 16 subjects had mild AEs, but none was severe enough to cause the stopping of the study [35].
(2) Sparsentan: The PROTECT phase 3 clinical trial, currently in progress, is investigating the effectiveness and safety of a unique non-immunosuppressive single-molecule drug called sparsentan. This drug is a dual antagonist for both endothelin and angiotensin receptors and is being tested on adults with IgAN. The trial is being carried out across 18 countries at 134 clinical practice sites. An interim analysis, predetermined for the primary proteinuria efficacy endpoint, revealed that sparsentan significantly reduced proteinuria compared to a control group that received irbesartan. The safety profile of sparsentan was found to be comparable to that of irbesartan. No cases of serious edema or heart failure were observed, and no cases were discontinued due to AEs [36].
6. Conclusions
Table 6 summarizes the treatment options for IgA nephropathy. The treatments can be divided into two main categories: immune and non-immune.

Table 6.
Summary of the treatment options.
In the past, glucocorticoids, which extensively suppress the immune system, have been used in the treatment of the immune system and have been shown to decrease proteinuria and suppress renal function decline, but serious side effects, such as infection, have been a problem. However, with the development of new therapeutic agents and their clinical application, therapies that target molecules on B cells, complement, etc., are attracting attention. Although these agents have not yet been fully tested in clinical trials, interim reports indicate that they are effective in reducing proteinuria and have milder side effects than glucocorticoids.
On the other hand, RAAS-Is, involved in non-immune therapies, have been shown to reduce proteinuria and be renoprotective. In addition, SGLT2 inhibitors for type 2 diabetes have been shown to be effective in non-diabetic CKD and are expected to provide new treatment options for IgAN. In addition, anti-endothelin agents such as atrasentan and sparsentan are also expected to be renoprotective because of their ability to decrease proteinuria, and they are also expected to have minimal side effects. Since these therapies have different mechanisms of action, synergistic effects from their combination are also expected.
Although the pathogenesis of IgAN is not yet fully understood, new treatments are being developed; more than 50 years after IgAN was first discovered, more effective treatments with fewer side effects are being developed. As research continues to advance, we can expect to find even more effective treatments.
Author Contributions
Y.S. (Yoshio Shimizu) was the architect of the study, formulating the primary conceptual ideas and outlining the proof. They also undertook the task of gathering relevant research. Y.T. and Y.S. (Yusuke Suzuki) provided their expertise in reviewing the study and contributed to the manuscript. Under the guidance of Y.S. (Yusuke Suzuki), who supervised the project, Y.S. (Yoshio Shimizu) penned the manuscript with assistance from Y.T. and Y.S. (Yusuke Suzuki). All authors have read and agreed to the published version of the manuscript.
Funding
This study received funding from the JSPS KAKENHI, under the grant numbers 17K09039, 20K08597, and 23K07707. Additionally, the Shizuoka Research Center for Disaster Medicine at Juntendo University is partially funded by a Grant-in-Aid for Special Research. This grant is part of the Subsides for Ordinary Express of Private School, provided by The Promotion and Mutual Aid Corporation for Private School of Japan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Manno, C.; Strippoli, G.F.; D’altri, C.; Torres, D.; Rossini, M.; Schena, F.P. A novel simpler histological classification for renal survival in IgA nephropathy: A retrospective study. Am. J. Kidney Dis. 2007, 49, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Berthoux, F.C.; Mohey, H.; Afiani, A. Natural history of primary IgA nephropathy. Semin. Nephrol. 2008, 28, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Tanaka, K.; Iwasaki, C.; Oshima, Y.; Ochi, A.; Kataoka, H.; Itabashi, M.; Takei, T.; Uchida, K.; Nitta, K. Prognosis in IgA nephropathy: 30-year analysis of 1012 patients at a single center in Japan. PLoS ONE 2014, 9, e91756. [Google Scholar] [CrossRef] [PubMed]
- Rizk, D.V.; Saha, M.K.; Hall, S.; Novak, L.; Brown, R.; Huang, Z.-Q.; Fatima, H.; Julian, B.A.; Novak, J. Glomerular Immunodeposits of Patients with IgA Nephropathy Are Enriched for IgG Autoantibodies Specific for Galactose-Deficient IgA1. J. Am. Soc. Nephrol. 2019, 30, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- KnoRobert, T.; Berthelot, L.; Cambier, A.; Rondeau, E.; Monteiro, R.C. Molecular Insights into the Pathogenesis of IgA Nephropathy. Trends Mol. Med. 2015, 21, 762–775. [Google Scholar]
- Placzek, W.J.; Yanagawa, H.; Makita, Y.; Renfrow, M.B.; Julian, B.A.; Rizk, D.V.; Suzuki, Y.; Novak, J.; Suzuki, H. Serum galactose-deficient-IgA1 and IgG autoantibodies correlate in patients with IgA nephropathy. PLoS ONE. 2018, 13, e0190967. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.T.; Leung, C.B.; Chow, K.M.; Cheng, Y.L.; Fung, S.K.; Mak, S.K.; Tang, A.W.; Wong, T.Y.; Yung, C.Y.; Yung, J.C.; et al. HKVIN Study Group. Hong Kong study using valsartan in IgAN (HKVIN): A double-blind, randomized, placebo-controlled study. Am. J. Kidney Dis. 2006, 47, 751–760. [Google Scholar] [CrossRef]
- Praga, M.; Gutiérrez, E.; González, E.; Morales, E.; Hernández, E. Treatment of IgAN with ACE inhibitors: A randomized and controlled trial. J. Am. Soc. Nephrol. 2003, 14, 1578–1583. [Google Scholar] [CrossRef]
- Woo, K.T.; Lau, Y.K.; Zhao, Y.; E Liu, F.; Tan, H.B.; Tan, E.K.; Stephanie, F.C.; Chan, C.M.; Wong, K.S. Disease progression, response to ACEI/ATRA therapy and influence of ACE gene in IgA nephritis. Cell Mol. Immunol. 2007, 4, 227–232. [Google Scholar]
- Kanno, Y.; Okada, H.; Yamaji, Y.; Nakazato, Y.; Suzuki, H. Angiotensin-converting-enzyme inhibitors slow renal decline in IgAN, independent of tubulointerstitial fibrosis at presentation. QJM 2005, 98, 199–203. [Google Scholar] [CrossRef]
- Woo, K.T.; Lau, Y.K.; Wong, K.S.; Chiang, G.S. ACEI/ATRA therapy decreases proteinuria by improving glomerular permselectivity in IgA nephritis. Kidney Int. 2000, 58, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Horita, Y.; Tadokoro, M.; Taura, K.; Ashida, R.; Hiu, M.; Taguchi, T.; Furusu, A.; Kohno, S. Prednisolone co-administered with losartan confers renoprotection in patients with IgA nephropathy. Ren. Fail. 2007, 29, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ushiyama, C.; Suzuki, S.; Hara, M.; Shimada, N.; Sekizuka, K.; Ebihara, I.; Koide, H. Effects of angiotensin-converting enzyme inhibitor, angiotensin II receptor antagonist and calcium antagonist on urinary podocytes in patients with IgAN. Am. J. Nephrol. 2000, 20, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Takei, T.; Uchida, K.; Tsuchiya, K.; Nitta, K. Low-dose losartan therapy reduces proteinuria in normotensive patients with immunoglobulin A nephropathy. Hypertens. Res. 2008, 31, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Yoshimura, M.; Miyazaki, Y.; Okamoto, H.; Kimura, K.; Hirano, K.; Matsushima, M.; Utsunomiya, Y.; Ogura, M.; Yokoo, T.; et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrol. Dial. Transplant. 2014, 29, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Matsuzaki, K.; Yasuda, T.; Nishikawa, M.; Yasuda, Y.; Koike, K.; Maruyama, S.; Yokoo, T.; Matsuo, S.; Kawamura, T.; et al. Association between tonsillectomy and outcomes in patients with immunoglobulin A nephropathy. JAMA Netw. Open 2019, 2, e194772. [Google Scholar] [CrossRef] [PubMed]
- Kovács, T.; Vas, T.; Kövesdy, C.P.; Degrell, P.; Nagy, G.; Rékási, Z.; Wittmann, I.; Nagy, J. Effect of tonsillectomy and its timing on renal outcomes in Caucasian IgA nephropathy patients. Int. Urol. Nephrol. 2014, 46, 2175–2182. [Google Scholar] [CrossRef]
- Feehally, J.; Coppo, R.; Troyanov, S.; Bellur, S.S.; Cattran, D.; Cook, T.; Roberts, I.S.; Verhave, J.C.; Camilla, R.; Vergano, L.; et al. Tonsillectomy in a European Cohort of 1147 Patients with IgA Nephropathy. Nephron 2016, 132, 15–24. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Toto, R.D.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Pecoits-Filho, R.; Correa-Rotter, R.; et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021, 100, 215–224. [Google Scholar] [CrossRef]
- Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, H.R.; Preiss, D.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; et al. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar]
- Lv, J.; Zhang, H.; Chen, Y.; Li, G.; Jiang, L.; Singh, A.K.; Wang, H. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgAN: A randomized controlled trial. Am. J. Kidney Dis. 2009, 53, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Manno, C.; Torres, D.D.; Rossini, M.; Pesce, F.; Schena, F.P. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol. Dial. Transplant. 2009, 24, 3694–3701. [Google Scholar] [CrossRef] [PubMed]
- Tesar, V.; Troyanov, S.; Bellur, S.; Verhave, J.C.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Cattran, D.; Coppo, R.; VALIGA study of the ERA-EDTA Immunonephrology Working Group. Corticosteroids in IgA Nephropathy: A retrospective Analysis from the VALIGA Study. J. Am. Soc. Nephrol. 2015, 26, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Rauen, T.; Eitner, F.; Fitzner, C.; Sommerer, C.; Zeier, M.; Otte, B.; Panzer, U.; Peters, H.; Benck, U.; Mertens, P.R.; et al. STOP-IgAN Investigators. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. N. Engl. J. Med. 2015, 373, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, H.; Wong, M.G.; Jardine, M.J.; Hladunewich, M.; Jha, V.; Monaghan, H.; Zhao, M.; Barbour, S.; Reich, H.; et al. Effect of Oral Methylprednisolone on Clinical Outcomes in Patients with IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA 2017, 318, 432–442. [Google Scholar] [CrossRef]
- Lv, J.; Wong, M.G.; Hladunewich, M.A.; Jha, V.; Hooi, L.S.; Monaghan, H.; Zhao, M.; Barbour, S.; Jardine, M.J.; Reich, H.N.; et al. Effect of Oral Methylprednisolone oEdsn Decline in Kidney Function or Kidney Failure in Patients with IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA 2022, 327, 1888–1898. [Google Scholar] [CrossRef]
- Fellström, B.C.; Barratt, J.; Cook, H.; Coppo, R.; Feehally, J.; de Fijter, J.W.; Floege, J.; Hetzel, G.; Jardine, A.G.; Locatelli, F.; et al. Targeted-release budesonide versus placebo in patients with IgAN (NEFIGAN): A double-blind, randomised, placebo-controlled phase 2b trial. Lancet 2017, 389, 2117–2127. [Google Scholar] [CrossRef]
- Barratt, J.; Lafayette, R.; Kristensen, J.; Stone, A.; Cattran, D.; Floege, J.; Tesar, V.; Trimarchi, H.; Zhang, H.; Eren, N.; et al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int. 2023, 103, 391–402. [Google Scholar] [CrossRef]
- Mathur, M.; Barratt, J.; Suzuki, Y.; Engler, F.; Pasetti, M.F.; Yarbrough, J.; Sloan, S.; Oldach, D. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of VIS649 (Sibeprenlimab), an APRIL-Neutralizing IgG2 Monoclonal Antibody, in Healthy Volunteers. Kidney Int. Rep. 2022, 7, 993–1003. [Google Scholar] [CrossRef]
- Barratt, J.; Hour, B.; Sibley, C.; Mittan, A.; Roy, S.; Stromatt, C.; Aaron Endsley, A.; Lo, J.; Glicklich, A. FC 040 Interim results of phase 1 and 3 trials to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics and clinical activity of BION-1301 in patients with IgAN. Nephrol. Dial. Transplant. 2021, 36 (Suppl. S1), gfab117.004. [Google Scholar] [CrossRef]
- Hartono, C.; Chung, M.; Perlman, A.S.; Chevalier, J.M.; Serur, D.; Seshan, S.V.; Muthukumar, T. Bortezomib for Reduction of Proteinuria in IgA Nephropathy. Kidney Int. Rep. 2018, 3, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Maixnerova, D.; Tesar, V. Emerging role of monoclonal antibodies in the treatment of IgAN. Expert Opin. Biol. Ther. 2023, 23, 419–427. [Google Scholar] [CrossRef] [PubMed]
- A Study of Mezagitamab in Adults with Primary Immunoglobulin A Nephropathy Receiving Stable Background Therapy. Available online: https://www.clinicaltrials.gov/ClinicalTrials.gov(NCT05174221) (accessed on 5 December 2023).
- IONIS Pharma Press Releases: Ionis presents positive Phase 2 data in patients with IgAN at American Society of Nephrology’s Kidney Week 2022. Available online: https://ir.ionispharma.com/news-releases/news-release-details/ionis-presents-positive-phase-2-data-patients-IgAN (accessed on 5 December 2023).
- Heerspink, L.; Kohan, D.; Lafyette, R.; Levin, A.; Zhang, H.; Glicklich, A.; King, A.; Camargo, M.; Barratt, J. A phase 3, randomized, double-blind placebo-controlled study of atrasentan in patients with IgA nephropathy. KI Rep. 2021, 6, S164–S165. [Google Scholar]
- Heerspink, H.J.L.; Radhakrishnan, J.; Alpers, C.E.; Barratt, J.; Bieler, S.; Diva, U.; Inrig, J.; Komers, R.; Mercer, A.; Noronha, I.L.; et al. Sparsentan in patients with IgAN: A prespecified interim analysis from a randomized, double-blind, active-controlled clinical trial. Lancet 2023, 401, 1584–1594. [Google Scholar] [CrossRef]
- Knoppova, B.; Reily, C.; King, R.G.; Julian, B.A.; Novak, J.; Green, T.J. Pathogenesis of IgA Nephropathy: Current Understanding and Implications for Development of Disease-Specific Treatment. Clin. Med. 2021, 10, 4501. [Google Scholar] [CrossRef]
- Suzuki, H.; Novak, J. IgA glycosylation and immune complex formation in IgAN. Semin. Immunopathol. 2021, 43, 669–678. [Google Scholar] [CrossRef]
- Gesualdo, L.; Di Leo, V.; Coppo, R. The mucosal immune system and IgA nephropathy. Semin. Immunopathol. 2021, 43, 657–668. [Google Scholar] [CrossRef]
- Hall, J.E. The renin-angiotensin system: Renal actions and blood pressure regulation. Compr. Ther. 1991, 17, 8. [Google Scholar]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, 753–779. [Google Scholar] [CrossRef]
- Fernández-Fernandez, B.; Sarafidis, P.; Soler, M.J.; Ortiz, A. EMPA-KIDNEY: Expanding the range of kidney protection by SGLT2 inhibitors. Clin. Kidney J. 2023, 16, 1187–1198. [Google Scholar] [CrossRef]
- Nuffield Department of Population Health Renal Studies Group. SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves First Drug to Decrease Urine Protein in IgA Nephropathy, A Rare Kidney Disease|FDA. Available online: https://www.fda.gov (accessed on 5 December 2023).
- Edsbäcker, S.; Andersson, T. Pharmacokinetics of budesonide (Entocort EC) capsules for Crohn’s disease. Clin. Pharmacokinet. 2004, 43, 803–821. [Google Scholar] [PubMed]
- Rizk, D.V.; Maillard, N.; Julian, B.A.; Knoppova, B.; Green, T.J.; Novak, J.; Wyatt, R.J. The Emerging Role of Complement Proteins as a Target for Therapy of IgA Nephropathy. Front. Immunol. 2019, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Patriquin, C.J.; Kuo, K.H.M. Eculizumab and Beyond: The Past, Present, and Future of Complement Therapeutics. Transfus. Med. Rev. 2019, 33, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Nakazawa, H.; Kurasawa, Y.; Sakai, H.; Nishina, S.; Senoo, N.; Senoo, Y.; Ishida, F. Severe Infection of Pseudomonas aeruginosa during Eculizumab Therapy for Paroxysmal Nocturnal Hemoglobinuria. Int. Med. 2018, 57, 127–130. [Google Scholar] [CrossRef]
- Hawkins, K.L.; Hoffman, M.; Okuyama, S.; Rowan, S.E. A Case of Fulminant Meningococcemia: It Is All in the Complement. Case Rep. Infect. Dis. 2017, 2017, 6093695. [Google Scholar] [CrossRef]
- Khedraki, R.; Noor, Z.; Rick, J. The Most Expensive Drug in the World: To Continue or Discontinue, That Is the Question. Fed. Pract. 2016, 33, 22–28. [Google Scholar]
- Martínez-Díaz, I.; Martos, N.; Llorens-Cebrià, C.; Álvarez, F.J.; Bedard, P.W.; Vergara, A.; Jacobs-Cachá, C.; Soler, M.J. Endothelin Receptor Antagonists in Kidney Disease. Int. J. Mol. Sci. 2023, 24, 3427. [Google Scholar] [CrossRef]
- Available online: https://www.chinooktx.com/file.cfm/52/docs/asn-2022-atrasentan-affinity-igan-update-th-po497.pdf (accessed on 5 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).