Effect of COVID-19 on Kidney Graft Function One Year after Onset
Abstract
:1. Introduction
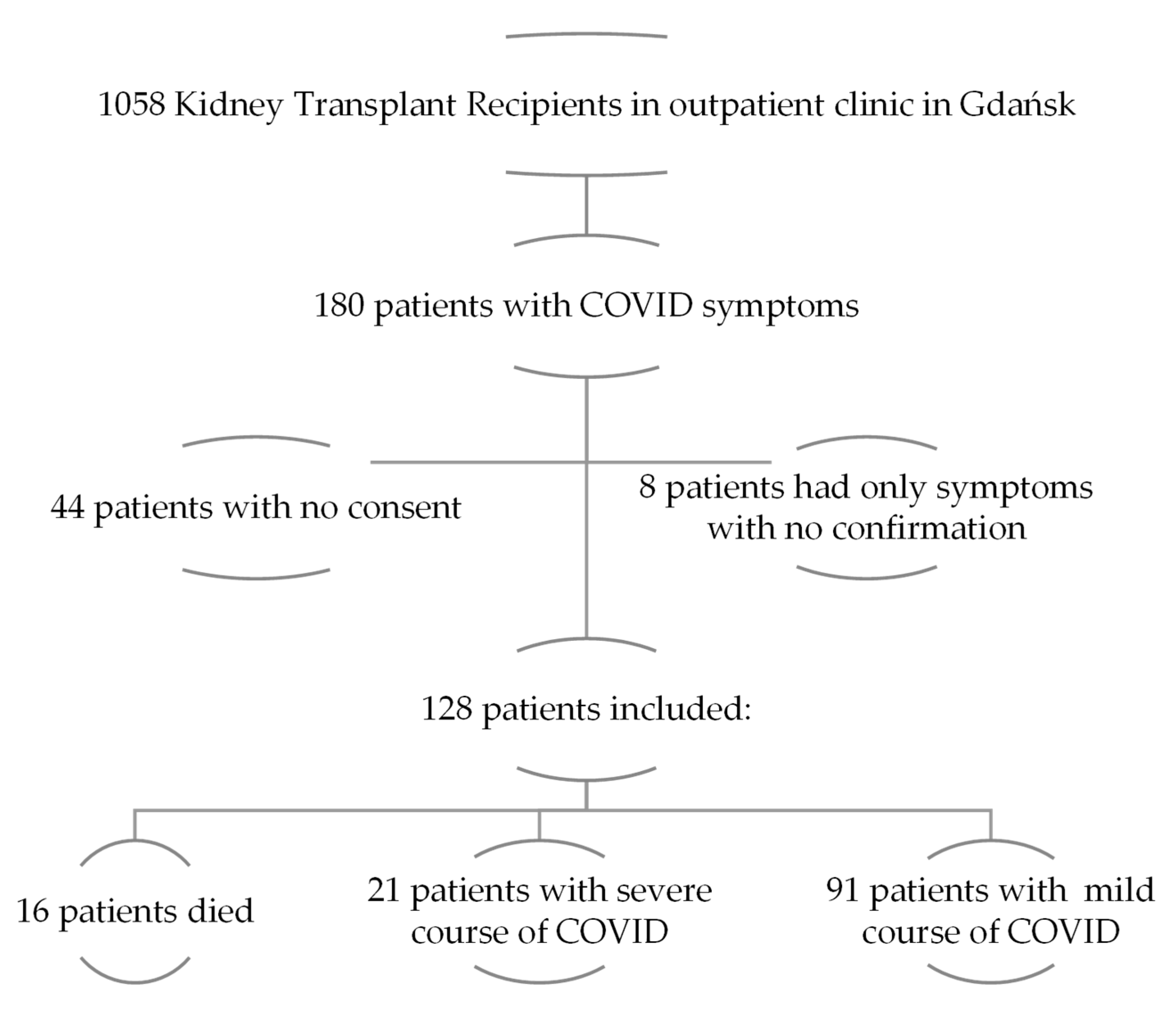
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
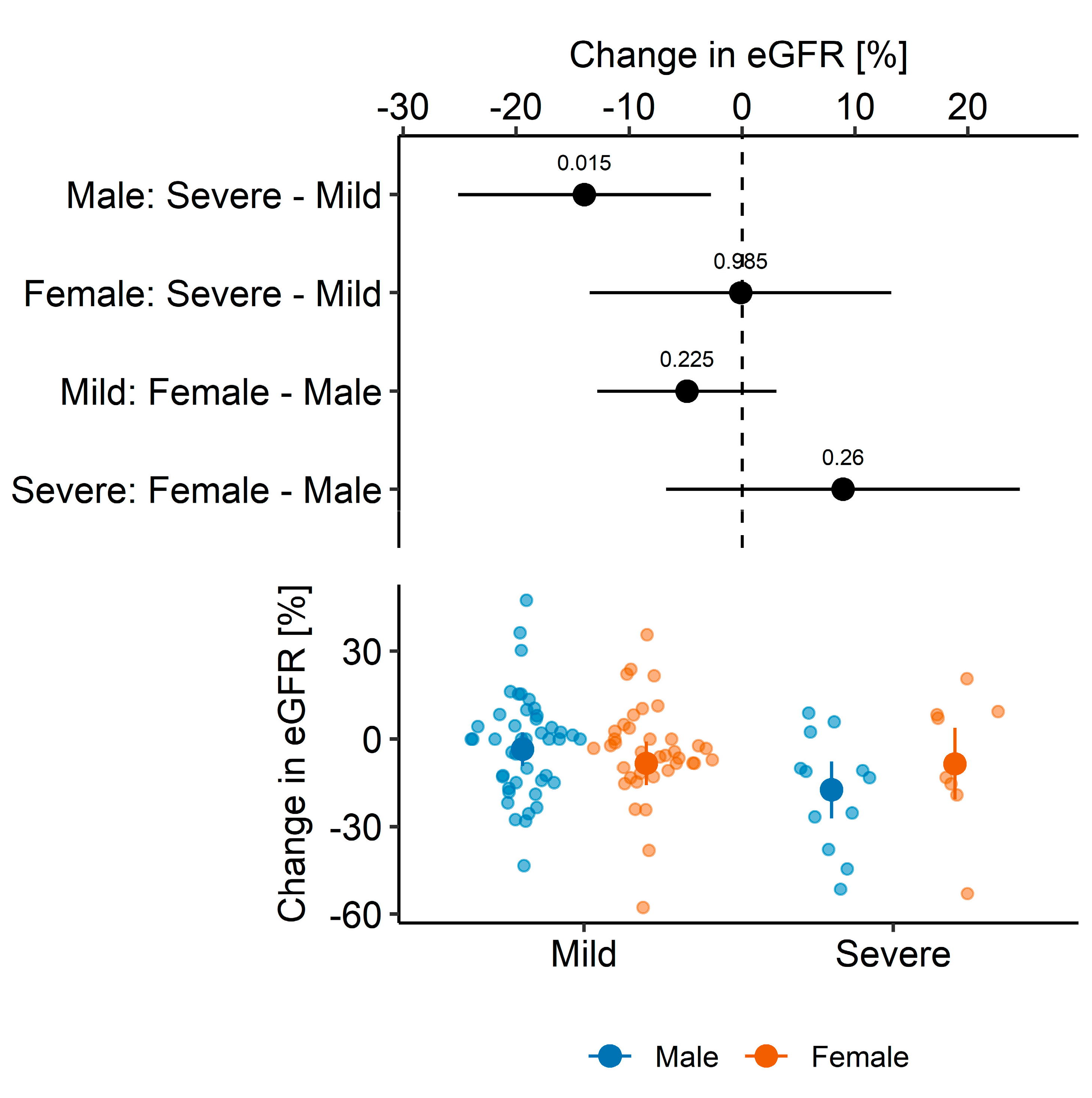
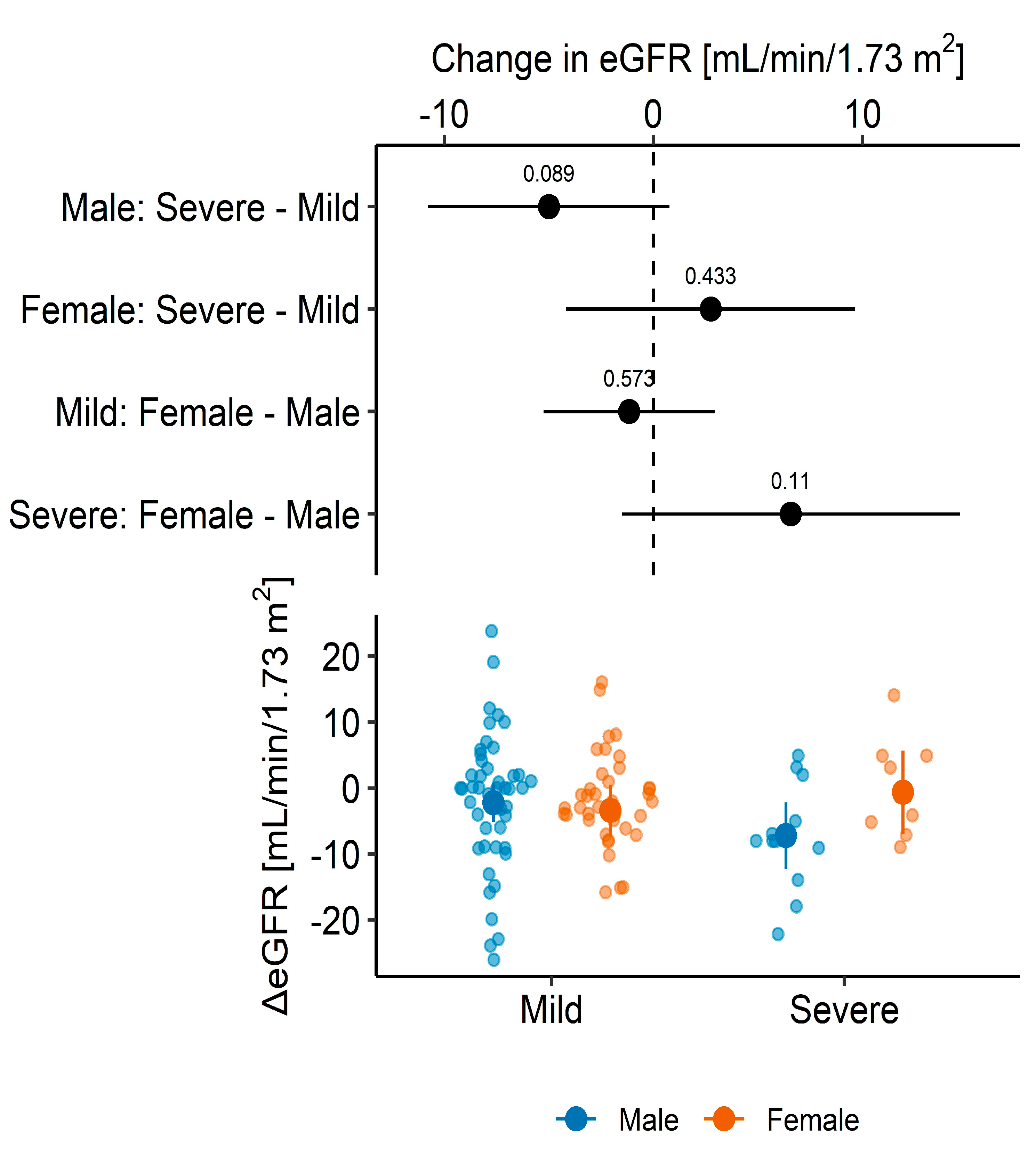
3. Results
3.1. Patients
3.2. Graft Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Sahota, A.; Tien, A.; Yao, J.; Dong, E.; Herald, J.; Javaherifar, S.; Neyer, J.; Hwang, J.; Lee, R.; Fong, T.-L. Incidence, Risk Factors, and Outcomes of COVID-19 Infection in a Large Cohort of Solid Organ Transplant Recipients. Transplantation 2022, 106, 2426–2434. [Google Scholar] [CrossRef] [PubMed]
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute kidney injury in critically ill patients with COVID-19. Intensiv. Care Med. 2020, 46, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Wang, Q.; Kim, T.-E.; Kang, J.-S. Clinical characteristics and outcome of coronavirus disease 2019 infection in patients with solid organ transplants: A systematic review and meta-analysis. J. Infect. Public Health 2022, 15, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Okeagu, C.N.; Tortorich, G.; Pham, A.D.; Ly, E.I.; Brondeel, K.C.; Eng, M.R.; Luedi, M.M.; Urman, R.D.; Cornett, E.M. COVID-19 impact on the renal system: Pathophysiology and clinical outcomes. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Tarasewicz, A.; Perkowska-Ptasińska, A.; Dębska-Ślizień, A. Thrombotic microangiopathy in a kidney transplant patient after COVID-19. Pol. Arch. Intern. Med. 2021, 131, 16125. [Google Scholar] [CrossRef]
- Nugent, J.; Aklilu, A.; Yamamoto, Y.; Simonov, M.; Li, F.; Biswas, A.; Ghazi, L.; Greenberg, J.H.; Mansour, S.G.; Moledina, D.G.; et al. Assessment of Acute Kidney Injury and Longitudinal Kidney Function After Hospital Discharge Among Patients With and Without COVID-19. JAMA Netw. Open 2021, 4, e211095. [Google Scholar] [CrossRef]
- Chen, J.-J.; Kuo, G.; Lee, T.H.; Yang, H.-Y.; Wu, H.H.; Tu, K.-H.; Tian, Y.-C. Incidence of mortality, acute kidney injury and graft Loss in adult kidney transplant recipients with coronavirus disease 2019: Systematic review and meta-analysis. J. Clin. Med. 2021, 10, 5162. [Google Scholar] [CrossRef]
- Duivenvoorden, R.; Vart, P.; Noordzij, M.; dos Santos, A.C.S.; Zulkarnaev, A.B.; Franssen, C.F.M.; Kuypers, D.; Demir, E.; Rahimzadeh, H.; Kerschbaum, J.; et al. Clinical, Functional, and Mental Health Outcomes in Kidney Transplant Recipients 3 Months After a Diagnosis of COVID-19. Transplantation 2022, 106, 1012–1023. [Google Scholar] [CrossRef]
- Malinowska, A.; Muchlado, M.; Ślizień, Z.; Biedunkiewicz, B.; Heleniak, Z.; Dębska-Ślizień, A.; Tylicki, L. Post-COVID-19 sydrome and decrease in health-related quality of life in kidney transplant recipients after SARS-COV-2 infection—A cohort longitudinal study from the north of Poland. J. Clin. Med. 2021, 10, 5205. [Google Scholar] [CrossRef]
- Tavares, J.; Oliveira, J.P.; Reis, P.; Ribeiro, B.; Silva, F.; Malheiro, J.; Almeida, M.; Martins, L.S.; Cabrita, A.; Henriques, A.C.; et al. COVID-19 in kidney transplant recipients: What have we learned one year later? A cohort study from a tertiary center. Braz. J. Nephrol. 2022, 44, 533–542. [Google Scholar] [CrossRef]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Pawłowska, M.; Parczewski, M.; Piekarska, A.; Simon, K.; Tomasiewicz, K.; Zarębska-Michaluk, D. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of 31 March 2020. Polish Arch. Intern. Med. 2020, 130, 352–357. [Google Scholar] [CrossRef]
- Available online: https://sarswpolsce.pl (accessed on 20 May 2021).
- Fabião, J.; Sassi, B.; Pedrollo, E.; Gerchman, F.; Kramer, C.; Leitão, C.; Pinto, L. Why do men have worse COVID-19-related outcomes? A systematic review and meta-analysis with sex adjusted for age. Braz. J. Med Biol. Res. 2022, 55, e11711. [Google Scholar] [CrossRef]
- Wagenmakers, E.-J.; Farrell, S. AIC model selection using Akaike weights. Psychon. Bull. Rev. 2004, 11, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013, 3 (Suppl.). [Google Scholar] [CrossRef]
- Available online: https://raw.githubusercontent.com/middleprofessor/applied-biostats/master/R/ggplot_the_model.R (accessed on 5 November 2022).
- Sylvester, S.V.; Rusu, R.; Chan, B.; Bellows, M.; O’keefe, C.; Nicholson, S. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: A review. Curr. Med. Res. Opin. 2022, 38, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Mourosi, J.T.; Anwar, S.; Hosen, M.J. The sex and gender dimensions of COVID-19: A narrative review of the potential underlying factors. Infect. Genet. Evol. 2022, 103, 105338. [Google Scholar] [CrossRef]
- Malinowska, A.; Heleniak, Z.; Muchlado, M.; Ślizień, Z.; Ruszkowski, J.; Biedunkiewicz, B.; Tylicki, L.; Król, E.; Dębska-Ślizień, A. Changes in Kidney Graft Function in COVID-19 Convalescents. Transplant. Proc. 2022, 54, 884–887. [Google Scholar] [CrossRef]
- Demir, E.; Ucar, Z.A.; Dheir, H.; Danis, R.; Yelken, B.; Uyar, M.; Parmaksiz, E.; Artan, A.S.; Sinangil, A.; Merhametsiz, O.; et al. COVID-19 in Kidney Transplant Recipients: A Multicenter Experience from the First Two Waves of Pandemic. BMC Nephrol. 2022, 23, 183. [Google Scholar] [CrossRef]
- Basic-Jukic, N.; Racki, S.; Tolj, I.; Aleckovic, M.; Babovic, B.; Juric, I.; Furic-Cunko, V.; Katalinic, L.; Mihaljevic, D.; Vujic, S.; et al. Hospitalization and death after recovery from acute COVID-19 among renal transplant recipients. Clin. Transplant. 2022, 36, e14572. [Google Scholar] [CrossRef]
- Elec, F.I.; Bolboacă, S.D.; Muntean, A.; Elec, A.D.; Cismaru, C.; Lupşe, M.; Oltean, M. Comparing the First and Second Wave of COVID-19 in Kidney Transplant Recipients: An East-European Perspective. Eur. Surg. Res. 2022, 63, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Oto, O.A.; Ozturk, S.; Arici, M.; Velioğlu, A.; Dursun, B.; Guller, N.; Şahin, I.; Eser, Z.E.; Paydaş, S.; Trabulus, S.; et al. Middle-term outcomes in renal transplant recipients with COVID-19: A national, multicenter, controlled study. Clin. Kidney J. 2022, 15, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Hajibaratali, B.; Amini, H.; Dalili, N.; Ziaie, S.; Anvari, S.; Keykha, E.; Rezaee, M.; Samavat, S. Clinical outcomes of kidney recipients with COVID-19 (COVID-19 in kidney recipients). Transpl. Immunol. 2023, 76, 101772. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.; Martin, S.J.; Whittemore, C.; Thankachan, R.; Dvorai, R.H.; Saidi, R.F. Immunosuppression regimen modification during COVID-19 infection in kidney transplant recipients. Transpl. Immunol. 2023, 80, 101883. [Google Scholar] [CrossRef]
- Li, D.; Liao, X.; Liu, Z.; Ma, Z.; Dong, J.; Zheng, G.; Zi, M.; Wang, F.; He, Q.; Li, G.; et al. Healthy outcomes of patients with COVID-19 two years after the infection: A prospective cohort study. Emerg. Microbes Infect. 2022, 11, 2680–2688. [Google Scholar] [CrossRef]
- Camargo, L.F.A.; de Sandes-Freitas, T.V.; Silva, C.D.R.; Bittante, C.D.; Ono, G.; Corrêa, L.; Silva, M.; Bellei, N.C.J.; Goto, J.M.; Medeiros, E.A.S.; et al. Morbimortality of pandemic influenza an H1N1 infection in kidney transplant recipients requiring hospitalization: A comparative analysis with nonimmunocompromised patients. Transplantation 2012, 93, 69–72. [Google Scholar] [CrossRef]
| ALL | MILD | SEVERE | p Value 1 | |
|---|---|---|---|---|
| n | 112 | 91 | 21 | |
| Age years, median (IQR) | 51 (41–62) | 50 (38–61) | 58 (49–67) | 0.022 |
| Male sex, n (%) | 64 (57.1) | 52 (57.1) | 12 (57.1) | >0.99 |
| BMI, median (IQR) No data, n | 25 (22–29) 1 | 24 (22–29) 1 | 26 (23–32) 0 | 0.19 |
| Comorbidities n (%) | ||||
| Hypertension | 106 (94.6) | 85 (93.4) | 21 (100) | 0.59 |
| Diabetes mellitus | 26 (23.2) | 16 (17.6) | 10 (47.6) | 0.008 |
| Heart disease | 25 (22.3) | 17 (18.7) | 8 (38.1) | 0.079 |
| Lung disease | 6 (5.4) | 6 (6.6) | 0 (0.0) | 0.59 |
| Smoking, n (%) | 0.028 | |||
| Current or in the past | 36 (32.1) | 25 (27.5) | 11 (52.4) | |
| Never | 76 (67.9) | 66 (72.5) | 10 (47.6) | |
| Primary nephropathy n (%) | 0.80 | |||
| Unknown or other | 36 (32.4) | 29 (32.2) | 7 (33.3) | |
| Glomerulonephritis | 36 (32.4) | 31 (34.4) | 5 (23.8) | |
| Pyelonephritis | 2 (1.8) | 2 (2.2) | 0 (0) | |
| Interstitial nephritis | 4 (3.6) | 3 (3.3) | 1 (4.8) | |
| Congenital anomalies | 5 (4.5) | 4 (4.4) | 1 (4.8) | |
| Diabetic nephropathy | 8 (7.2) | 7 (7.8) | 1 (4.8) | |
| Hereditary nephropathies | 20 (18.0) | 14 (15.6) | 6 (28.6) | |
| No data, n | 1 | 1 | 0 | |
| Transplantation vintage years, median (IQR) | 6 (2–10) | 6 (2–10) | 6 (3–11) | 0.55 |
| Deceased donor, n (%) | 105 (93.7) | 84 (92.3) | 21 (100) | 0.34 |
| Medications, n (%) | ||||
| ACEi | 31 (27.9) | 23 (25.6) | 8 (38.1) | 0.25 |
| ARB | 10 (9.0) | 7 (7.8) | 3 (14.3) | 0.40 |
| Vitamin D | 60 (54.1) | 50 (55.6) | 10 (47.6) | 0.51 |
| No data, n | 1 | 1 | 0 | |
| Immunosuppressive agents, n (%) | >0.99 | |||
| TAC+MMF/MPS + steroids | 61 (54.5) | 49 (53.8) | 12 (57.1) | |
| CyA+MMF/MPS + steroids | 26 (23.2) | 21 (23.1) | 5 (23.8) | |
| CNI+steroids, without antiproliferative | 14 (12.5) | 12 (13.2) | 2 (9.5) | |
| Other protocols | 11 (9.8) | 9 (9.9) | 2 (9.5) | |
| Hospitalized, n (%) No data, n | 46 (41.1) 0 | 26 (28.6) 0 | 20 (95.2) 0 | <0.001 |
| Duration of hospitalization in days, median (IQR) Valid data/No data, n | 12 (9–19) 39/73 | 10 (8–14) 22/69 | 14 (12–26) 17/4 | 0.027 |
| SARS-CoV-2 variants, n (%) | >0.99 | |||
| Wild-type | 80 (71.4) | 65 (71.43) | 15 (71.4) | |
| British variant | 32 (28.6) | 26 (28.6) | 6 (28.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinowska, A.; Ruszkowski, J.; Muchlado, M.; Ślizień, Z.; Heleniak, Z.; Parczewska, A.; Kanclerz, K.; Biedunkiewicz, B.; Tylicki, L.; Król, E.; et al. Effect of COVID-19 on Kidney Graft Function One Year after Onset. Medicina 2024, 60, 26. https://doi.org/10.3390/medicina60010026
Malinowska A, Ruszkowski J, Muchlado M, Ślizień Z, Heleniak Z, Parczewska A, Kanclerz K, Biedunkiewicz B, Tylicki L, Król E, et al. Effect of COVID-19 on Kidney Graft Function One Year after Onset. Medicina. 2024; 60(1):26. https://doi.org/10.3390/medicina60010026
Chicago/Turabian StyleMalinowska, Agnieszka, Jakub Ruszkowski, Marta Muchlado, Zuzanna Ślizień, Zbigniew Heleniak, Aleksandra Parczewska, Katarzyna Kanclerz, Bogdan Biedunkiewicz, Leszek Tylicki, Ewa Król, and et al. 2024. "Effect of COVID-19 on Kidney Graft Function One Year after Onset" Medicina 60, no. 1: 26. https://doi.org/10.3390/medicina60010026
APA StyleMalinowska, A., Ruszkowski, J., Muchlado, M., Ślizień, Z., Heleniak, Z., Parczewska, A., Kanclerz, K., Biedunkiewicz, B., Tylicki, L., Król, E., & Dębska-Ślizień, A. (2024). Effect of COVID-19 on Kidney Graft Function One Year after Onset. Medicina, 60(1), 26. https://doi.org/10.3390/medicina60010026









