Role of Elevated Serum TGF-β1 and the Common Promoter TGFB1-509C/T Polymorphism in the Development and Progression of Primary Glial Tumors and Brain Metastases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Blood Samples and DNA Extraction
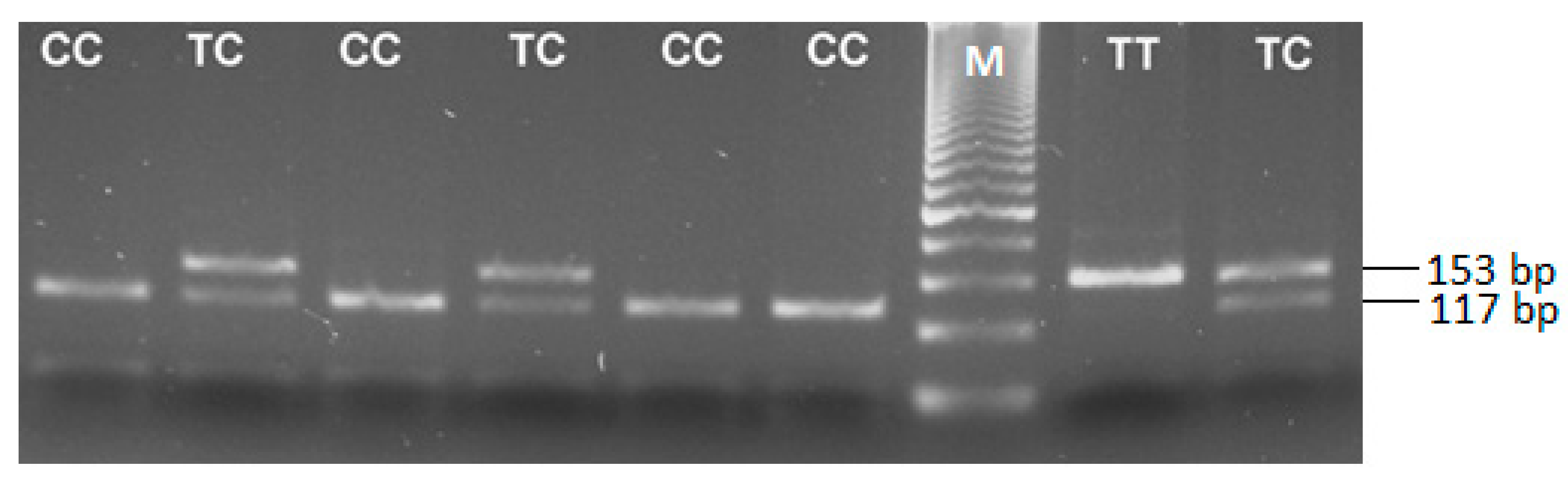
2.3. Genotyping for the TGFB1 -509 C/T Polymorphism (rs1800469)
2.4. Quantification of Serum TGF-β1 Levels
2.5. Immunohistochemistry
2.6. Statistical Analyses
3. Results
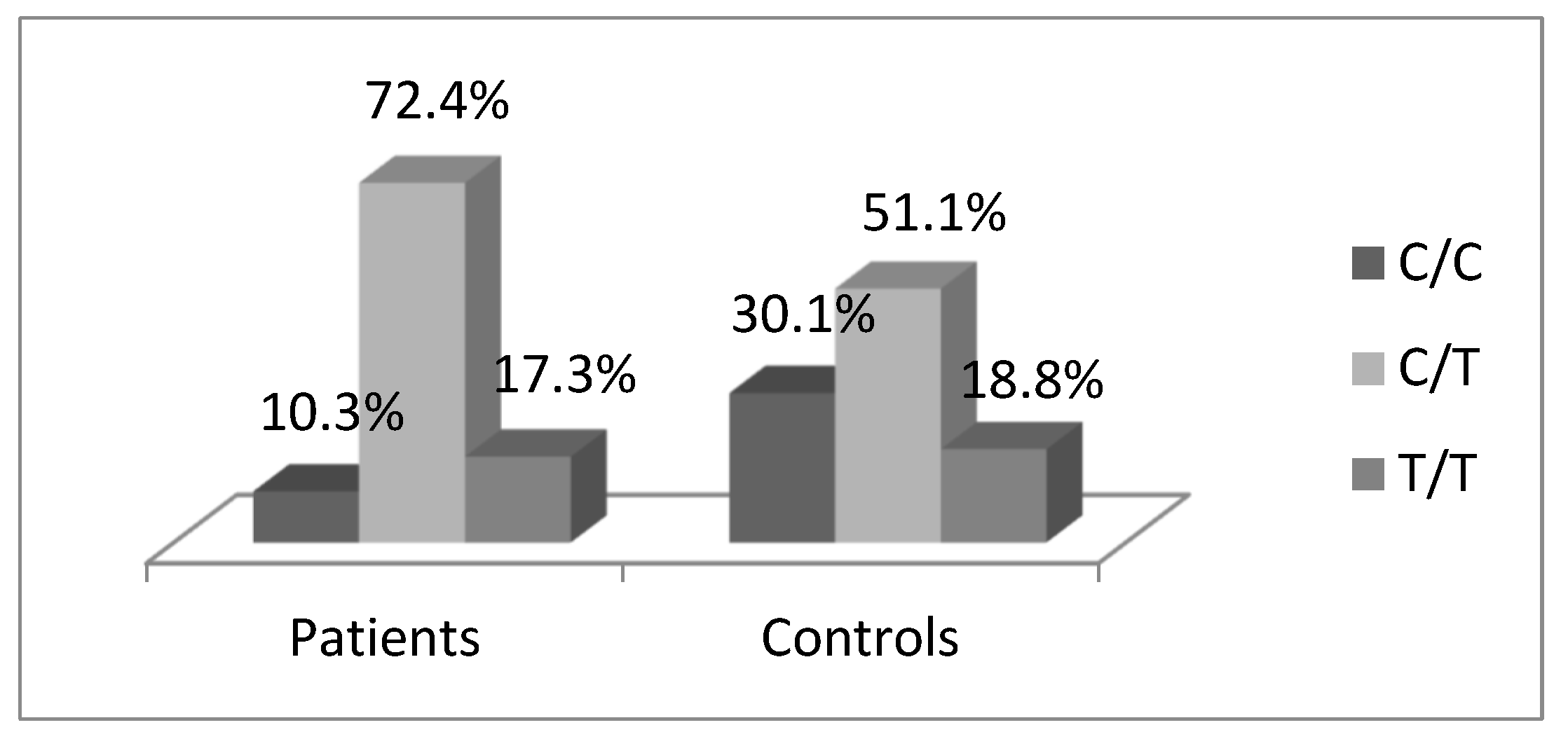
3.1. Distribution of TGFB1-509C/T Polymorphism
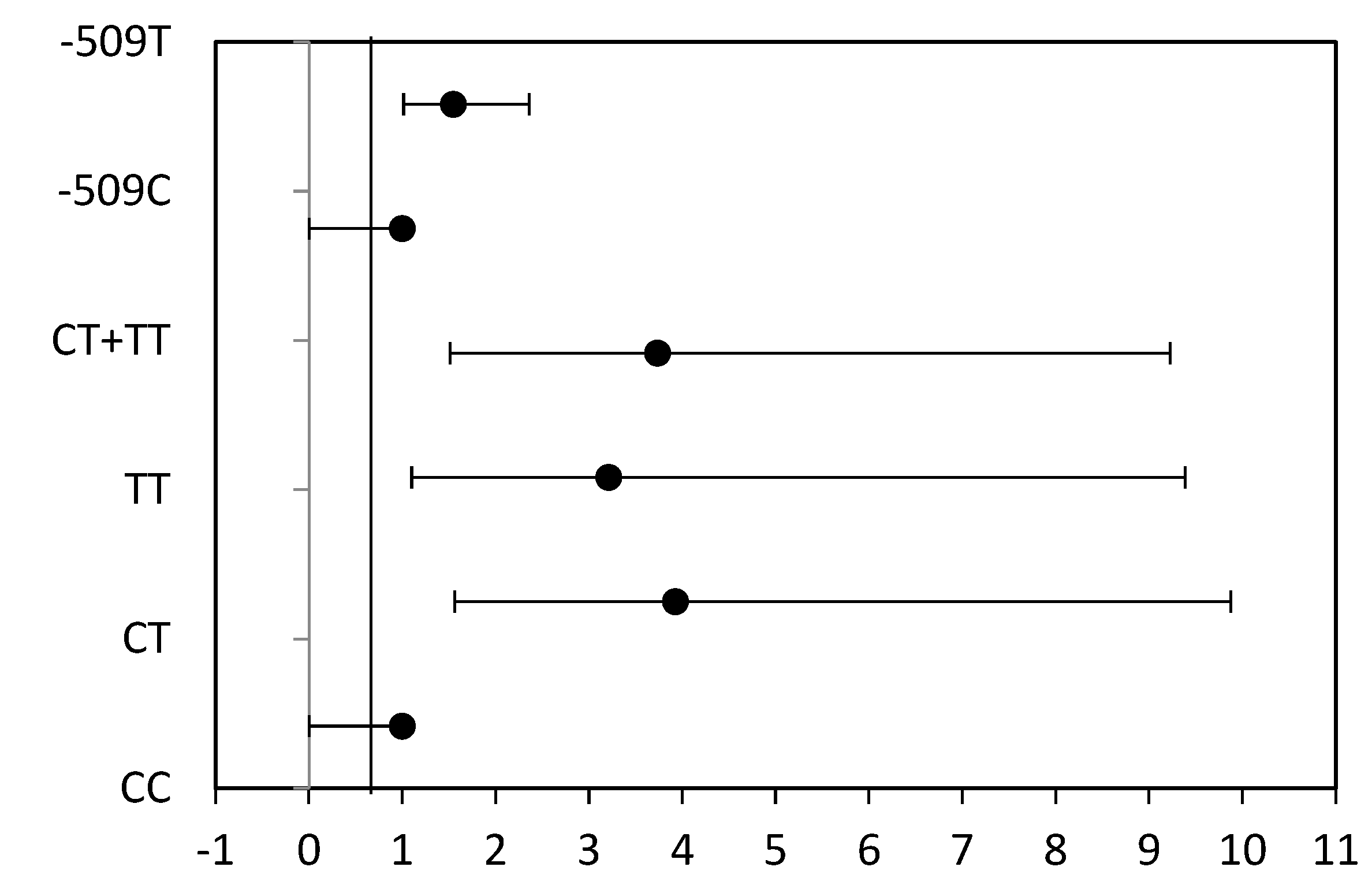
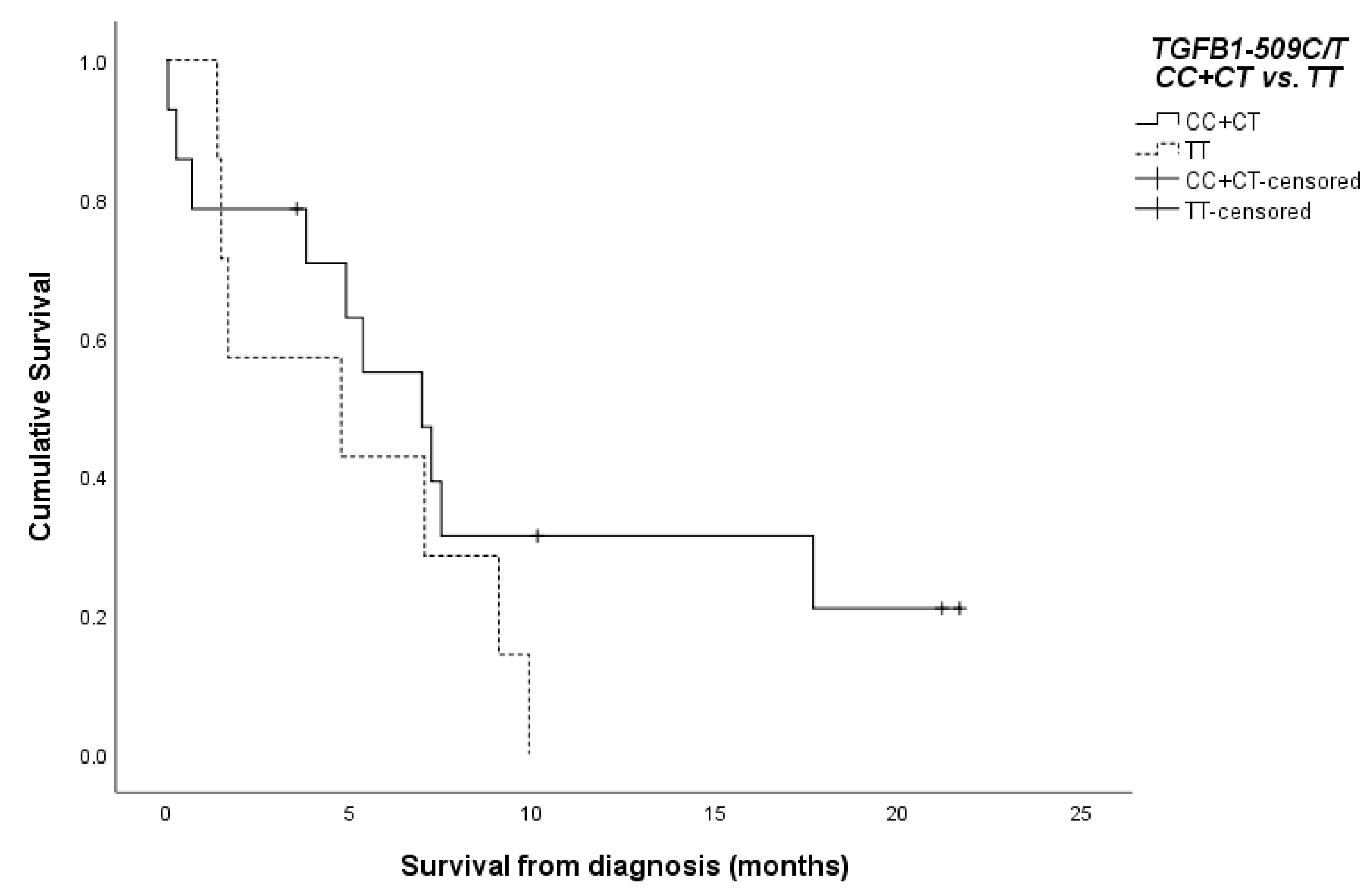
3.2. Survival of the Patients According to the TGFB1-509C/T SNP
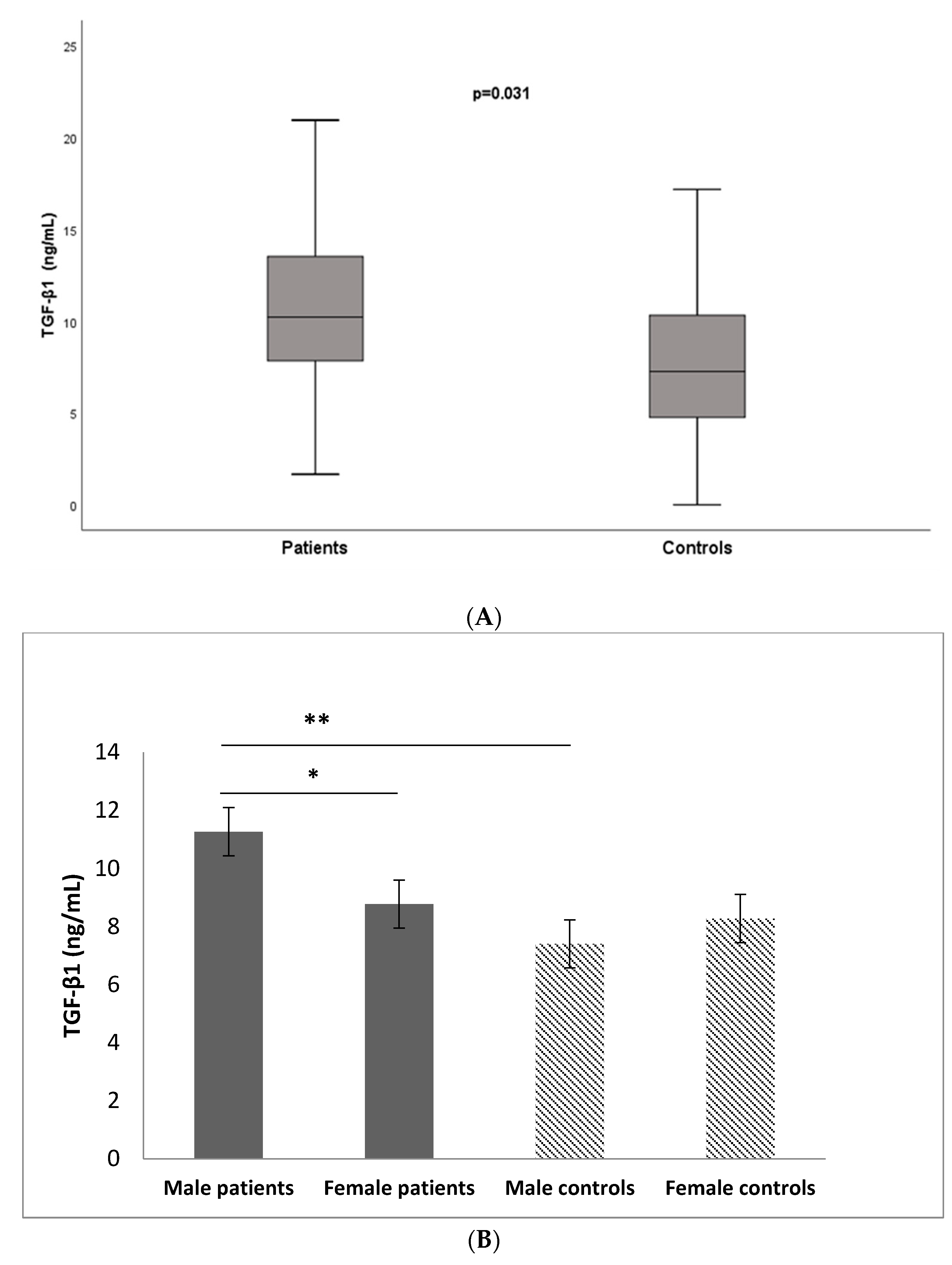
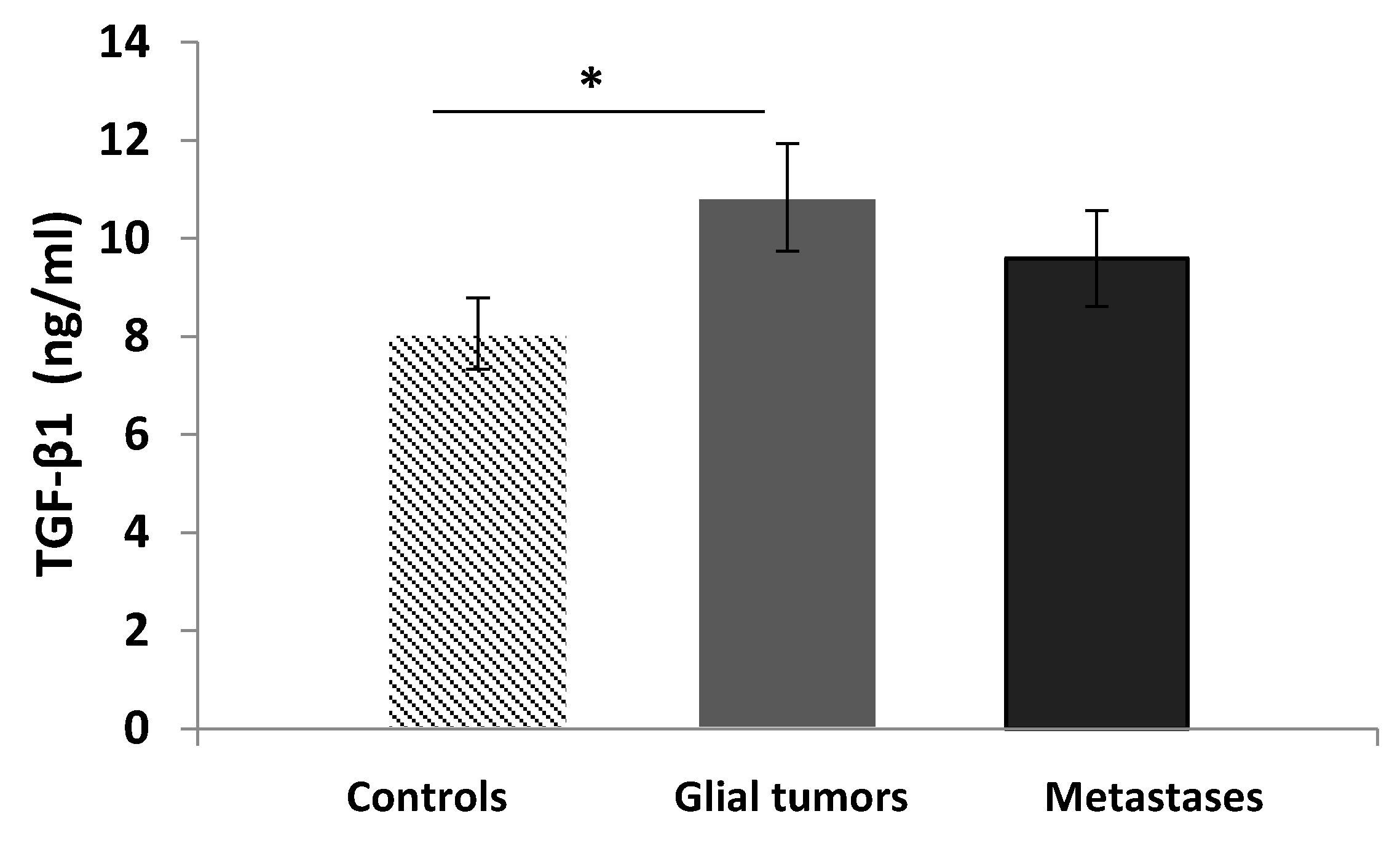
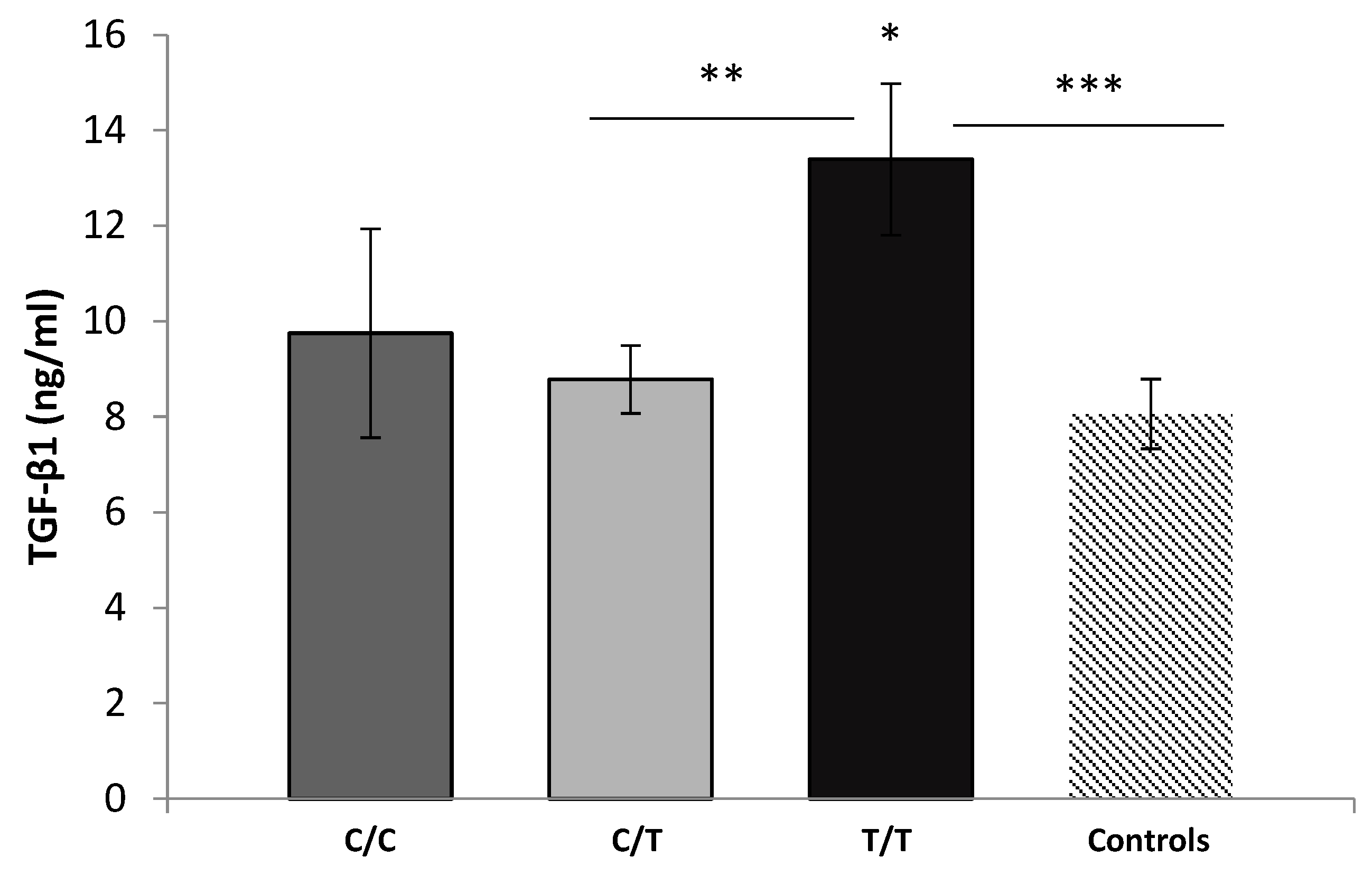
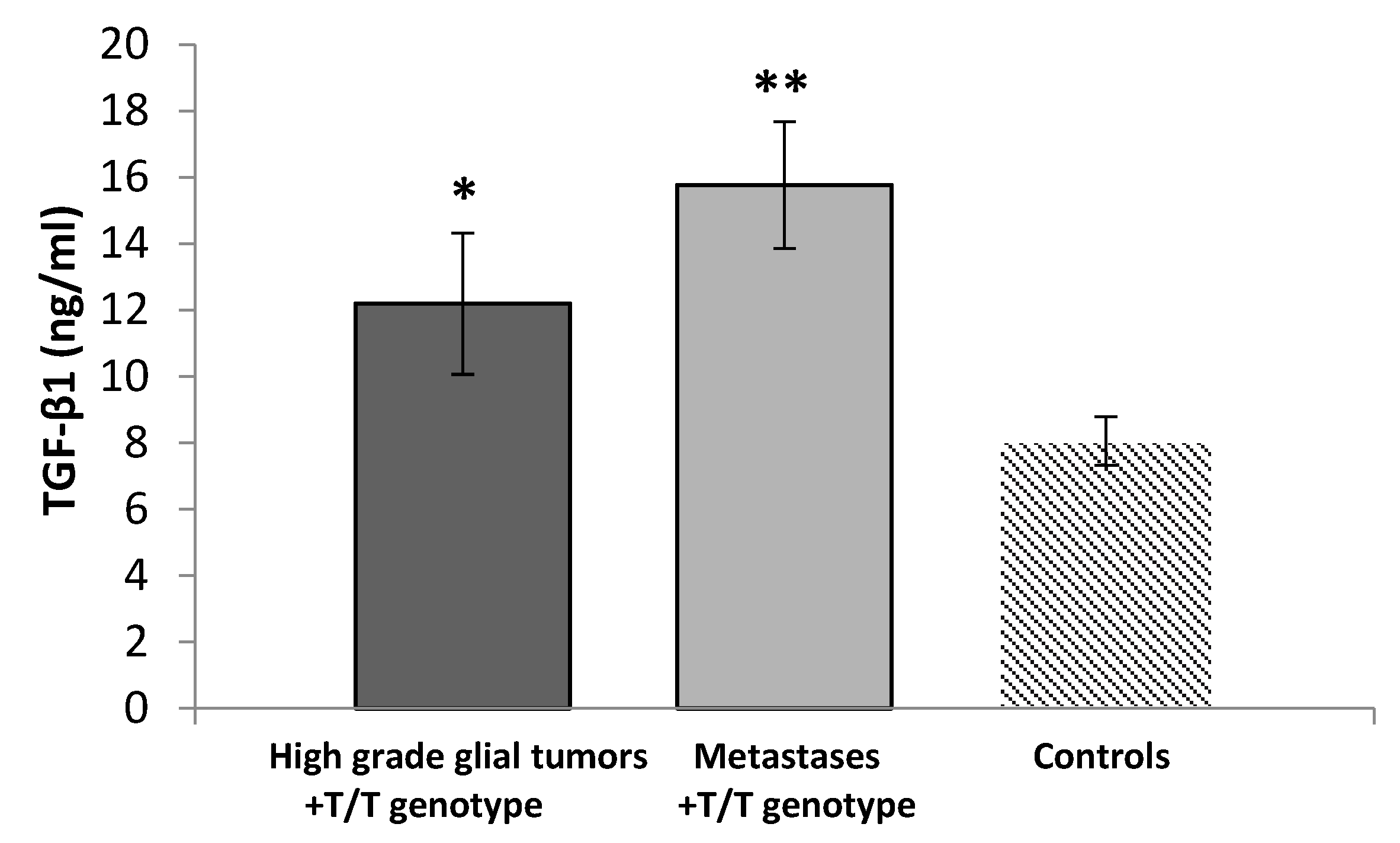
3.3. Serum Levels of TGF-β1
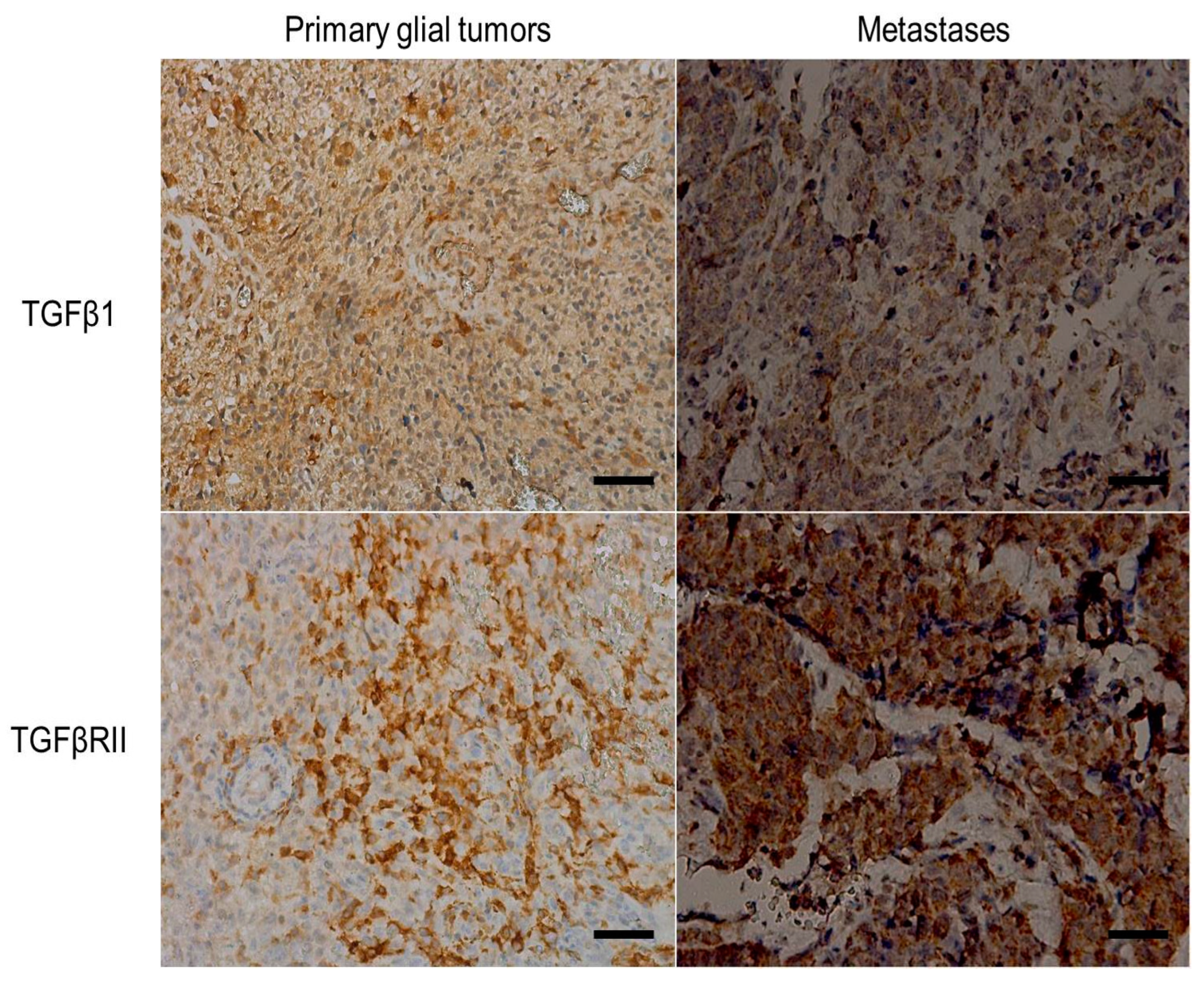
3.4. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaminska, B.; Wesolowska, A.; Danilkiewicz, M. TGF beta signalling and its role in tumour pathogenesis. Acta Biochim. Pol. 2005, 52, 329–337. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.M. Transforming growth factor beta (TGF-beta) in inflammation: A cause and a cure. J. Clin. Immunol. 1992, 12, 61–74. [Google Scholar] [CrossRef]
- Rubtsov, Y.P.; Rudensky, A.Y. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat. Rev. Immunol. 2007, 7, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Reibman, J.; Meixler, S.; Lee, T.C.; Gold, L.I.; Cronstein, B.N.; Haines, K.A.; Kolasinski, S.L.; Weissmann, G. Transforming growth factor beta 1, a potent chemoattractant for human neutrophils, bypasses classic signal-transduction pathways. Proc. Natl. Acad. Sci. USA 1991, 88, 6805–6809. [Google Scholar] [CrossRef]
- Bettelli, E.; Oukka, M.; Kuchroo, V.K. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007, 8, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chu, X.; Meng, X.; Zou, F. Association of TGF-β1-509C/T polymorphisms with breast cancer risk: Evidence from an updated meta-analysis. Tumor Biol. 2014, 35, 935–942. [Google Scholar] [CrossRef]
- Shah, R.; Hurley, C.K.; Posch, P.E. A molecular mechanism for the differential regulation of TGF-β1 expression due to the common SNP-509C-T (c.-1347C > T). Hum Genet. 2006, 120, 461–469. [Google Scholar] [CrossRef]
- Fleming, N.I.; Jorissen, R.N.; Mouradov, D.; Christie, M.; Sakthianandeswaren, A.; Palmieri, M.; Day, F.; Li, S.; Tsui, C.; Lipton, L.; et al. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Cancer Res. 2013, 73, 725–735. [Google Scholar] [CrossRef]
- Biswas, S.; Chytil, A.; Washington, K.; Romero-Gallo, J.; Gorska, A.E.; Wirth, P.S.; Gautam, S.; Moses, H.L.; Grady, W.M. Transforming growth factor beta receptor type II inactivation promotes the establishment and progression of colon cancer. Cancer Res. 2004, 64, 4687–4692. [Google Scholar] [CrossRef]
- Mager, L.F.; Wasmer, M.H.; Rau, T.T.; Krebs, P. Cytokine-Induced Modulation of Colorectal Cancer. Front. Oncol. 2016, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yuan, X.Q.; Zhang, C.Y.; Ye, F.; Zhou, H.F.; Li, W.L.; Liu, Z.Y.; Zhang, Y.Q.; Pan, X.; Li, G.C. High TGF-β1 expression predicts poor disease prognosis in hepatocellular carcinoma patients. Oncotarget 2017, 8, 34387–34397. [Google Scholar] [CrossRef] [PubMed]
- Merzak, A.; McCrea, S.; Koocheckpour, S.; Pilkington, G.J. Control of human glioma cell growth, migration and invasion in vitro by transforming growth factor beta 1. Br. J. Cancer 1994, 70, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, B.S.; Yamamoto, J.F.; Decker, R.; Yokochi, L.; Theriault, A.G.; Vogt, T.M.; Le Marchand, L. Association of genetic variation in the transforming growth factor beta-1 gene with serum levels and risk of colorectal neoplasia. Cancer Res. 2008, 68, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C.H. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007, 98, 1512–1520. [Google Scholar] [CrossRef]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1419. [Google Scholar] [CrossRef]
- Burghardt, I.; Schroeder, J.J.; Weiss, T.; Gramatzki, D.; Weller, M. A tumor-promoting role for soluble TβRIII in glioblastoma. Mol. Cell. Biochem. 2021, 476, 2963–2973. [Google Scholar] [CrossRef]
- Wrana, J.L.; Attisano, L.; Wieser, R.; Ventura, F.; Massague, J. Mechanism of activation of the TGF-b receptor. Nature 1994, 370, 341–347. [Google Scholar] [CrossRef]
- Dobolyi, A.; Vincze, C.; Pál, G.; Lovas, G. The neuroprotective functions of transforming growth factor beta proteins. Int. J. Mol. Sci. 2012, 13, 8219–8258. [Google Scholar] [CrossRef]
- Guo, S.K.; Shen, M.F.; Yao, H.W.; Liu, Y.S. Enhanced Expression of TGFBI Promotes the Proliferation and Migration of Glioma Cells. Cell. Physiol. Biochem. 2018, 49, 1097–1109. [Google Scholar] [CrossRef]
- Peñuelas, S.; Anido, J.; Prieto-Sanchez, R.M.; Folch, G.; Barba, I.; Cuartas, I.; Garcia-Dorado, D.; Poca, M.A.; Sahuquillo, J.; Baselga, J.; et al. TGF-β increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell 2009, 15, 315–327. [Google Scholar] [CrossRef]
- Pickup, M.; Novitskiy, S.; Moses, H.L. The roles of TGFβ in the tumour microenvironment. Nat. Rev. Cancer. 2013, 13, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Calon, A.; Tauriello, D.V.; Batlle, E. TGF-beta in CAF-mediated tumor growth and metastasis. Semin. Cancer Biol. 2014, 25, 15–22. [Google Scholar] [CrossRef]
- Gulubova, M.; Aleksandrova, E.; Vlaykova, T. Promoter polymorphisms in TGFB1 and IL10 genes influence tumor dendritic cells infiltration, development and prognosis of colorectal cancer. J. Gene Med. 2018, 20, e3005. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Song, W.G.; Liu, H.Q.; Fang, F.; Xiao, Y. Role of TGFB1 polymorphism in the development of metastatic brain tumors in non-small cell lung cancer patients. Genet. Mol. Res. 2015, 14, 3545–3550. [Google Scholar] [CrossRef]
- Vieira de Castro, J.; Gonçalves, C.S.; Costa, S.; Linhares, P.; Vaz, R.; Nabiço, R.; Amorim, J.; Viana-Pereira, M.; Reis, R.M.; Costa, B.M. Impact of TGF-β1-509C/T and 869T/C polymorphisms on glioma risk and patient prognosis. Tumor Biol. 2015, 36, 6525–6532. [Google Scholar] [CrossRef]
- Yuan, X.; Wei, Q.; Komaki, R.; Liu, Z.; Yang, J.; Tucker, S.L.; Xu, T.; Heymach, J.V.; Lu, C.; Cox, J.D.; et al. TGFβ1 Polymorphisms Predict Distant Metastasis-Free Survival in Patients with Inoperable Non-Small-Cell Lung Cancer after Definitive Radiotherapy. PLoS ONE 2013, 8, e65659. [Google Scholar] [CrossRef] [PubMed]
- Ananiev, J.; Manolova, I.; Aleksandrova, E.; Gulubova, M. Impact of TGF-β1 expression and -509C>T polymorphism in the TGF-β1 gene on the progression and survival of gastric cancer. Pol. J. Pathol. 2017, 68, 234–240. [Google Scholar] [CrossRef]
- Grainger, D.J.; Heathcote, K.; Chiano, M.; Snieder, H.; Kemp, P.R.; Metcalfe, J.C.; Carter, N.D.; Spector, T.D. Genetic control of the circulating concentration of transforming growth factor type beta1. Hum. Mol. Genet. 1999, 8, 93–97. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, Y.; Yin, Q.; Liu, S.; Wang, Q.; Tang, Y.; Cao, C. Clinical and prognostic significance of serum transforming growth factor-β1 levels in patients with pancreatic ductal adenocarcinoma. Braz. J. Med. Biol. Res. 2016, 49, e5485. [Google Scholar] [CrossRef]
- Lin, Y.; Kikuchi, S.; Obata, Y.; Yagyu, K. Serum levels of transforming growth factor beta1 are significantly correlated with venous invasion in patients with gastric cancer. J. Gastroenterol. Hepatol. 2006, 21, 432–437. [Google Scholar] [CrossRef]
- Xu, S.; Yang, S.; Sun, G.; Huang, W.; Zhang, Y. Transforming growth factor-beta polymorphisms and serum level in the development of osteosarcoma. DNA Cell Biol. 2014, 33, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, A.; Samsó, P.; Fontova, P.; Simon-Molas, H.; Manzano, A.; Castaño, E.; Rosa, J.L.; Martinez-Outshoorn, U.; Ventura, F.; Navarro-Sabaté, À.; et al. TGF-β1 targets Smad, p38 MAPK, and PI3K/Akt signaling pathways to induce PFKFB3 gene expression and glycolysis in glioblastoma cells. FEBS J. 2017, 284, 3437–3454. [Google Scholar] [CrossRef] [PubMed]
- Jurisic, D.; Erjavec, I.; Trkulja, V.; Dumic-Cule, I.; Hadzibegovic, I.; Kovacevic, L.; Svagusa, T.; Stanec, Z.; Vukicevic, S.; Grgurevic, L.; et al. Soluble type III TGF β receptor in diagnosis and follow-up of patients with breast cancer. Growth Factors 2015, 33, 200–209. [Google Scholar] [PubMed]
- Roberts, A.B.; Wakefield, L.M. The two faces of transforming growth factor β in carcinogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 8621–8623. [Google Scholar] [CrossRef]
- Principe, D.R.; DeCant, B.; Staudacher, J.; Vitello, D.; Mangan, R.J.; Wayne, E.A.; Mascariñas, E.; Diaz, A.M.; Bauer, J.; McKinney, R.D.; et al. Loss of TGFβ signaling promotes colon cancer progression and tumor-associated inflammation. Oncotarget 2017, 8, 3826–3839. [Google Scholar] [CrossRef]
- Oshima, H.; Nakayama, M.; Han, T.S.; Naoi, K.; Ju, X.; Maeda, Y.; Robine, S.; Tsuchiya, K.; Sato, T.; Sato, H.; et al. Suppressing TGFβ signaling in regenerating epithelia in an inflammatory microenvironment is sufficient to cause invasive intestinal cancer. Cancer Res. 2015, 75, 766–776. [Google Scholar] [CrossRef]
- Gu, Y.H.; Feng, Y.G. Down-regulation of TGF-β RII expression is correlated with tumor growth and invasion in non-functioning pituitary adenomas. J. Clin. Neurosci. 2018, 47, 264–268. [Google Scholar] [CrossRef]
| Parameter | Number (%) |
|---|---|
| Patients | n = 61 |
| Gender | |
| Male | 40 (65.6) |
| Female | 21 (34.4) |
| Age at diagnosis (years) | |
| Range | 25–82 |
| Mean (±SD) | 62.1 (±11.76) |
| <62.1 | 27 (44.3) |
| >62.1 | 34 (55.7) |
| Primary/Metastases | |
| Primary glial tumors | 31(50.8) |
| Grade III | 2 (3.2) |
| Grade IV | 29 (47.6) |
| Metastases | 30 (49.2) |
| Chemotherapy | |
| yes | 52 (85.2) |
| no | 9 (14.8) |
| Radiotherapy | |
| yes | 28 (45.9) |
| no | 33 (54.1) |
| Survival status | n = 48 |
| alive | 15 (31.2) |
| deceased | 33 (68.8) |
| TGF-beta1 expression | n = 39 |
| positive | 19 (48.7) |
| negative | 20 (51.3) |
| TGF-beta RII expression | n = 39 |
| positive | 27 (69.2) |
| negative | 12 (30.8) |
| TGFB1-509 C/T SNP | Patients | Controls | OR (95% CI), p-Value | OR * (95% CI), p-Value | ||
|---|---|---|---|---|---|---|
| n | Frequency | n | Frequency | |||
| 61 | 176 | |||||
| Genotype | ||||||
| CC | 7 | 0.10 | 53 | 0.30 | 1.0 (Ref.) | 1.0 (Ref.) |
| CT | 41 | 0.73 | 90 | 0.51 | 3.926 (1.560–9.878), p = 0.002 | 4.292 (1.092–9.685), p = 0.002 |
| TT | 13 | 0.17 | 33 | 0.19 | 3.212 (1.100–9.384), p = 0.037 | 3.215 (1.060–9.110), p = 0.033 |
| CT + TT | 54 | 0.90 | 123 | 0.70 | 3.734 (1.512–9.225), p = 0.003 | 3.968 (1.010–9.624), p = 0.003 |
| Alleles | ||||||
| -509C | 35 | 0.47 | 196 | 0.56 | 1.0 (Ref.) | … |
| -509T | 46 | 0.53 | 156 | 0.44 | 1.546 (1.014–2.358), p = 0.053 | … |
| TGF-β1 Expression | TGFβ-RII Expression | TGF-β1/TGFβ-RII Co-Expression | p | |
|---|---|---|---|---|
| Positive | 19 | 27 | 17 | 0.006 |
| Negative | 20 | 12 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrova, E.; Mindov, I.; Petrov, B.; Dimitrova, I.; Petrov, N.; Ananiev, J.; Vlaykova, T.; Valkanov, S. Role of Elevated Serum TGF-β1 and the Common Promoter TGFB1-509C/T Polymorphism in the Development and Progression of Primary Glial Tumors and Brain Metastases. Medicina 2024, 60, 146. https://doi.org/10.3390/medicina60010146
Aleksandrova E, Mindov I, Petrov B, Dimitrova I, Petrov N, Ananiev J, Vlaykova T, Valkanov S. Role of Elevated Serum TGF-β1 and the Common Promoter TGFB1-509C/T Polymorphism in the Development and Progression of Primary Glial Tumors and Brain Metastases. Medicina. 2024; 60(1):146. https://doi.org/10.3390/medicina60010146
Chicago/Turabian StyleAleksandrova, Elina, Ivan Mindov, Bozhidar Petrov, Ivelina Dimitrova, Nikolay Petrov, Julian Ananiev, Tatyana Vlaykova, and Stefan Valkanov. 2024. "Role of Elevated Serum TGF-β1 and the Common Promoter TGFB1-509C/T Polymorphism in the Development and Progression of Primary Glial Tumors and Brain Metastases" Medicina 60, no. 1: 146. https://doi.org/10.3390/medicina60010146
APA StyleAleksandrova, E., Mindov, I., Petrov, B., Dimitrova, I., Petrov, N., Ananiev, J., Vlaykova, T., & Valkanov, S. (2024). Role of Elevated Serum TGF-β1 and the Common Promoter TGFB1-509C/T Polymorphism in the Development and Progression of Primary Glial Tumors and Brain Metastases. Medicina, 60(1), 146. https://doi.org/10.3390/medicina60010146








