Physiotherapy Intervention on Premature Infants—A Pilot Study
Abstract
1. Introduction
- Analysis of the factors that influence the level of motor development of premature babies up to 9 months.
- Identification of the motor development curve of premature babies according to the three stages of motor development: the position of symmetrical support on the elbows at 3 months, sitting with support at 6 months, verticalization at 9 months, and identifying the factors that influence motor development in premature infants.
2. Materials and Methods
- -
- With head adjustment, the tonic reflexes of the neck were activated to favor flexion or extension of the upper or lower limbs.
- -
- In order to activate the asymmetrical neck reflexes, the head was positioned on the side of the affected limb to achieve relaxation of the flexor tone. Alternatively, mobilization was facilitated by turning the head in the other direction.
- -
- To stimulate the tonic labyrinthine reflex, the infant was positioned in the supine position (DD), and through anterior flexion of the head and neck, with the upper limbs crossed on the ribcage, relaxation of the lower limbs was achieved.
- -
- In the case of the opisthotonus position, to relax the extensor muscles of the neck and at the level of the trunk and limbs, the fetal position was adopted and performed with slight antero-posterior rocking movements.
- -
- For an infant who showed a tendency to lean forward, the lifting was performed with maintenance in the axillary area to obtain the extension of the head and limbs in a reflex inhibitory position, thus favoring easy movement of the limbs. The same relaxation was obtained from the recumbent position ventral (DV) by lifting the infant’s head and supporting the abdomen.
- -
- Small, brief pressures were applied to the infant’s shoulder while sitting, kneeling, or in a quadruped position, pushing it in different directions to teach it to respond by raising its arm in the direction of the push. This exercise was performed in two sets of five repetitions, with a one-minute break between sets.
- -
- In order to stimulate the infant to sit up from the supine position, its return to the lateral decubitus was initiated, with support on the body, elbow, and then on the palm, while being maintained at the level of the lower limbs by the physiotherapist. This exercise was performed in two sets of five repetitions, with a one-minute break between sets (Figure 1).
- -
- In order to transfer from the sitting position to the quadrupedal position, the infant was given a lateral movement either to the left or to the right, with progressive loading of the upper limbs. This movement can be performed on a horizontal surface or on a slope, tilted down. The exercise was performed in two sets of five repetitions, with a one-minute break between sets.
- -
- From the ventral decubitus position, transitioning the infant into quadrupedal posture was achieved with support in the anterior thoracic region, facilitating loading on the upper limbs. Simultaneously, by gently elevating the pelvis through flexion of the hip joints, loading at the knee level was facilitated. This exercise was performed in sets of five repetitions, either on a mat or with the assistance of a Bobath ball (Figure 2).
- -
- The initiation of verticalization was achieved through the “Servant Knight” position. The initial position involved support on both knees and on the upper limbs. The infant was lifted through one of the flexed lower limbs (triple flexion at the hip, knee, and ankle) and pushed to adopt a balanced and stable position, being supported by both supporting limbs. This exercise was performed in two sets of five repetitions for each lower limb, with a one-minute break between sets (position executed only at 9 months).
- -
- In the standing position, an inflatable disc was used to stimulate balance. Because balance is an essential reaction for walking, the infant was slightly thrown off balance by anteroposterior and lateral thrusting movements, a position executed only at 9 months (Figure 3).
Statistical Analysis
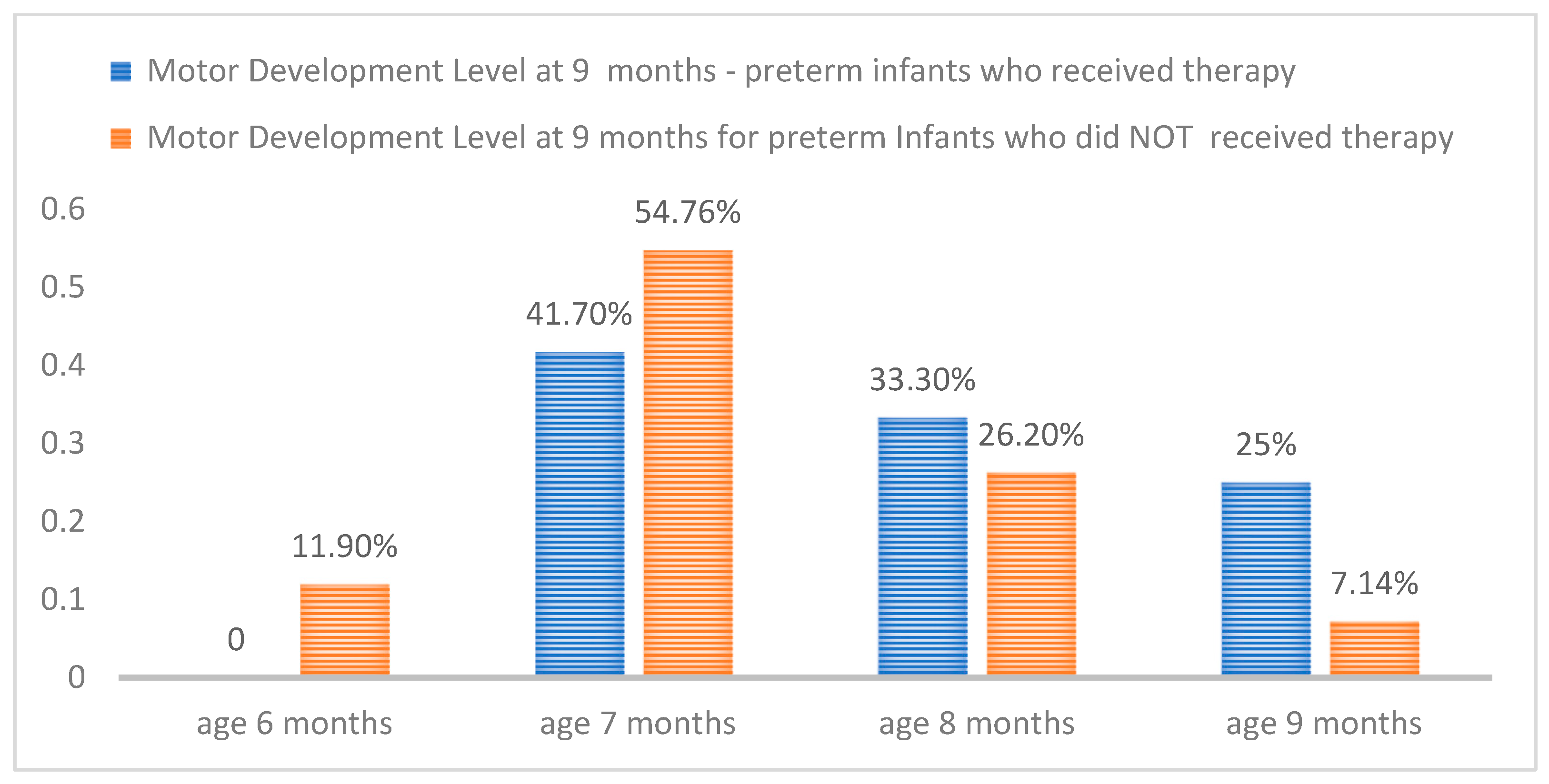
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Purisch, S.E.; Gyamfi-Bannerman, C. Epidemiology of preterm birth. Semin. Perinatol. 2017, 41, 387–391. [Google Scholar] [CrossRef]
- Torchin, H.; Ancel, P.Y.; Jarreau, P.H.; Goffinet, F. Épidémiologie de la prématurité: Prévalence, évolution, devenir des enfants [Epidemiology of preterm birth: Prevalence, recent trends, short- and long-term outcomes]. J. Gynécologie Obs. Étrique Biol. Reprod. 2015, 44, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, G.; Shankaran, S. Short- and long-term outcomes of moderate and late preterm infants. Am. J. Perinatol. 2016, 33, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Jackson, R.A.; Gibson, K.A.; Wu, Y.W.; Croughan, M.S. Perinatal outcomes in singletons following in vitro fertilization: A meta-analysis. Obstet. Gynecol. 2004, 103, 551–563. [Google Scholar] [CrossRef]
- Dumuids-Vernet, M.V.; Forma, V.; Provasi, J.; Anderson, D.I.; Hinnekens, E.; Soyez, E.; Strassel, M.; Guéret, L.; Hym, C.; Huet, V.; et al. Stimulating the motor development of very premature infants: Effects of early crawling training on a mini-skateboard. Front. Pediatr. 2023, 11, 1198016. [Google Scholar] [CrossRef]
- Wang, H.H.; Hwang, Y.S.; Ho, C.H.; Lai, M.C.; Chen, Y.C.; Tsai, W.H. Prevalence and initial diagnosis of cerebral palsy in preterm and term-born children in Taiwan: A nationwide, population-based cohort study. Int. J. Environ. Res. Public Health 2021, 18, 8984. [Google Scholar] [CrossRef]
- Arnaud, C.; Ehlinger, V.; Delobel-Ayoub, M.; Klapouszczak, D.; Perra, O.; Hensey, O.; Neubauer, D.; Hollódy, K.; Virella, D.; Rackauskaite, G.; et al. Trends in prevalence and severity of pre/perinatal cerebral palsy among children born preterm from 2004 to 2010: A SCPE collaboration study. Front. Neurol. 2021, 12, 624884. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. 2007, 49, 8–14. [Google Scholar] [CrossRef]
- Pierrat, V.; Marchand-Martin, L.; Arnaud, C.; Kaminski, M.; Resche-Rigon, M.; Lebeaux, C. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. Br. Med. J. 2017, 358, j3448. [Google Scholar] [CrossRef] [PubMed]
- Pierrat, V.; Marchand-Martin, L.; Marret, S.; Arnaud, C.; Benhammou, V.; Cambonie, G.; Debillon, T.; Dufourg, M.-N.; Gire, C.; Goffinet, F.; et al. Neurodevelopmental outcomes at age 5 among children born preterm: EPIPAGE-2 cohort study. Br. Med. J. 2021, 373, n741. [Google Scholar] [CrossRef] [PubMed]
- Bobath Tutors. The Bobath Concept. Available online: https://www.bobathtutors.com/concept.php (accessed on 15 November 2023).
- Vaughan-Graham, J.; Cheryl, C.; Holland, A. Developing a revised definition of the Bobath concept: Phase three. Physiother. Res. Int. 2020, 25, e1832. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Gyanpuri, V.; Dev, P.; Dhiman, N.R. The Bobath Concept (NDT) as rehabilitation in stroke patients: A systematic review. J. Fam. Med. Prim. Care 2021, 10, 3983–3990. [Google Scholar] [CrossRef]
- Drăgan, C.F.; Pădure, L. Metodologie și tehnici de Kinetoterapie. Ed. Național 2008, 9–10, 35–39. [Google Scholar]
- Kashuba, V.; Dolynskyi, B.; Todorova, V.; Bukhovets, B.; Andrieieva, O.; Shankovskyi, A. Physical Rehabilitation of Children with Cerebral Palsy by Bobath-Therapy Method. Int. J. Appl. Exerc. Physiol. 2020, 9, 6–13. [Google Scholar]
- Indicators of Cerebral Blood Flow Changes in Venous Vessels of Children with ICP in the Course of Physical Rehabilitation Using the Bobath Therapy Method. Available online: http://dspace.pdpu.edu.ua/jspui/handle/123456789/4499 (accessed on 30 September 2023).
- Parau, D.; Todoran, A.B.; Barcutean, L.; Avram, C.; Balasa, R. The Benefits of Combining Bobath and Vojta Therapies in Infants with Motor Development Impairment—A Pilot Study. Medicina 2023, 59, 1883. [Google Scholar] [CrossRef]
- Kayenne Martins Roberto Formiga, C.; Linhares, M.B. Motor development curve from 0 to 12 months in infants born preterm. Acta Paediatr. 2011, 100, 379–384. [Google Scholar] [CrossRef]
- Ko, J.; Lim, H.K. Motor Development Comparison between Preterm and Full-Term Infants Using Alberta Infant Motor Scale. Int. J. Environ. Res. Public Health 2023, 20, 3819. [Google Scholar] [CrossRef]
- Dumuids-Vernet, M.V.; Provasi, J.; Anderson, D.I.; Barbu-Roth, M. Effects of Early Motor Interventions on Gross Motor and Locomotor Development for Infants at-Risk of Motor Delay: A Systematic Review. Front. Pediatr. 2022, 10, 877345. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Early human motor development: From variation to the ability to vary and adapt. Neurosci. Biobehav. Rev. 2018, 90, 411–427. [Google Scholar] [CrossRef]
- Øberg, G.K.; Campbell, S.K.; Girolami, G.L. Study protocol: An early intervention program to improve motor outcome in preterm infants: A randomized controlled trial and a qualitative study of physiotherapy performance and parental experiences. BMC Pediatr. 2012, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Kinney, H.C.; Brody, B.A.; Kloman, A.S.; Gilles, F.H. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J. Neuropathol. Exp. Neurol. 1988, 47, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Moczko, J.; Naczk, M.; Naczk, A.; Sobieska, M. Impact of selected risk factors on motor performance in the third month of life and motor development in the ninth month. PeerJ 2023, 11, e15460. [Google Scholar] [CrossRef] [PubMed]
- Zdzienicka-Chyła, A.M.; Mitosek-Szewczyk, K. Development in the first year of life of newborns born prematurely-preliminary report. Dev. Period Med. 2018, 22, 247–254. [Google Scholar] [CrossRef]
- Yaari, M.; Mankuta, D.; Harel-Gadassi, A.; Friedlander, E.; Bar-Oz, B.; Eventov-Friedman, S. Early developmental trajectories of preterm infants. Res. Dev. Disabil. 2018, 81, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Barroso, R.M.; Tiernan, C.; Chen, L.C.; Valentin-Gudiol, M.; Ulrich, D. Treadmill training in moderate-risk preterm infants promotes stepping quality--results of a small randomized controlled trial. Res. Dev. Disabil. 2013, 34, 3629–3638. [Google Scholar] [CrossRef] [PubMed]
- Schlittler, D.X.C.; Lopes, T.F.; Raniero, E.P.; Barela, J.A. Treadmill training effects on walking acquisition and motor development in infants at risk of developmental delay. Rev. Paul. Pediatr. 2011, 29, 91–99. [Google Scholar] [CrossRef]
- Kałucka, A.M.; Kałużyński, W.; Prokop, A.M.; Kikowski, Ł. Physiotherapy of Prematurely Born Children Taking into Account the Degree of Biological Immaturity. Wiadomości Lek. 2022, 75, 2315–2321. [Google Scholar] [CrossRef]
- Groeschel, S.; Holmström, L.; Northam, G. Motor Abilities in Adolescents Born Preterm Are Associated with Microstructure of the Corpus Callosum. Front. Neurol. 2019, 10, 367. [Google Scholar] [CrossRef]
- Fuentefria, R.D.N.; Silveira, R.C.; Procianoy, R.S. Motor development of preterm infants assessed by the Alberta Infant Motor Scale: Systematic review article. J. Pediatr. 2017, 93, 328–342. [Google Scholar] [CrossRef]
- Bélanger, R.; Mayer-Crittenden, C.; Minor-Corriveau, M.; Robillard, M. Gross Motor Outcomes of Children Born Prematurely in Northern Ontario and Followed by a Neonatal Follow-Up Programme. Physiother. Can. 2018, 70, 233–239. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Shamsi, B.H.; Hao, M.C.; Cao, C.-H.; Yang, W.-Y. A study on the neurodevelopment outcomes of late preterm infants. BMC Neurol. 2019, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Acar, G.; Altun, G.P.; Yurdalan, S.; Polat, M.G. Efficacy of neurodevelopmental treatment combined with the Nintendo® Wii in patients with cerebral palsy. J. Phys. Ther. Sci. 2016, 28, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Kavlak, E.; Ünal, A.; Tekin, F.; Altuğ, F. Effectiveness of Bobath therapy on balance in cerebral palsy. Çukurova Med. J. 2018, 43, 975–981. [Google Scholar] [CrossRef]
- Tekin, F. Effectiveness of Neuro-Developmental Treatment (Bobath Concept) on Postural Control and Balance in Cerebral Palsied Children. J. Back Musculoskelet. Rehabil. 2018, 1, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, A.; Rusu, L.; Rusu, M.R.; Marin, M.I. Balance Rehabilitation Approach by Bobath and Vojta Methods in Cerebral Palsy: A Pilot Study. Children 2022, 9, 1481. [Google Scholar] [CrossRef]
- Szuflak, K.; Malak, R.; Fechner, B.; Sikorska, D.; Samborski, W.; Mojs, E.; Gerreth, K. The Masticatory Structure and Function in Children with Cerebral Palsy—A Pilot Study. Healthcare 2023, 11, 1029. [Google Scholar] [CrossRef]
- Acar, G.; Ejraei, N.; Turkdoğan, D.; Enver, N.; Öztürk, G.; Aktaş, G. The Effects of Neurodevelopmental Therapy on Feeding and Swallowing Activities in Children with Cerebral Palsy. Dysphagia 2022, 37, 800–811. [Google Scholar] [CrossRef]
- Vaughan-Graham, J.; Cott, C.; Holland, A.; Michielsen, M.; Magri, A.; Suzuki, M.; Brooks, D. Developing a revised definition of the Bobath concept. Physiother. Res. Int. 2019, 24, e1762. [Google Scholar] [CrossRef]
- Giannantonio, C.; Papacci, P.; Ciarniello, R. Chest physiotherapy in preterm infants with lung diseases. Ital. J. Pediatr. 2010, 36, 65. [Google Scholar] [CrossRef] [PubMed]




| Frequency | Percent | ||
|---|---|---|---|
| Gender | Male | 22 | 61.1 |
| Female | 14 | 38.9 | |
| Age at birth (weeks) | 34 | 17 | 47.2 |
| 35 | 11 | 30.6 | |
| 36 | 8 | 22.2 | |
| APGAR score | 8 | 21 | 58.3 |
| 9 | 9 | 25.0 | |
| 10 | 6 | 16.7 | |
| Muscle tone | Hypotonic form | 18 | 50.0 |
| Spastic form | 18 | 50.0 | |
| Age (months): initial assessment | 1 | 7 | 19.4 |
| 2 | 11 | 30.6 | |
| 3 | 18 | 50.0 | |
| Neuromotor development level: initial assessment of the infant | 0 | 19 | 52.8 |
| 1 | 9 | 25.0 | |
| 2 | 4 | 11.1 | |
| 3 | 4 | 11.1 | |
| Neuromotor development level: assessment of the infant at age 3 months | 0 | 8 | 22.2 |
| 1 | 11 | 30.6 | |
| 2 | 13 | 36.1 | |
| 3 | 4 | 11.1 | |
| Neuromotor development level: assessment of the infant at age 6 months | 3 | 6 | 16.7 |
| 4 | 11 | 30.6 | |
| 5 | 19 | 52.8 | |
| Neuromotor development level: assessment of the infant at age 9 months | 7 | 15 | 41.7 |
| 8 | 12 | 33.3 | |
| 9 | 9 | 25.0 | |
| Variable | Level of Development–Evaluation at Infants Age of 3 Months | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total | p | ||
| Female | Frequency % | 0 0.0% | 6 42.9% | 8 57.1% | 0 0.0% | 14 100.0% | 0.009 |
| Male | Frequency % | 8 36.4% | 5 22.7% | 5 22.7% | 4 18.2% | 22 100.0% | |
| Total | Frequency % | 8 22.2% | 11 30.6% | 13 36.1% | 4 11.1% | 36 100.0% | |
| Level of development–evaluation at infants age of 6 months | |||||||
| 3 | 4 | 5 | Total | ||||
| Female | Frequency % | 0 0.0% | 6 42.9% | 8 57.1% | 14 100.0% | 0.081 | |
| Male | Frequency % | 6 27.3% | 5 22.7% | 11 50.0% | 22 100.0% | ||
| Total | Frequency % | 6 16.7% | 11 30.6% | 19 52.8% | 36 100.0% | ||
| Level of development–evaluation at infants age of 9 months | |||||||
| 7 | 8 | 9 | Total | ||||
| Female | Frequency % | 5 35.7% | 5 35.7% | 4 28.6% | 14 100.0% | 0.839 | |
| Male | Frequency % | 10 45.5% | 7 31.8% | 5 22.7% | 22 100.0% | ||
| Total | Frequency % | 15 41.7% | 12 33.3% | 9 25.0% | 36 100.0% | ||
| Age at Birth (Weeks) | Developmental Level–Assessment at Infants Age of 3 Months | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total | p | ||
| 34 | Frequency % | 5 29.4% | 6 35.3% | 2 11.8% | 4 23.5% | 17 100.0% | 0.001 |
| 35 | Frequency % | 3 27.3% | 5 45.5% | 3 27.3% | 0 0.0% | 11 100.0% | |
| 36 | Frequency % | 0 0.0% | 0 0.0% | 8 100.0% | 0 0.0% | 8 100.0% | |
| Total | Frequency % | 8 22.2% | 11 30.6% | 13 36.1% | 4 11.1% | 36 100.0% | |
| Developmental level–assessment at infants age of 6 months | |||||||
| 4 | 5 | 6 | Total | ||||
| 34 | Frequency % | 3 17.6% | 7 41.2% | 7 41.2% | 17 100.0% | 0.040 | |
| 35 | Frequency % | 3 27.3% | 4 36.4% | 4 36.4% | 11 100.0% | ||
| 36 | Frequency % | 0 0.0% | 0 0.0% | 8 100.0% | 8 100.0% | ||
| Total | Frequency % | 6 16.7% | 11 30.6% | 19 52.8% | 36 100.0% | ||
| Developmental level–assessment at infants age of 9 months | |||||||
| 7 | 8 | 9 | Total | ||||
| 34 | Frequency % | 12 70.6% | 2 11.8% | 3 17.6% | 17 100.0% | 0.010 | |
| 35 | Frequency % | 2 18.2% | 9 81.8% | 0 0.0% | 11 100.0% | ||
| 36 | Frequency % | 1 12.5% | 1 12.5% | 6 75.0% | 8 100.0% | ||
| Total | Frequency % | 15 41.7% | 12 33.3% | 9 25.0% | 36 100.0% | ||
| APGAR Score | Developmental Level–Assessment at Infants Age of 3 Months | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total | p | ||
| 8 | Frequency % | 6 28.6% | 8 38.1% | 3 14.3% | 4 19.0% | 21 100.0% | 0.440 |
| 9 | Frequency % | 2 22.2% | 2 22.2% | 5 55.6% | 0 0.0% | 9 100.0% | |
| 10 | Frequency % | 0 0.0% | 1 16.7% | 5 83.3% | 0 0.0% | 6 100.0% | |
| Total | Frequency % | 8 22.2% | 11 30.6% | 13 36.1% | 4 11.1% | 36 100.0% | |
| Developmental level–assessment at infants age of 6 months | |||||||
| 3 | 4 | 5 | Total | ||||
| 8 | Frequency % | 4 19.0% | 8 38.1% | 9 42.9% | 21 100.0% | 0.451 | |
| 9 | Frequency % | 2 22.2% | 2 22.2% | 5 55.6% | 9 100.0% | ||
| 10 | Frequency % | 0 0.0% | 1 16.7% | 5 83.3% | 6 100.0% | ||
| Total | Frequency % | 6 16.7% | 11 30.6% | 19 52.8% | 36 100.0% | ||
| Developmental level–assessment at infants age of 9 months | |||||||
| 7 | 8 | 9 | Total | ||||
| 8 | Frequency % | 12 57.1% | 6 28.6% | 3 14.3% | 21 100.0% | 0.191 | |
| 9 | Frequency % | 2 22.2% | 4 44.4% | 3 33.3% | 9 100.0% | ||
| 10 | Frequency % | 1 16.7% | 2 33.3% | 3 50.0% | 6 100.0% | ||
| Total | Frequency % | 15 41.7% | 12 33.3% | 9 25.0% | 36 100.0% | ||
| Muscle Tone | Developmental Level–Assessment at Infants Age of 3 Months | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total | p | ||
| Hypotonic form | Frequency % | 2 11.1% | 8 44.4% | 7 38.9% | 1 5.6% | 18 100.0% | 0.148 |
| Spastic form | Frequency % | 6 33.3% | 3 16.7% | 6 33.3% | 3 16.7% | 18 100.0% | |
| Total | Frequency % | 8 22.2% | 11 30.6% | 13 36.1% | 4 11.1% | 36 100.0% | |
| Developmental level–assessment at infants age of 6 months | |||||||
| 3 | 4 | 5 | Total | ||||
| Hypotonic form | Frequency % | 1 5.6% | 9 50.0% | 8 44.4% | 18 100.0% | 0.022 | |
| Spastic form | Frequency % | 5 27.8% | 2 11.1% | 11 61.1% | 18 100.0% | ||
| Total | Frequency % | 6 16.7% | 11 30.6% | 19 52.8% | 36 100.0% | ||
| Developmental level–assessment at infants age of 9 months | |||||||
| 7 | 8 | 9 | Total | ||||
| Hypotonic form | Frequency % | 9 50.0% | 5 27.8% | 4 22.2% | 18 100.0% | 0.593 | |
| Spastic form | Frequency % | 6 33.3% | 7 38.9% | 5 27.8% | 18 100.0% | ||
| Total | Frequency % | 15 41.7% | 12 33.3% | 9 25.0% | 36 100.0% | ||
| Age–Initial Assessment | Developmental Level–Assessment at Infants Age of 3 Months | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total | p | ||
| 1 | Frequency % | 0 0.0% | 4 57.1% | 3 42.9% | 0 0.0% | 7 100.0% | 0.005 |
| 2 | Frequency % | 0 0.0% | 5 45.5% | 6 54.5% | 0 0.0% | 11 100.0% | |
| 3 | Frequency % | 8 44.4% | 2 11.1% | 4 22.2% | 4 22.2% | 18 100.0% | |
| Total | Frequency % | 8 22.2% | 11 30.6% | 13 36.1% | 4 11.1% | 36 100.0% | |
| Developmental level–assessment at infants age of 6 months | |||||||
| 3 | 4 | 5 | Total | ||||
| 1 | Frequency % | 0 0.0% | 4 57.1% | 3 42.9% | 7 100.0% | 0.029 | |
| 2 | Frequency % | 0 0.0% | 5 45.5% | 6 54.5% | 11 100.0% | ||
| 3 | Frequency % | 6 33.3% | 2 11.1% | 10 55.6% | 18 100.0% | ||
| Total | Frequency % | 6 16.7% | 11 30.6% | 19 52.8% | 36 100.0% | ||
| Developmental level–assessment at infants age of 9 months | |||||||
| 7 | 8 | 9 | Total | ||||
| 1 | Frequency % | 4 57.1% | 1 14.3% | 2 28.6% | 7 100.0% | 0.673 | |
| 2 | Frequency % | 3 27.3% | 5 45.5% | 3 27.3% | 11 100.0% | ||
| 3 | Frequency % | 8 44.4% | 6 33.3% | 4 22.2% | 18 100.0% | ||
| Total | Frequency % | 15 41.7% | 12 33.3% | 9 25.0% | 36 100.0% | ||
| Level of Development at Infants Age of 3 Months/Weight (g) | ||||
|---|---|---|---|---|
| N | Mediate | Standard Deviation | p | |
| 0 | 8 | 1738.75 | 150,849 | 0.002 |
| 1 | 11 | 1851.82 | 175,375 | |
| 2 | 13 | 2053.08 | 196,909 | |
| 3 | 4 | 1830.00 | 55,976 | |
| Development level at infants age of 6 months/Weight (g) | ||||
| 3 | 6 | 1768.33 | 164,367 | 0.125 |
| 4 | 11 | 1864.55 | 163,668 | |
| 5 | 19 | 1956.32 | 225,862 | |
| Development level at infants age of 9 months/Weight (g) | ||||
| 7 | 15 | 1802.67 | 126,517 | 0.029 |
| 8 | 12 | 1917.50 | 253,812 | |
| 9 | 9 | 2026.67 | 185,876 | |
| Total | 36 | 1896.94 | 206,948 | |
| Age at Assessment Initial | N | Mediate | Standard Deviation | p | |
|---|---|---|---|---|---|
| Developmental level: evaluation at infants age of 3 months | 1 | 7 | 1.43 | 0.535 | 0.678 |
| 2 | 11 | 1.55 | 0.522 | ||
| 3 | 18 | 1.22 | 1.263 | ||
| Total | 36 | 1.36 | 0.961 | ||
| Developmental level: evaluation at infants age of 6 months | 1 | 7 | 4.43 | 0.535 | 0.536 |
| 2 | 11 | 4.55 | 0.522 | ||
| 3 | 18 | 4.22 | 0.943 | ||
| Total | 36 | 4.36 | 0.762 | ||
| Developmental level: evaluation at infants age of 9 months | 1 | 7 | 7.71 | 0.951 | 0.716 |
| 2 | 11 | 8.00 | 0.775 | ||
| 3 | 18 | 7.78 | 0.808 | ||
| Total | 36 | 7.83 | 0.811 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parau, D.; Todoran, A.B.; Balasa, R. Physiotherapy Intervention on Premature Infants—A Pilot Study. Medicina 2024, 60, 138. https://doi.org/10.3390/medicina60010138
Parau D, Todoran AB, Balasa R. Physiotherapy Intervention on Premature Infants—A Pilot Study. Medicina. 2024; 60(1):138. https://doi.org/10.3390/medicina60010138
Chicago/Turabian StyleParau, Daniela, Anamaria Butila Todoran, and Rodica Balasa. 2024. "Physiotherapy Intervention on Premature Infants—A Pilot Study" Medicina 60, no. 1: 138. https://doi.org/10.3390/medicina60010138
APA StyleParau, D., Todoran, A. B., & Balasa, R. (2024). Physiotherapy Intervention on Premature Infants—A Pilot Study. Medicina, 60(1), 138. https://doi.org/10.3390/medicina60010138






