Exploring the Interplay of the CRISPR-CAS System with Antibiotic Resistance in Staphylococcus aureus: A Poultry Meat Study from Lahore, Pakistan
Abstract
1. Introduction
2. Results
2.1. Bacterial Isolates Conformation
2.2. Antibiotic Resistance Pattern
2.3. Detection of Antibiotic Resistance Genes
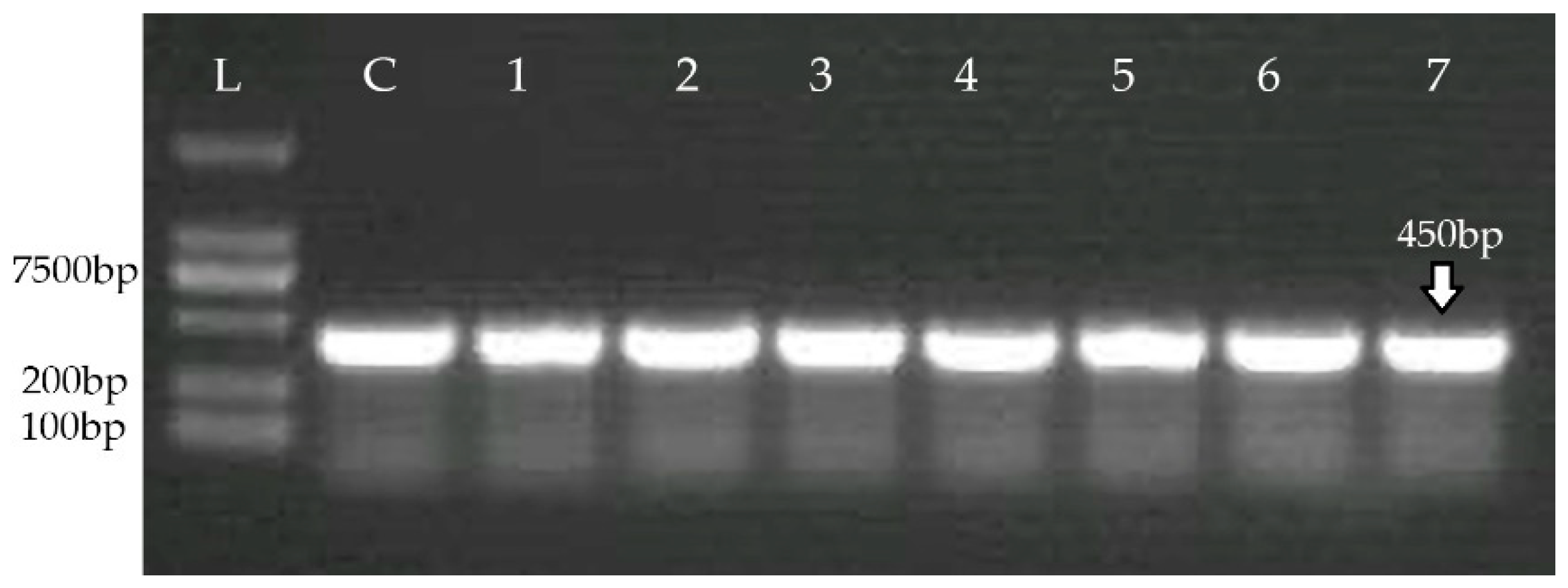
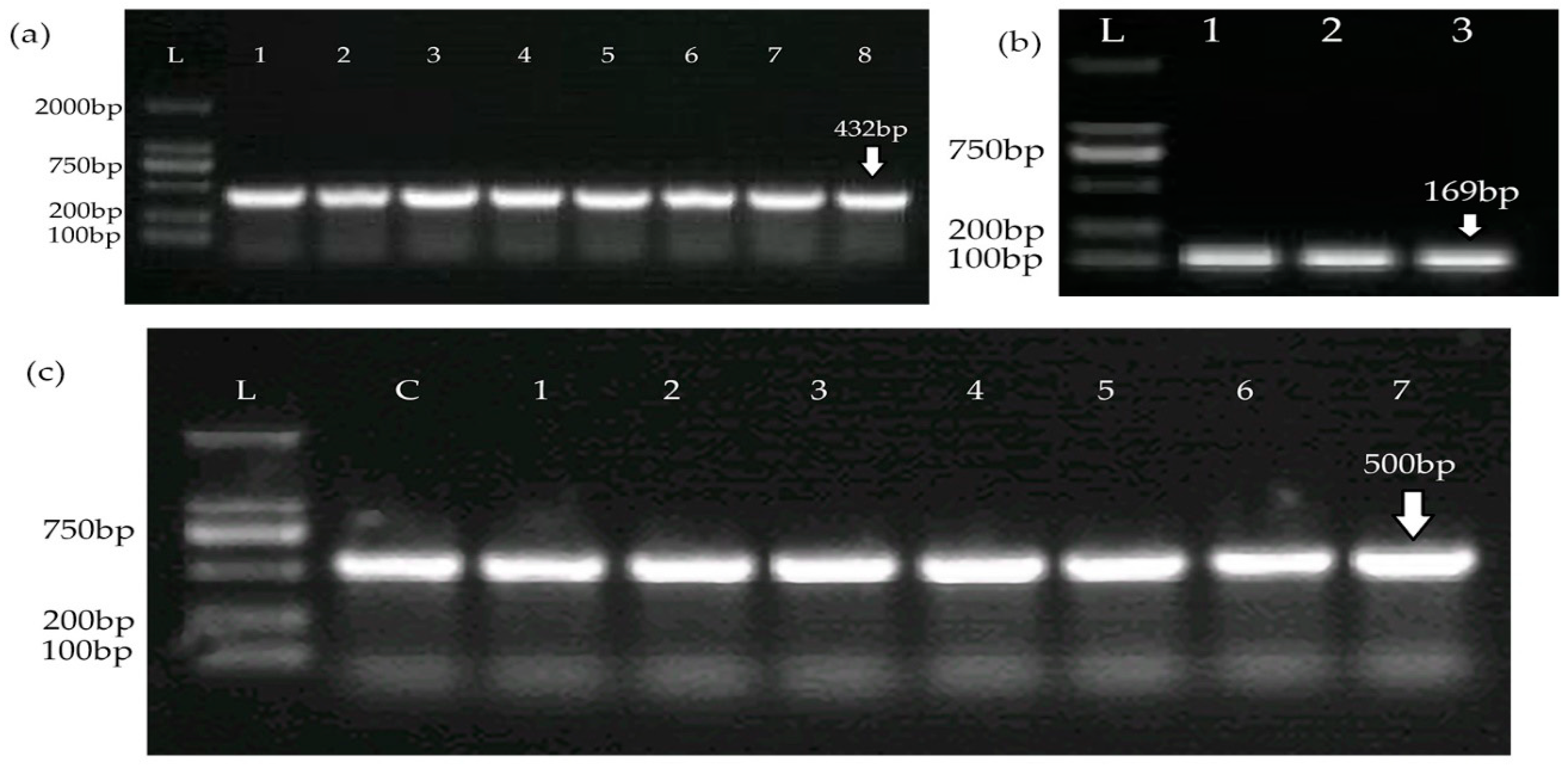
2.4. Detection of CRISPR-Cas System
2.5. Identification of Spacers and Analysis of Chicken Meat Isolates of S. aureus
2.6. Expression of CRISPR-Cas Genes in Standard and MDR Strains Exposed to Antimicrobials
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolation and Growth Conditions
4.2. Identification of S. aureus Isolates
4.3. Antibiotic Resistance Profiling
4.4. Detection of Antibiotic Resistance Genes
4.5. Detection of CRISPR-Cas System
4.6. Detection of CRISPR Spacers
4.7. Determination of CRISPR Gene Expression via qRT-PCR after Exposure to Antibiotics
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, T.; Ameer, H.A.; Javed, S. Pakistan’s backyard poultry farming initiative: Impact analysis from a public health perspective. Trop. Anim. Health Prod. 2021, 53, 210. [Google Scholar] [CrossRef] [PubMed]
- Parvin, M.S.; Ali, M.Y.; Talukder, S.; Nahar, A.; Chowdhury, E.H.; Rahman, M.T.; Islam, M.T. Prevalence and multidrug resistance pattern of methicillin resistant S. aureus isolated from frozen chicken meat in Bangladesh. Microorganisms 2021, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.F.; Abbas, M.A.; Ghafoor, T.; Dil, S.; Shahid, M.A.; Bullo, M.M.; Ain, Q.U.; Ranjha, M.A.; Khan, M.A.; Naseem, M.T. Seroprevalence and risk factors of avian influenza H9 virus among poultry professionals in Rawalpindi, Pakistan. J. Infect. Public Health 2019, 12, 482–485. [Google Scholar] [CrossRef]
- Szafraniec, G.M.; Szeleszczuk, P.; Dolka, B. Review on skeletal disorders caused by Staphylococcus spp. in poultry. Vet. Q. 2022, 42, 21–40. [Google Scholar] [CrossRef]
- Bilal, H.; Khan, M.N.; Rehman, T.; Hameed, M.F.; Yang, X. Antibiotic resistance in Pakistan: A systematic review of past decade. BMC Infect. Dis. 2021, 21, 244. [Google Scholar] [CrossRef]
- Mkize, N.; Zishiri, O.T.; Mukaratirwa, S. Genetic characterisation of antimicrobial resistance and virulence genes in Staphylococcus aureus isolated from commercial broiler chickens in the Durban metropolitan area, South Africa. J. S. Afr. Vet. Assoc. 2017, 88, 1–7. [Google Scholar] [CrossRef]
- Schmidt, T.; Kock, M.M.; Ehlers, M.M. Molecular characterization of Staphylococcus aureus isolated from bovine mastitis and close human contacts in South African dairy herds: Genetic diversity and inter-species host transmission. Front. Microbiol. 2017, 8, 511. [Google Scholar] [CrossRef] [PubMed]
- Fluit, A.C. Livestock-associated Staphylococcus aureus. Clin. Microbiol. Infect. 2012, 18, 735–744. [Google Scholar] [CrossRef]
- Kim, Y.B.; Seo, K.W.; Jeon, H.Y.; Lim, S.K.; Lee, Y.J. Characteristics of the antimicrobial resistance of Staphylococcus aureus isolated from chicken meat produced by different integrated broiler operations in Korea. Poult. Sci. 2018, 97, 962–969. [Google Scholar] [CrossRef]
- Iskandar, K.; Murugaiyan, J.; Hammoudi Halat, D.; Hage, S.E.; Chibabhai, V.; Adukkadukkam, S.; Roques, C.; Molinier, L.; Salameh, P.; Van Dongen, M. Antibiotic discovery and resistance: The chase and the race. Antibiotics 2022, 11, 182. [Google Scholar] [CrossRef]
- Mubarak, N.; Arif, S.; Irshad, M.; Aqeel, R.M.; Khalid, A.; Ijaz, U.e.B.; Mahmood, K.; Jamshed, S.; Zin, C.S.; Saif-Ur-Rehman, N. How Are We Educating Future Physicians and Pharmacists in Pakistan? A Survey of the Medical and Pharmacy Student’s Perception on Learning and Preparedness to Assume Future Roles in Antibiotic Use and Resistance. Antibiotics 2021, 10, 1204. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Sukiman, M.Z.; Kamarun Baharin, A.H.; Ramlan, I.; Lai, L.Z.; Liew, Y.; Malayandy, P.; Mohamad, N.M.; Choong, S.; Ariffin, S.M.Z.; et al. Methicillin-resistant Staphylococcus aureus from peninsular Malaysian animal handlers: Molecular profile, antimicrobial resistance, immune evasion cluster and genotypic categorization. Antibiotics 2022, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Caron, K.; Craw, P.; Richardson, M.B.; Bodrossy, L.; Voelcker, N.H.; Thissen, H.; Sutherland, T.D. The Requirement of Genetic Diagnostic Technologies for Environmental Surveillance of Antimicrobial Resistance. Sensors 2021, 21, 6625. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.A.B.; Wu, Q.; Mahmood, S.; Sajid, A.; Maan, M.K.; Ahmed, S.; Naveed, U.; Hao, H.; Yuan, Z. CRISPR-cas system: Biological function in microbes and its use to treat antimicrobial resistant pathogens. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 21. [Google Scholar] [CrossRef]
- Achek, R.; Hotzel, H.; Cantekin, Z.; Nabi, I.; Hamdi, T.M.; Neubauer, H.; El-Adawy, H. Emerging of antimicrobial resistance in staphylococci isolated from clinical and food samples in Algeria. BMC Res Notes 2018, 11, 663. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, B.R.; Singh, R.S. Genetic determinants of antibiotic resistance in Staphylococcus aureus isolates from milk of mastitic crossbred cattle. Curr. Microbiol. 2010, 60, 379–386. [Google Scholar] [CrossRef]
- Shehreen, S.; Chyou, T.; Fineran, P.C.; Brown, C.M. Genome-wide correlation analysis suggests different roles of CRISPR-Cas systems in the acquisition of antibiotic resistance genes in diverse species. Philos. Trans. R. Soc. B 2019, 374, 20180384. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Gao, C.-H.; Zhu, J.; Zhao, L.; Wu, Q.; Li, M.; Sun, B. Identification and functional study of type III-A CRISPR-Cas systems in clinical isolates of Staphylococcus aureus. Int. J. Med. Microbiol. 2016, 306, 686–696. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Xu, Z. Study the features of 57 confirmed CRISPR loci in 38 strains of Staphylococcus aureus. Front. Microbiol. 2018, 9, 1591. [Google Scholar] [CrossRef]
- Li, Q.; Xie, X.; Yin, K.; Tang, Y.; Zhou, X.; Chen, Y.; Xia, J.; Hu, Y.; Ingmer, H.; Li, Y.; et al. Characterization of CRISPR-Cas system in clinical Staphylococcus epidermidis strains revealed its potential association with bacterial infection sites. Microbiol. Res. 2016, 193, 103–110. [Google Scholar] [CrossRef]
- Sampson, T.R.; Napier, B.A.; Schroeder, M.R.; Louwen, R.; Zhao, J.; Chin, C.-Y.; Ratner, H.K.; Llewellyn, A.C.; Jones, C.L.; Laroui, H.; et al. A CRISPR-Cas system enhances envelope integrity mediating antibiotic resistance and inflammasome evasion. Proc. Natl. Acad. Sci. USA 2014, 111, 11163–11168. [Google Scholar] [CrossRef]
- Bikard, D.; Hatoum-Aslan, A.; Mucida, D.; Marraffini, L.A. CRISPR interference can prevent natural transformation and virulence acquisition during in vivo bacterial infection. Cell Host Microbe 2012, 12, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Hatoum-Aslan, A.; Marraffini, L.A. Impact of CRISPR immunity on the emergence and virulence of bacterial pathogens. Curr. Opin. Microbiol. 2014, 17, 82–90. [Google Scholar] [CrossRef]
- Shabbir, M.A.B.; Wu, Q.; Shabbir, M.Z.; Sajid, A.; Ahmed, S.; Sattar, A.; Tang, Y.; Li, J.; Maan, M.K.; Hao, H.; et al. The CRISPR-cas system promotes antimicrobial resistance in Campylobacter jejuni. Future Microbiol. 2018, 13, 1757–1774. [Google Scholar] [CrossRef] [PubMed]
- Dweba, C.C.; Zishiri, O.T.; El Zowalaty, M.E. Isolation and molecular identification of virulence, antimicrobial and heavy metal resistance genes in livestock-associated methicillin-resistant Staphylococcus aureus. Pathogens 2019, 8, 79. [Google Scholar] [CrossRef]
- Louwen, R.; Staals, R.H.J.; Endtz, H.P.; van Baarlen, P.; van der Oost, J. The role of CRISPR-Cas systems in virulence of pathogenic bacteria. Microbiol. Mol. Biol. Rev. 2014, 78, 74–88. [Google Scholar] [CrossRef]
- Strich, J.R.; Chertow, D.S. CRISPR-Cas biology and its application to infectious diseases. J. Clin. Microbiol. 2019, 57, 10–128. [Google Scholar] [CrossRef]
- Mortensen, K.; Lam, T.J.; Ye, Y. Comparison of CRISPR–Cas immune systems in healthcare-related pathogens. Front. Microbiol. 2021, 12, 758782. [Google Scholar] [CrossRef] [PubMed]
- Torki Baghbaderani, Z.; Shakerian, A.; Rahimi, E. Phenotypic and genotypic assessment of antibiotic resistance of Staphylococcus aureus bacteria isolated from retail meat. Infect. Drug Resist. 2020, 13, 1339–1349. [Google Scholar] [CrossRef]
- Bala, H.K.; Igwe, J.C.; Onaolapo, J.A. Molecular characterization of Methicillin resistant Staph. aureus from poultry farms in Kano State, Nigeria. Bayero J. Pure Appl. Sci. 2019, 12, 359–365. [Google Scholar] [CrossRef]
- Amen, O.; Hussein, A.; Ibrahim, R.; Sayed, A. Detection of antibiotics resistance genes in Staphylococcus aureus isolated from poultry farms. Assiut Vet. Med. J. 2019, 65, 1–9. [Google Scholar] [CrossRef]
- Suleiman, A.; Zaria, L.T.; Grema, H.A.; Ahmadu, P. Antimicrobial resistant coagulase positive Staphylococcus aureus from chickens in Maiduguri, Nigeria. Sokoto J. Vet. Sci. 2013, 11, 51–55. [Google Scholar] [CrossRef]
- Momtaz, H.; Dehkordi, F.S.; Rahimi, E.; Asgarifar, A.; Momeni, M. Virulence genes and antimicrobial resistance profiles of Staphylococcus aureus isolated from chicken meat in Isfahan province, Iran. J. Appl. Poult. Res. 2013, 22, 913–921. [Google Scholar] [CrossRef]
- Benrabia, I.; Hamdi, T.M.; Shehata, A.A.; Neubauer, H.; Wareth, G. Methicillin-resistant Staphylococcus aureus (MRSA) in poultry species in Algeria: Long-term study on prevalence and antimicrobial resistance. Vet Sci 2020, 7, 54. [Google Scholar] [CrossRef]
- Ruban, S.W.; Babu, R.N.; Abraham, R.J.; Senthilkumar TM, A.; Kumaraswamy, P.; Porteen, K.; Vemala, G. Prevalence and Antimicrobial Susceptibility of Staphylococcus aureus Isolated from Retail Chicken Meat in Chennai, India. J. Anim. Res. 2018, 8, 423–427. [Google Scholar] [CrossRef]
- Ruban, S.W.; Babu, R.N.; Abraham, R.J.; Senthilkumar TM, A.; Kumaraswamy, P.; Rao, V.A.; Porteen, K. Prevalence of panton valentine leukocidin (PVL) gene in methicillin resistant Staphylococcus aureus isolated from market samples of chicken meat. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2459–2466. [Google Scholar]
- Murugesan, A.C.; Varughese, H.S. Analysis of CRISPR–Cas system and antimicrobial resistance in Staphylococcus coagulans isolates. Lett. Appl. Microbiol. 2022, 75, 126–134. [Google Scholar] [CrossRef]
- Palmer, K.L.; Gilmore, M.S. Multidrug-resistant enterococci lack CRISPR-cas. MBio 2010, 1, 10–128. [Google Scholar] [CrossRef]
- Cruz-López, E.A.; Rivera, G.; Cruz-Hernández, M.A.; Martínez-Vázquez, A.V.; Castro-Escarpulli, G.; Flores-Magallón, R.; Vázquez-Cisneros, K.; Cruz-Pulido, W.L.; Bocanegra-García, V. Identification and characterization of the CRISPR/Cas System in Staphylococcus aureus strains from diverse sources. Front. Microbiol. 2021, 12, 656996. [Google Scholar] [CrossRef]
- McGinn, J.; Marraffini, L.A. Molecular mechanisms of CRISPR–Cas spacer acquisition. Nat. Rev. Microbiol. 2019, 17, 7–12. [Google Scholar] [CrossRef]
- Samson, J.E.; Magadan, A.H.; Moineau, S. The CRISPR-Cas immune system and genetic transfers: Reaching an equilibrium. Microbiol. Spectr. 2015, 3, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kitai, S.; Shimizu, A.; Kawano, J.; Sato, E.; Nakano, C.; Kitagawa, H.; Fujio, K.; Matsumura, K.; Yasuda, R.; Inamoto, T. Prevalence and characterization of Staphylococcus aureus and enterotoxigenic Staphylococcus aureus in retail raw chicken meat throughout Japan. J. Vet. Med. Sci. 2005, 67, 269–274. [Google Scholar] [CrossRef]
- Zakki, S.A.; Qureshi, R.; Hussain, A.; Ghias, W.; Sharif, M.; Ansari, F. Microbial quality evaluation and prevalence of bacteria and fungus in different varieties of chicken meat in Lahore. RADS J. Pharm. Pharm. Sci. 2017, 5, 30–37. [Google Scholar]
- Li, Q.; Li, Y.; Tang, Y.; Meng, C.; Ingmer, H.; Jiao, X. Prevalence and characterization of Staphylococcus aureus and Staphylococcus argenteus in chicken from retail markets in China. Food Control 2019, 96, 158–164. [Google Scholar] [CrossRef]
- El-Hadedy, D.; El-Nour, S.A. Identification of Staphylococcus aureus and Escherichia coli isolated from Egyptian food by conventional and molecular methods. J. Genet. Eng. Biotechnol. 2012, 10, 129–135. [Google Scholar] [CrossRef]
- Harrigan, W.F. Laboratory Methods in Food Microbiology; Gulf Professional Publishing: Houston, TX, USA, 1998. [Google Scholar]
- Akbar, A.; Anal, A.K. Prevalence and antibiogram study of Salmonella and Staphylococcus aureus in poultry meat. Asian Pac. J. Trop. Biomed. 2013, 3, 163–168. [Google Scholar] [CrossRef]
- Li, F.; Xie, G.; Zhou, B.; Yu, P.; Yu, S.; Aguilar, Z.P.; Wei, H.; Xu, H. Rapid and simultaneous detection of viable Cronobacter sakazakii, Staphylococcus aureus, and Bacillus cereus in infant food products by PMA-mPCR assay with internal amplification control. LWT 2016, 74, 176–182. [Google Scholar] [CrossRef]
- Approved Standard M100-S20; FRC Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010.
- Wang, G.; Song, G.; Xu, Y. Association of CRISPR/Cas system with the drug resistance in Klebsiella pneumoniae. Infect. Drug Resist. 2020, 13, 1929–1935. [Google Scholar] [CrossRef]


| Antibiotic | Disk Concentration (µg) | Antibiotic Resistance Profile | ||
|---|---|---|---|---|
| n = 40 | ||||
| Susceptible (%) | Intermediate (%) | Resistant (%) | ||
| CHL | 30 | 47.5 | 10 | 42.5 |
| CIP | 5 | 35 | 15 | 50 |
| CN | 10 | 65 | 7.5 | 27.5 |
| E | 15 | 0 | 0 | 100 |
| FOX | 30 | 10 | 15 | 75 |
| TE | 30 | 0 | 0 | 97.5 |
| VAN | 30 | 35 | 10 | 55 |
| Homology to Various Strains | |||||||
|---|---|---|---|---|---|---|---|
| Poultry Isolates | +S. aureus 08BA02176 (%) | +S. aureus AR-0470 (%) | +S. aureus 110900 (%) | +S. aureus AR-0473 (%) | +S. aureus WH39 (%) | +S. aureus JS395 (%) | +S. aureus AR-0472 (%) |
| S. A 1 | 99.74 | 98.9 | - | 99.74 | - | 99.74 | 99.74 |
| S. A 6 | 98.43 | 99 | - | - | 98.43 | 98.43 | - |
| S. A 12 | - | - | 98.49 | 98.66 | - | - | 98.66 |
| S.A 26 | 99.6 | - | 99.6 | - | 99.6 | - | - |
| Target Gene | Primers (5′……3′) | Annealing Temperature | Amplicon (bp) | Reference |
|---|---|---|---|---|
| Nuc | F: AGTATATAGTGCAACTTCAACTAAA | 55 | 450 | [2] |
| R: ATCAGCGTTGTCTTCGCTCCAAATA | ||||
| mecA | F: AAAATCGATGGTAAAGGTTGGC | 55 | 532 | [5] |
| R: AGTTCTGCAGTACCGGATTTGC | ||||
| tetM | F: AGTGGAGCGATTACAGAA | 55 | 158 | [15] |
| R: CATATGTCCTGGCGTGTCTA | ||||
| gyrA | F: AATGAACAAGGTATGACACC | 55 | 223 | [8] |
| R: TACGCGCTTCAGTATAACGC | ||||
| ermA | F: AAGCGGTAAACCCCTCTGA | 55 | 190 | [27] |
| R: TTCGCAAATCCCTTCTCAAC | ||||
| Cas1 | F: GCACTCTCCATTAACGCAACT | 54 | 432 | This study |
| R: AGGGGTGTTTTCTTCATAGCA | ||||
| Cas2 | F: ACGAGAGGTATGTCAGCGAT | 54 | 169 | This study |
| R: GGTTCTTTTCGCACAACAACC | ||||
| Cas10 | F: AGAAGAGGGCGACGAAGAAT | 54 | 500 | This study |
| R: ACTGCTGACATTACGCCAAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabbir, M.A.B.; Ul-Rahman, A.; Iftikhar, M.R.; Rasheed, M.; Maan, M.K.; Sattar, A.; Ahmad, M.; Khan, F.A.; Ahmad, W.; Riaz, M.I.; et al. Exploring the Interplay of the CRISPR-CAS System with Antibiotic Resistance in Staphylococcus aureus: A Poultry Meat Study from Lahore, Pakistan. Medicina 2024, 60, 130. https://doi.org/10.3390/medicina60010130
Shabbir MAB, Ul-Rahman A, Iftikhar MR, Rasheed M, Maan MK, Sattar A, Ahmad M, Khan FA, Ahmad W, Riaz MI, et al. Exploring the Interplay of the CRISPR-CAS System with Antibiotic Resistance in Staphylococcus aureus: A Poultry Meat Study from Lahore, Pakistan. Medicina. 2024; 60(1):130. https://doi.org/10.3390/medicina60010130
Chicago/Turabian StyleShabbir, Muhammad Abu Bakr, Aziz Ul-Rahman, Muhammad Rizwan Iftikhar, Majeeda Rasheed, Muhammad Kashif Maan, Adeel Sattar, Mehmood Ahmad, Farid Ahmed Khan, Waqas Ahmad, Muhammad Ilyas Riaz, and et al. 2024. "Exploring the Interplay of the CRISPR-CAS System with Antibiotic Resistance in Staphylococcus aureus: A Poultry Meat Study from Lahore, Pakistan" Medicina 60, no. 1: 130. https://doi.org/10.3390/medicina60010130
APA StyleShabbir, M. A. B., Ul-Rahman, A., Iftikhar, M. R., Rasheed, M., Maan, M. K., Sattar, A., Ahmad, M., Khan, F. A., Ahmad, W., Riaz, M. I., & Aslam, H. B. (2024). Exploring the Interplay of the CRISPR-CAS System with Antibiotic Resistance in Staphylococcus aureus: A Poultry Meat Study from Lahore, Pakistan. Medicina, 60(1), 130. https://doi.org/10.3390/medicina60010130






