Identifying the Risk Factors for Orbital Complications in Isolated Sphenoid Rhinosinusitis
Abstract
1. Introduction
2. Materials and Methods
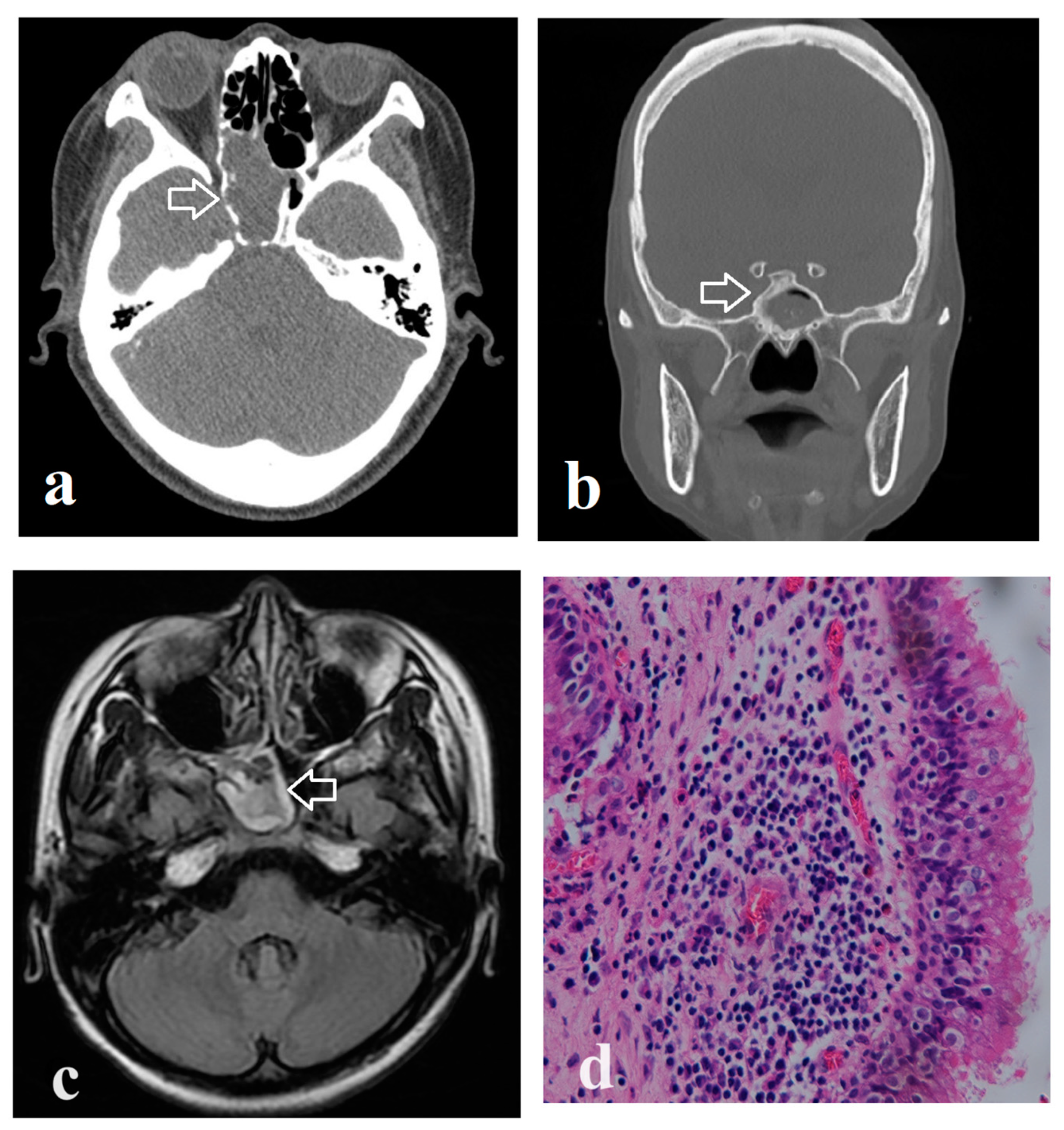
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppilasalmi, S.; Bernalsprekelsen, M.; Mullol, J.; Alobid, I. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef]
- Hellings, P.; Fokkens, W.; Orlandi, R.; Adriaensen, G.; Alobid, I.; Baroody, F.; Bjermer, L.; Senior, B.; Cervin, A.; Cohen, N.; et al. The EUFOREA pocket guide for chronic rhinosinusitis. Rhinology 2023, 61, 85–89. [Google Scholar] [CrossRef]
- Fooanant, S.; Angkurawaranon, S.; Angkurawaranon, C.; Roongrotwattanasiri, K.; Chaiyasate, S. Sphenoid Sinus Diseases: A Review of 1442 Patients. Int. J. Otolaryngol. 2017, 2017, 9650910. [Google Scholar]
- Krishnan, R.D.Y.; Kumarasekaran, P.; Raja, R.V. Isolated Sphenoid Sinusitis. Indian J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. S3), 1762–1764. [Google Scholar] [CrossRef]
- Fadda, G.L.; D’Eramo, A.; Grosso, A.; Galizia, A.; Cavallo, G. Isolated Sphenoid Sinus Inflammatory Disease-A Report of 14 Cases. Iran. J. Otorhinolaryngol. 2020, 32, 101–107. [Google Scholar]
- Fan, Y.; Wu, P.; Huang, Y.; Lee, C.; Lee, T.; Huang, C.; Chang, P. Identifying a sphenoid sinus fungus ball using a nomogram model. Rhinology 2023, 61, 153–160. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, D.W.; Kong, I.G.; Kim, D.-Y.; Park, S.-W.; Rhee, C.-S.; Min, Y.-G. Isolated sphenoid sinus diseases: Report of 76 cases. Acta Otolaryngol. 2008, 128, 455–459. [Google Scholar]
- Charakorn, N.; Snidvongs, K. Chronic sphenoid rhinosinusitis: Management challenge. J. Asthma Allergy. 2016, 9, 199–205. [Google Scholar] [CrossRef]
- Nour, Y.A.; Al-Madani, A.; El-Daly, A.; Gaafar, A. Isolated sphenoid sinus pathology: Spectrum of diagnostic and treatment modalities. Auris Nasus Larynx 2008, 35, 500–508. [Google Scholar] [CrossRef]
- Hu, L.; Wang, D.; Yu, H. Isolated sphenoid fungal sinusitis and vision loss: The case for early intervention. J. Laryngol. Otol. 2009, 123, e8. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, L.; Yang, B.; Subramanian, P.S. Clinical features of visual disturbances secondary to isolated sphenoid sinus inflammatory diseases. BMC Ophthalmol. 2017, 17, 237. [Google Scholar] [CrossRef]
- Suarez, R.I.; Polmann, M.B.; Portnoy, W.M.; Quintero, E.; Bedran, K. Isolated Abducens Nerve Palsy in the Setting of Isolated Sphenoid Sinusitis: A Case Report. Cureus 2023, 15, e46993. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.S.; Mulla, O. Lateral rectus muscle palsy secondary to sphenoid sinusitis. Ann. R. Coll. Surg. Engl. 2022, 104, e239–e243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Chen, P.Y.; Ting, P.J.; Huang, F.L. A review of eight cases of cavernous sinus thrombosis secondary to sphenoid sinusitis, including a 12-year-old girl at the present department. Infect. Dis. 2017, 49, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Dua, K.; Malhotra, V.; Gupta, R.P.; Puri, H. Invasive fungal sinusitis of isolated sphenoid sinus in immunocompetent subjects. Mycoses 2006, 49, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Huang, Y.; Yang, S.; Lee, Y.; Huang, C.; Chang, P.; Lee, T. Acute invasive fungal rhinosinusitis in twenty-one diabetic patients. Clin. Otolaryngol. 2018, 43, 1163–1167. [Google Scholar] [CrossRef]
- Montone, K.T. Pathology of fungal rhinosinusitis: A review. Head Neck Pathol. 2016, 10, 40–46. [Google Scholar] [CrossRef]
- Ada, M.; Kaytaz, A.; Tuskan, K.; Güvenç, M.G.; Selçuk, H. Isolated sphenoid sinusitis presenting with unilateral VIth nerve palsy. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 507–510. [Google Scholar] [CrossRef]
- Nowacka, B.; Lubiński, W.; Lubiński, J. Optic Neuritis Related to Chronic Sphenoid Sinusitis as an Uncommon Cause of Vision Loss: A Case Report and Literature Review. Am. J. Case Rep. 2023, 24, e939267. [Google Scholar] [CrossRef]
- Celenk, F.; Gulsen, S.; Gonuldas, B.; Baysal, E.; Durucu, C.; Kanlikama, M.; Mumbuc, S. Isolated sphenoid sinus disease: An overlooked cause of headache. J. Craniomaxillofac. Surg. 2015, 43, 1914–1917. [Google Scholar] [CrossRef]
- Wu, P.-W.; Lee, T.-J.; Yang, S.-W.; Huang, Y.; Lee, Y.-S.; Ho, C.-F.; Huang, C.-C. Differences in clinical and imaging presentation of maxillary sinus fungus ball with and without intralesional hyperdensity. Sci. Rep. 2021, 11, 23945. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-H.; Shih, K.-Y.; Wu, P.-W.; Huang, Y.-L.; Lee, T.-J.; Huang, C.-C.; Chang, P.-H.; Huang, C.-C. Predicting the Probability of the Incidence of Maxillary Sinus Fungus Ball in Patients Using Nomogram Models. Diagnostics 2023, 13, 3156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, Q.; Li, C.; Li, T.; Sun, S.; Chen, A.; Ji, H.; Wan, Y.; Shi, L.; Yu, L. Clinical Characteristics of Sphenoid Sinus Fungus Ball: A Nine-year Retrospective Study of 77 Cases. Laryngoscope 2023, 133, 3292–3298. [Google Scholar] [CrossRef]
- Shen, T.Y.; Strong, C.; Yu, T. Age at menopause and mortality in Taiwan: A cohort analysis. Maturitas 2020, 136, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Haeggström, A.; Östberg, B.; Stjerna, P.; Graf, P.; Hallén, H. Nasal mucosal swelling and reactivity during a menstrual cycle. ORL-J. Oto-Rhino-Laryngol. Head Neck Surg. 2000, 62, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Özler, G.S.; Akbay, E.; Akkoca, A.N.; Karapınar, O.S.; Şimşek, G. Does menopause effect nasal mucociliary clearance time? Eur. Arch. Otorhinolaryngol. 2015, 272, 363–366. [Google Scholar] [CrossRef]
- Gumussoy, S.; Gumussoy, M.; Hortu, I.; Ergenoglu, A.M. The effect of surgical menopause after bilateral oophorectomy on hormonal changes, mucociliary clearance, and quality of life. Eur. Arch Otorhinolaryngol. 2020, 277, 2793–2800. [Google Scholar] [CrossRef]
- Haimi-Cohen, Y.; Amir, J.; Zeharia, A.; Danziger, Y.; Ziv, N.; Mimouni, M. Isolated sphenoidal sinusitis in children. Eur. J. Pediatr. 1999, 158, 298–301. [Google Scholar] [CrossRef]
- Kotowski, M.; Szydlowski, J. Isolated Sphenoid Sinus Disease in Children. Int. J. Environ. Res. Public Health 2023, 20, 847. [Google Scholar] [CrossRef]
- Chiang, P.T.; Luo, S.D.; Ho, R.W.; Wu, C.N.; Fang, K.C.; Chen, W.C. A Multi-Institutional Database Review of Orbital Complications and Survival Outcomes in Adult Patients with Invasive or Non-Invasive Fungal Rhinosinusitis. J. Fungi 2022, 8, 1239. [Google Scholar] [CrossRef]
- Muszewska, A.; Pawłowska, J.; Krzyściak, P. Biology, systematics, and clinical manifestations of Zygomycota infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1273–1287. [Google Scholar] [CrossRef]
- Yohai, R.A.; Bullock, J.D.; Aziz, A.A.; Markert, R.J. Survival factors in rhino-orbital-cerebral mucormycosis. Surv. Ophthalmol. 1994, 39, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Nyunt, T.P.K.; Mullol, J.; Snidvongs, K. Immune response to fungi in diabetic patients with invasive fungal rhinosinusitis. Asian Pac. J. Allergy Immunol. 2020, 38, 233–238. [Google Scholar] [PubMed]
- Yaguchi, T.; Sumimoto, H.; Kudo-Saito, C.; Tsukamoto, N.; Ueda, R.; Iwata-Kajihara, T.; Nishio, H.; Kawamura, N.; Kawakami, Y. The mechanisms of cancer immunoescape and development of overcoming strategies. Int. J. Hematol. 2011, 93, 294–300. [Google Scholar] [CrossRef]
- Wu, P.-W.; Huang, C.-C.; Lee, Y.-S.; Chou, Y.-C.; Fan, K.-H.; Lin, C.-Y.; Huang, B.-S.; Yang, S.-W.; Huang, C.-C.; Chang, P.-H.; et al. Post-Irradiation Sinus Mucosa Disease in Nasopharyngeal Carcinoma Patients Treated with Intensity-Modulated Proton Therapy. Cancers 2022, 14, 225. [Google Scholar] [CrossRef]
- Munzen, M.E.; Goncalves Garcia, A.D.; Martinez, L.R. An update on the global treatment of invasive fungal infections. Future Microbiol. 2023, 18, 1095–1117. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.F.; Lee, T.J.; Wu, P.W.; Huang, C.C.; Chang, P.H.; Huang, Y.L.; Lee, Y.L.; Huang, C.C. Diagnosis of a maxillary sinus fungus ball without intralesional hyperdensity on computed tomography. Laryngoscope 2019, 129, 1041–1045. [Google Scholar] [CrossRef]
- Bhandarkar, N.D.; Sautter, N.B.; Kennedy, D.W.; Smith, T.L. Osteitis in chronic rhinosinusitis: A review of the literature. Int. Forum Allergy Rhinol. 2013, 3, 355–363. [Google Scholar] [CrossRef]
- Huang, C.C.; Wang, C.H.; Wu, P.W.; He, J.R.; Huang, C.C.; Chang, P.H.; Fu, C.H. Increased nasal matrix metalloproteinase-1 and -9 expression in smokers with chronic rhinosinusitis and asthma. Sci. Rep. 2019, 9, 15357. [Google Scholar] [CrossRef]
- Erlebacher, A.; Filvaroff, E.H.; Ye, J.Q.; Derynck, R. Osteoblastic responses to TGF-beta during bone remodeling. Mol. Biol. Cell 1998, 9, 1903–1918. [Google Scholar] [CrossRef]
- Lee, J.T.; Kennedy, D.W.; Palmer, J.N.; Feldman, M.; Chiu, A.G. The incidence of concurrent osteitis in patients with chronic rhinosinusitis: A clinicopathological study. Am. J. Rhinol. 2006, 20, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Snidvongs, K.; Sacks, R.; Harvey, R.J. Osteitis in Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2019, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Odat, H.; Almardeeni, D.; Tanash, M.; Al-Qudah, M. Anatomical variation of the sphenoid sinus in paediatric patients and its association with age and chronic rhinosinusitis. J. Laryngol. Otol. 2019, 133, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Grillone, G.A.; Kasznica, P. Isolated sphenoid sinus disease. Otolaryngol. Clin. N. Am. 2004, 37, 435–451. [Google Scholar] [CrossRef]
- Aribandi, M.; Bazan, C., 3rd. CT and MRI features in Bipolaris fungal sinusitis. Australas. Radiol. 2007, 51, 127–132. [Google Scholar] [CrossRef] [PubMed]
| Variables | Orbital Complication | No Orbital Complication | p Value |
|---|---|---|---|
| (n = 15) | (n = 103) | ||
| Age, years (mean ± SD) | 58.9 ± 21.1 | 52.6 ± 16.1 | 0.116 |
| Gender | |||
| Male, n | 6 (40.0%) | 33 (32.0%) | 0.565 |
| Female, n | 9 (60.0%) | 70 (68.0%) | |
| Site of sphenoid lesion | |||
| Left, n | 5 (33.3%) | 47 (45.6%) | 0.149 |
| Right, n | 8 (53.3%) | 53 (51.5%) | |
| Single sphenoid sinus, n | 2 (13.3%) | 3 (2.9%) | |
| Histopathology | |||
| Fungus ball, n | 7 (46.7%) | 62 (60.2%) | 0.403 |
| Non-fungus, n | 8 (53.3%) | 41 (39.8%) | |
| Laboratory data | |||
| WBC count, k per µl | 7.52 ± 1.79 | 6.83 ± 2.15 | 0.050 |
| Underlying conditions | |||
| Diabetes mellitus, n | 6 (40.0%) | 13 (12.6%) | 0.016 * |
| Malignant neoplasms, n | 4 (26.7%) | 8 (7.8%) | 0.046 * |
| Previous sphenoid sinus surgery, n | 2 (13.3%) | 19 (18.4%) | 1.000 |
| Clinical presentations | |||
| Headache and facial pain, n | 14 (93.3%) | 47 (45.6%) | <0.001 *** |
| Rhinorrhea, n | 3 (20.0%) | 49 (47.6%) | 0.054 |
| Purulent rhinorrhea, n | 3 (20.0%) | 25 (24.3%) | 1.000 |
| Bloody rhinorrhea, n | 0 (0%) | 11 (10.7%) | 0.355 |
| Nasal obstruction, n | 0 (0%) | 45 (43.7%) | <0.001 *** |
| Post nasal dripping, n | 2 (13.3%) | 40 (38.8%) | 0.081 |
| Hyposmia, n | 2 (13.3%) | 15 (14.6%) | 1.000 |
| Foul odor smell, n | 0 (0%) | 9 (8.7%) | 0.601 |
| Tinnitus, n | 0 (0%) | 2 (1.9%) | 1.000 |
| Incidental found, n | 0 (0%) | 8 (7.8%) | 0.594 |
| Features of CT image | |||
| Total opacification, n | 7 (46.7%) | 49 (47.6%) | 1.000 |
| Partial opacification, n | 8 (53.3%) | 54 (52.4%) | 1.000 |
| Irregular surface, n | 3 (20.0%) | 34 (33.0%) | 0.385 |
| Bony dehiscence, n | 10 (66.7%) | 30 (29.1%) | 0.007 ** |
| Lateral wall sclerosis, n | 9 (60.0%) | 58 (56.3%) | 1.000 |
| Intralesional hyperdensity, n | 4 (26.7%) | 38 (36.9%) | 0.569 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value |
| Characteristics of patients | ||||
| Age | 1.02 (0.99–1.06) | 0.179 | 1.00 (0.96–1.04) | 0.892 |
| Diabetes mellitus | 4.62 (1.41–15.10) | 0.011 * | 5.27 (1.40–19.93) | 0.014 * |
| Malignant neoplasms | 4.32 (1.12–16.71) | 0.034 * | 5.23 (1.17–23.31) | 0.030 * |
| Female sex | 0.71 (0.23–2.15) | 0.542 | ||
| WBC (x1000 per μL) | 1.14 (0.91–1.43) | 0.243 | ||
| CT imaging features | ||||
| Total opacification | 0.96(0.33–2.86) | 0.948 | ||
| Partial opacification | 1.04 (0.35–3.07) | 0.948 | ||
| Irregular surface | 0.51 (0.13–1.92) | 0.317 | ||
| Bony dehiscence | 4.87 (1.53–15.44) | 0.007 ** | 5.21 (1.44–18.84) | 0.012 * |
| Lateral wall sclerosis | 1.16 (0.39–3.51) | 0.788 | ||
| Intralesional hyperdensity | 0.62 (0.19–2.09) | 0.443 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-Y.; Huang, C.-C.; Fan, Y.-H.; Wu, P.-W.; Lee, T.-J.; Chang, P.-H.; Huang, C.-C. Identifying the Risk Factors for Orbital Complications in Isolated Sphenoid Rhinosinusitis. Medicina 2024, 60, 128. https://doi.org/10.3390/medicina60010128
Chang S-Y, Huang C-C, Fan Y-H, Wu P-W, Lee T-J, Chang P-H, Huang C-C. Identifying the Risk Factors for Orbital Complications in Isolated Sphenoid Rhinosinusitis. Medicina. 2024; 60(1):128. https://doi.org/10.3390/medicina60010128
Chicago/Turabian StyleChang, Shiaw-Yu, Chi-Che Huang, Yu-Hsi Fan, Pei-Wen Wu, Ta-Jen Lee, Po-Hung Chang, and Chien-Chia Huang. 2024. "Identifying the Risk Factors for Orbital Complications in Isolated Sphenoid Rhinosinusitis" Medicina 60, no. 1: 128. https://doi.org/10.3390/medicina60010128
APA StyleChang, S.-Y., Huang, C.-C., Fan, Y.-H., Wu, P.-W., Lee, T.-J., Chang, P.-H., & Huang, C.-C. (2024). Identifying the Risk Factors for Orbital Complications in Isolated Sphenoid Rhinosinusitis. Medicina, 60(1), 128. https://doi.org/10.3390/medicina60010128








