The Role of Laser Photocoagulation in Treating Diabetic Macular Edema in the Era of Intravitreal Drug Administration: A Descriptive Review
Abstract
1. Introduction
2. Focal/Grid Laser Therapy
2.1. ETDRS
2.2. DRCR Network
2.3. Can Focal/Grid Laser Therapy Reduce the Number of Anti-VEGF Injections?
2.4. Steroid vs. Focal/Grid Laser
3. New Laser Technologies
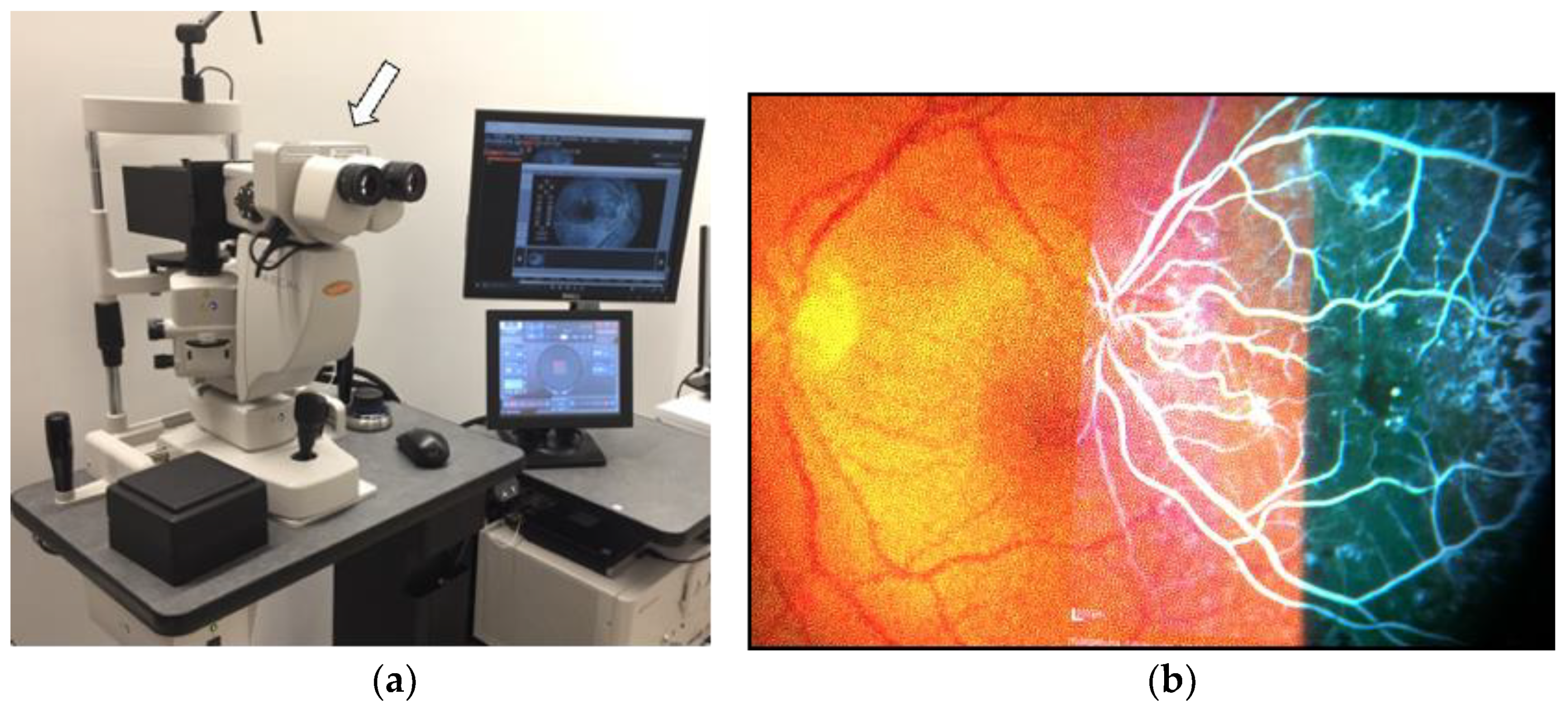
3.1. Navigated Laser Systems
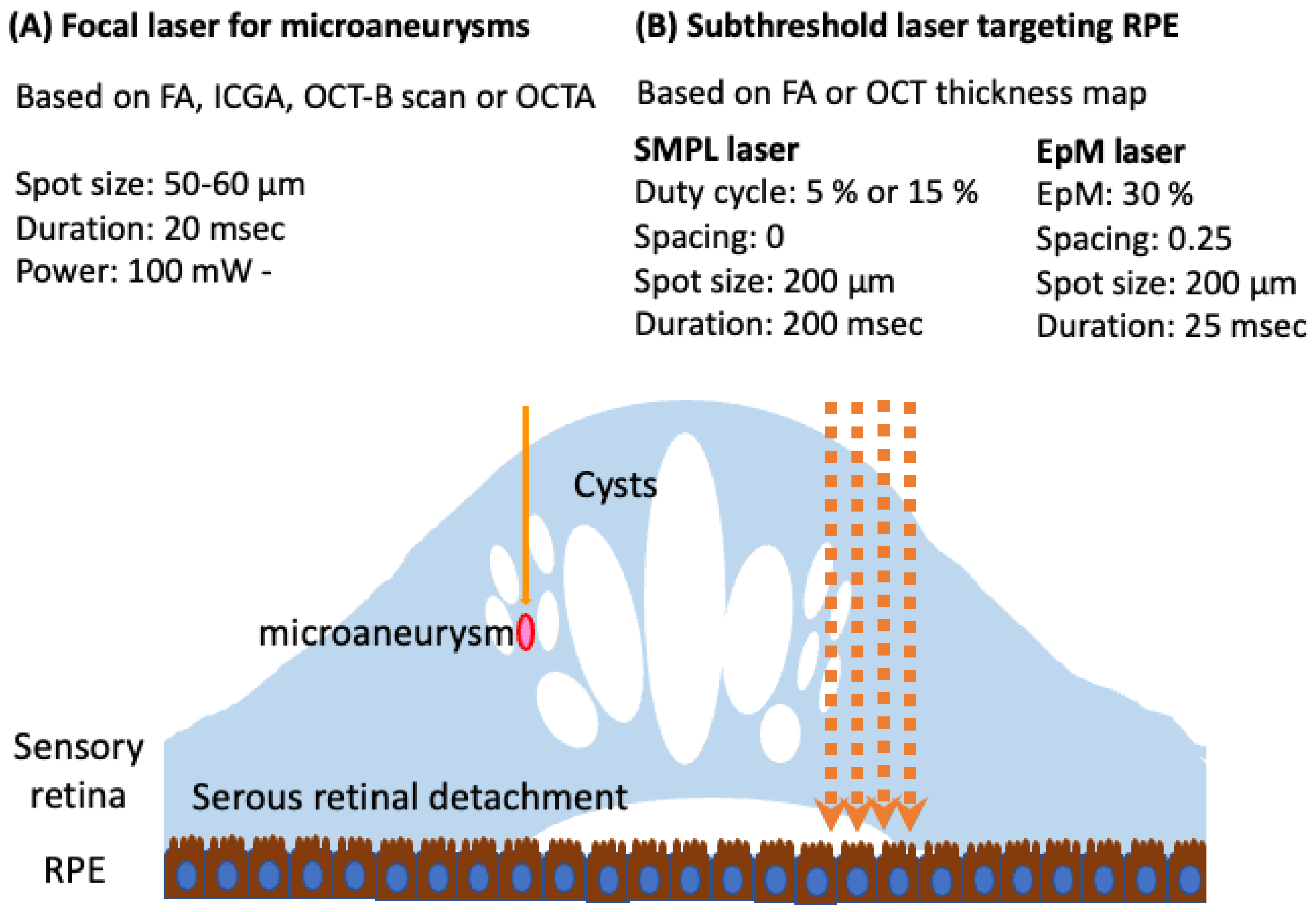
3.2. Subthreshold Laser
3.3. Pattern Scan Laser
4. Multimodal Imaging-Guided Laser Therapy
4.1. Indocyanine Green Angiography-Guided Laser Therapy
4.2. OCT-Guided Laser
4.3. Multimodal Imaging Integrated System
5. The Current Role of Laser Photocoagulation in the Era of Intravitreal Drug Administration
5.1. Comparison of Anti-VEGF/Steroid and Laser Treatment
5.2. A Real-World Approach: Laser Photocoagulation in Combination with Intravitreal Drug Administration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabanayagam, C.; Yip, W.; Ting, D.S.W.; Tan, G.; Wong, T.Y. Ten Emerging Trends in the Epidemiology of Diabetic Retinopathy. Ophthalmic Epidemiol. 2016, 23, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group: Photocoagulation for Diabetic Macular Edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch. Ophthalmol. 1985, 103, 1796–1806. [Google Scholar] [CrossRef]
- Ogura, Y.; Shiraga, F.; Terasaki, H.; Ohji, M.; Ishida, S.; Sakamoto, T.; Hirakata, A.; Ishibashi, T. Clinical practice pattern in management of diabetic macular edema in Japan: Survey results of Japanese retinal specialists. Jpn. J. Ophthalmol. 2017, 61, 43–50. [Google Scholar] [CrossRef]
- Sugimoto, M.; Tsukitome, H.; Okamoto, F.; Oshika, T.; Ueda, T.; Niki, M.; Mitamura, Y.; Ishikawa, H.; Gomi, F.; Kitano, S.; et al. Clinical preferences and trends of anti-vascular endothelial growth factor treatments for diabetic macular edema in Japan. J. Diabetes Investig. 2019, 10, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Moulin, T.A.; Boakye, E.A.; Wirth, L.S.; Chen, J.; Burroughs, T.E.; Vollman, D.E. Yearly Treatment Patterns for Patients with Recently Diagnosed Diabetic Macular Edema. Ophthalmol. Retin. 2019, 3, 362–370. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef]
- Diabetic Retinopathy Clinical Research Network; Elman, M.J.; Aiello, L.P.; Beck, R.W.; Bressler, N.M.; Bressler, S.B.; Edwards, A.R.; Ferris, F.L., 3rd; Friedman, S.M.; Glassman, A.R.; et al. Randomized Trial Evaluating Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone Plus Prompt Laser for Diabetic Macular Edema. Ophthalmology 2010, 117, 1064–1077.e35. [Google Scholar] [CrossRef] [PubMed]
- Elman, M.J.; Bressler, N.M.; Qin, H.; Beck, R.W.; Ferris, F.L.; Friedman, S.M., 3rd; Glassman, A.R.; Scott, I.U.; Stockdale, C.R.; Sun, J.K. Expanded 2-Year Follow-up of Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone Plus Prompt Laser for Diabetic Macular Edema. Ophthalmology 2011, 118, 609–614. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Shah, S.M.; Khwaja, A.A.; Channa, R.; Hatef, E.; Do, D.V.; Boyer, D.; Heier, J.S.; Abraham, P.; Thach, A.B.; et al. Two-Year Outcomes of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) Study. Ophthalmology 2010, 117, 2146–2151. [Google Scholar] [CrossRef]
- Mitchell, P.; Bandello, F.; Schmidt-Erfurth, U.; Lang, G.E.; Massin, P.; Schlingemann, R.O.; Sutter, F.; Simader, C.; Burian, G.; Gerstner, O.; et al. The RESTORE Study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011, 118, 615–625. [Google Scholar] [CrossRef]
- Holekamp, N.M.; Campbell, J.; Almony, A.; Ingraham, H.; Marks, S.; Chandwani, H.; Cole, A.L.; Kiss, S. Vision Outcomes Following Anti–Vascular Endothelial Growth Factor Treatment of Diabetic Macular Edema in Clinical Practice. Am. J. Ophthalmol. 2018, 191, 83–91. [Google Scholar] [CrossRef]
- Kawasaki, R.; Bauer, M.; Bezlyak, V.; Ogura, Y. Treatment patterns for retinal diseases in patients newly-treated with anti-VEGF agents: A retrospective analysis of claims data from the Japan Medical Data Center database. Jpn. J. Ophthalmol. 2021, 65, 215–226. [Google Scholar] [CrossRef]
- Sakamoto, T.; Shimura, M.; Kitano, S.; Ohji, M.; Ogura, Y.; Yamashita, H.; Suzaki, M.; Mori, K.; Ohashi, Y.; Yap, P.S.; et al. Impact on visual acuity and psychological outcomes of ranibizumab and subsequent treatment for diabetic macular oedema in Japan (MERCURY). Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 477–487. [Google Scholar] [CrossRef]
- Kernt, M.; Cheuteu, R.; Vounotrypidis, E.; Haritoglou, C.; Kampik, A.; Ulbig, M.W.; Neubauer, A.S. Focal and panretinal photocoagulation with a navigated laser (NAVILAS®). Acta Ophthalmol. 2011, 89, e662–e664. [Google Scholar] [CrossRef]
- Ohkoshi, K.; Yamaguchi, T. Subthreshold Micropulse Diode Laser Photocoagulation for Diabetic Macular Edema in Japanese Patients. Am. J. Ophthalmol. 2010, 149, 133–139.e1. [Google Scholar] [CrossRef]
- Lavinsky, D.; Sramek, C.; Wang, J.B.; Huie, P.M.; Dalal, R.M.; Mandel, Y.; Palanker, D. Subvisible retinal laser therapy: Titration algorithm and tissue response. Retina 2014, 34, 87–97. [Google Scholar] [CrossRef]
- Blumenkranz, M.S.; Yellachich, D.; Andersen, D.E.; Wiltberger, M.W.; Mordaunt, D.; Marcellino, G.R.; Palanker, D. Semiautomated patterned scanning laser for retinal photocoagulation. Retina 2006, 26, 370–376. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effects to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Arch Ophthalmol. 1995, 113, 1144–1155. [Google Scholar] [CrossRef]
- McDONALD, H.R.; Schatz, H. Grid photocoagulation for diffuse macular edema. Retina 1985, 5, 65–72. [Google Scholar] [CrossRef]
- Lövestam-Adrian, M.; Agardh, E. Photocoagulation of diabetic macular oedema; complications and visual outcome. Acta Ophthalmol. Scand. 2000, 78, 667–671. [Google Scholar] [CrossRef]
- Fong, D.S.; Strauber, S.F.; Aiello, L.P.; Beck, R.W.; Callanan, D.G.; Danis, R.P.; Davis, M.D.; Feman, S.S.; Ferris, F.; Writing Committee for the Diabetic Retinopathy Clinical Research Network; et al. Comparison of the Modified Early Treatment Diabetic Retinopathy Study and Mild Macular Grid Laser Photocoagulation Strategies for Diabetic Macular Edema. Arch. Ophthalmol. 2007, 125, 469–480. [Google Scholar] [CrossRef]
- Scott, I.U.; Danis, R.P.; Bressler, S.B.; Bressler, N.M.; Browning, D.J.; Qin, H.; Diabetic Retinopathy Clinical Research Network. Effect of focal/grid photocoagulation on visual acuity and retinal thickening in eyes with non–center-involved diabetic macular edema. Retina 2009, 29, 613–617. [Google Scholar] [CrossRef]
- Elman, M.J.; Ayala, A.; Bressler, N.M.; Browning, D.; Flaxel, C.J.; Glassman, A.R.; Jampol, L.M.; Stone, T.W.; Diabetic Retinopathy Clinical Research Network. Intravitreal Ranibizumab for Diabetic Macular Edema with Prompt versus Deferred Laser Treatment: 5-Year Randomized Trial Results. Ophthalmology 2015, 122, 375–381. [Google Scholar] [CrossRef]
- Bressler, N.M.; Varma, R.; Suñer, I.J.; Dolan, C.M.; Ward, J.; Ehrlich, J.S.; Colman, S.; Turpcu, A.; RIDE and RISE Research Groups. Vision-related function after ranibizumab treatment for diabetic macular edema: Results from RIDE and RISE. Ophthalmology 2014, 121, 2461–2472. [Google Scholar] [CrossRef]
- Do, D.V.; Schmidt-Erfurth, U.; Gonzalez, V.H.; Gordon, C.M.; Tolentino, M.; Berliner, A.J.; Vitti, R.; Rückert, R.; Sandbrink, R.; Stein, D.; et al. The DA VINCI Study: Phase 2 Primary Results of VEGF Trap-Eye in Patients with Diabetic Macular Edema. Ophthalmology 2011, 118, 1819–1826. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Abreu, F.; Adamis, A.P.; Basu, K.; Eichenbaum, D.A.; Haskova, Z.; Lin, H.; Loewenstein, A.; Mohan, S.; Pearce, I.A.; et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): Two randomised, double-masked, phase 3 trials. Lancet 2022, 399, 741–755. [Google Scholar] [CrossRef]
- Brown, D.M.; Emanuelli, A.; Bandello, F.; Barranco, J.J.E.; Figueira, J.; Souied, E.; Wolf, S.; Gupta, V.; Ngah, N.F.; Liew, G.; et al. KESTREL and KITE: 52-Week Results from Two Phase III Pivotal Trials of Brolucizumab for Diabetic Macular Edema. Am. J. Ophthalmol. 2022, 238, 157–172. [Google Scholar] [CrossRef]
- Callanan, D.G.; Gupta, S.; Boyer, D.S.; Ciulla, T.A.; Singer, M.A.; Kuppermann, B.D.; Liu, C.-C.; Li, X.-Y.; Hollander, D.A.; Schiffman, R.M.; et al. Dexamethasone Intravitreal Implant in Combination with Laser Photocoagulation for the Treatment of Diffuse Diabetic Macular Edema. Ophthalmology 2013, 120, 1843–1851. [Google Scholar] [CrossRef]
- Chalam, K.V.; Murthy, R.K.; Brar, V.; Radhakrishnan, R.; Khetpal, V.; Grover, S. Evaluation of a novel, non contact, automated focal laser with integrated (NAVILAS®) fluorescein angiography for diabetic macular edema. Middle East. Afr. J. Ophthalmol. 2012, 19, 158–162. [Google Scholar] [CrossRef]
- Kozak, I.; Oster, S.F.; Cortes, M.A.; Dowell, D.; Hartmann, K.; Kim, J.S.; Freeman, W.R. Clinical Evaluation and Treatment Accuracy in Diabetic Macular Edema Using Navigated Laser Photocoagulator NAVILAS. Ophthalmology 2011, 118, 1119–1124. [Google Scholar] [CrossRef]
- Kernt, M.; Kampik, A.; Neubauer, A.S.; Langer, W.; Kozak, S.; Ulbig, F.; Langer, J. Navigated macular laser decreases retreatment rate for diabetic macular edema: A comparison with conventional macular laser. Clin. Ophthalmol. 2013, 7, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Liegl, R.; Langer, J.; Seidensticker, F.; Reznicek, L.; Haritoglou, C.; Ulbig, M.W.; Neubauer, A.S.; Kampik, A.; Kernt, M. Comparative Evaluation of Combined Navigated Laser Photocoagulation and Intravitreal Ranibizumab in the Treatment of Diabetic Macular Edema. PLoS ONE 2014, 9, e113981. [Google Scholar] [CrossRef]
- Herold, T.R.; Langer, J.; Vounotrypidis, E.; Kernt, M.; Liegl, R.; Priglinger, S.G. 3-year-data of combined navigated laser photocoagulation (Navilas) and intravitreal ranibizumab compared to ranibizumab monotherapy in DME patients. PLoS ONE 2018, 13, e0202483. [Google Scholar] [CrossRef]
- Payne, J.F.; Wykoff, C.C.; Clark, W.L.; Bruce, B.B.; Boyer, D.S.; Brown, D.M. TREX-DME Study Group. Long-term outcomes of treat-and-extend ranibizumab with and without navigated laser for diabetic macular oedema: TREX-DME 3-year results. Br. J. Ophthalmol. 2021, 105, 253–257. [Google Scholar] [CrossRef]
- Kato, F.; Nozaki, M.; Kato, A.; Hasegawa, N.; Morita, H.; Yoshida, M.; Ogura, Y. Evaluation of Navigated Laser Photocoagulation (Navilas 577+) for the Treatment of Refractory Diabetic Macular Edema. J. Ophthalmol. 2018, 2018, 3978514. [Google Scholar] [CrossRef]
- Menzler, J.; Neubauer, A.; Ziemssen, F. Navigated laser in diabetic macular edema: The impact of reduced injection burden on patients and physicians-who wins and who loses? Int. J. Ophthalmol. 2019, 12, 342–345. [Google Scholar] [CrossRef]
- Nozaki, M.; Kato, A.; Yasukawa, T.; Suzuki, K.; Yoshida, M.; Ogura, Y. Indocyanine green angiography-guided focal navigated laser photocoagulation for diabetic macular edema. Jpn. J. Ophthalmol. 2019, 63, 243–254. [Google Scholar] [CrossRef]
- Hirano, T.; Toriyama, Y.; Takamura, Y.; Sugimoto, M.; Nagaoka, T.; Sugiura, Y.; Okamoto, F.; Saito, M.; Noda, K.; Yoshida, S.; et al. Outcomes of a 2-year treat-and-extend regimen with aflibercept for diabetic macular edema. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Schatz, H.; Madeira, D.; McDonald, H.R.; Johnson, R.N. Progressive Enlargement of Laser Scars Following Grid Laser Photocoagulation for Diffuse Diabetic Macular Edema. Arch. Ophthalmol. 1991, 109, 1549–1551. [Google Scholar] [CrossRef]
- Pankratov, M.M. Pulsed delivery of laser energy in experimental thermal retinal photocoagulation. Proc. Soc. Photo Opt. Instrum. Eng. 1990, 1202, 205–213. [Google Scholar] [CrossRef]
- Friberg, T.R.; Karatza, E.C. The Treatment of Macular Disease Using a Micropulsed and Continuous Wave 810-nm Diode Laser. Ophthalmology 1997, 104, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Moisseiev, E.; Abbassi, S.; Thinda, S.; Yoon, J.; Yiu, G.; Morse, L.S. Subthreshold micropulse laser reduces anti-VEGF injection burden in patients with diabetic macular edema. Eur. J. Ophthalmol. 2018, 28, 68–73. [Google Scholar] [CrossRef]
- Inagaki, K.; Hamada, M.; Ohkoshi, K. Minimally invasive laser treatment combined with intravitreal injection of anti-vascular endothelial growth factor for diabetic macular oedema. Sci. Rep. 2019, 9, 7585. [Google Scholar] [CrossRef]
- Khattab, A.M.; Hagras, S.M.; AbdElhamid, A.; Torky, M.A.; Awad, E.A.; Abdelhameed, A.G. Aflibercept with adjuvant micropulsed yellow laser versus aflibercept monotherapy in diabetic macular edema. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1373–1380. [Google Scholar] [CrossRef]
- Kanar, H.S.; Arsan, A.; Altun, A.; Akı, S.F.; Hacısalihoglu, A. Can subthreshold micropulse yellow laser treatment change the anti-vascular endothelial growth factor algorithm in diabetic macular edema? A randomized clinical trial. Indian. J. Ophthalmol. 2020, 68, 145–151. [Google Scholar] [CrossRef]
- Abouhussein, M.A.; Gomaa, A.R. Aflibercept plus micropulse laser versus aflibercept monotherapy for diabetic macular edema: 1-year results of a randomized clinical trial. Int. Ophthalmol. 2020, 40, 1147–1154. [Google Scholar] [CrossRef]
- Koushan, K.; Eshtiaghi, A.; Fung, P.; Berger, A.R.; Chow, D.R. Treatment of Diabetic Macular Edema with Aflibercept and Micropulse Laser (DAM Study). Clin. Ophthalmol. 2022, 16, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Sampat, K.M.; Malik, K.J.; Steiner, J.N.; Glaser, B.M. Efficacy of subthreshold micropulse laser in the treatment of diabetic macular edema is influenced by pre-treatment central foveal thickness. Eye 2014, 28, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Luttrull, J.K.; Sinclair, S.H. Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina 2014, 34, 2010–2020. [Google Scholar] [CrossRef]
- Busch, C.; Fraser-Bell, S.; Zur, D.; Rodríguez-Valdés, P.J.; Cebeci, Z.; Lupidi, M.; Fung, A.T.; Gabrielle, P.-H.; Giancipoli, E.; International Retina Group; et al. Real-world outcomes of observation and treatment in diabetic macular edema with very good visual acuity: The OBTAIN study. Acta Diabetol. 2019, 56, 777–784. [Google Scholar] [CrossRef]
- Chhablani, J.; Roh, Y.J.; Jobling, A.I.; Fletcher, E.L.; Lek, J.J.; Bansal, P.; Guymer, R.; Luttrull, J.K. Restorative retinal laser therapy: Present state and future directions. Surv. Ophthalmol. 2018, 63, 307–328. [Google Scholar] [CrossRef]
- Roider, J.; Michaud, N.A.; Flotte, T.J.; Birngruber, R. Response of the Retinal Pigment Epithelium to Selective Photocoagulation. Arch. Ophthalmol. 1992, 110, 1786–1792. [Google Scholar] [CrossRef]
- Roider, J.; Brinkmann, R.; Wirbelauer, C.; Laqua, H.; Birngruber, R. Retinal sparing by selective retinal pigment epithelial photocoagulation. Arch. Ophthalmol. 1999, 117, 1028–1034. [Google Scholar] [CrossRef]
- Brinkmann, R.; Roider, J.; Birngruber, R.; Lin, C.P. Origin of retinal pigment epithelium cell damage by pulsed laser irradiance in the nanosecond to microsecond time regimen. Lasers Surg. Med. 2000, 27, 451–464. [Google Scholar] [CrossRef]
- Roider, J.; Liew, S.H.M.; Klatt, C.; Elsner, H.; Poerksen, E.; Hillenkamp, J.; Brinkmann, R.; Birngruber, R. Selective retina therapy (SRT) for clinically significant diabetic macular edema. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Kim, J.R.; Kang, S.; Seifert, E.; Theisen-Kunde, D.; Brinkmann, R.; Roh, Y.-J. Safety and efficacy of selective retina therapy (SRT) for the treatment of diabetic macular edema in Korean patients. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1703–1713. [Google Scholar] [CrossRef]
- Yamamoto, M.; Miura, Y.; Hirayama, K.; Kohno, T.; Kabata, D.; Theisen-Kunde, D.; Brinkmann, R.; Honda, S. Predictive factors of outcome of selective retina therapy for diabetic macular edema. Int. Ophthalmol. 2020, 40, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, Y.G.; Jeon, S.H.; Choi, S.Y.; Roh, Y.-J. The efficacy of selective retina therapy for diabetic macular edema based on pretreatment central foveal thickness. Lasers Med. Sci. 2020, 35, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Casson, R.J.; Raymond, G.; Newland, H.S.; Gilhotra, J.S.; Gray, T.L. Pilot randomized trial of a nanopulse retinal laser versus conventional photocoagulation for the treatment of diabetic macular oedema. Clin. Exp. Ophthalmol. 2012, 40, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, M.; Marlecha, S.; Nagpal, K. Comparison of laser photocoagulation for diabetic retinopathy using 532-nm standard laser versus multispot pattern scan laser. Retina 2010, 30, 452–458. [Google Scholar] [CrossRef]
- Ito, A.; Hirano, Y.; Nozaki, M.; Ashikari, M.; Sugitani, K.; Ogura, Y. Short Pulse Laser Induces Less Inflammatory Cytokines in the Murine Retina after Laser Photocoagulation. Ophthalmic Res. 2015, 53, 65–73. [Google Scholar] [CrossRef]
- Takamura, Y.; Arimura, S.; Miyake, S.; Matsumura, T.; Gozawa, M.; Iwasaki, K.; Inatani, M. Panretinal Photocoagulation Using Short-Pulse Laser Induces Less Inflammation and Macular Thickening in Patients with Diabetic Retinopathy. J. Ophthalmol. 2017, 2017, 8530261. [Google Scholar] [CrossRef]
- Chappelow, A.V.; Tan, K.; Waheed, N.K.; Kaiser, P.K. Panretinal Photocoagulation for Proliferative Diabetic Retinopathy: Pattern Scan Laser Versus Argon Laser. Am. J. Ophthalmol. 2012, 153, 137–142.e2. [Google Scholar] [CrossRef]
- Maeshima, K.; Utsugi-Sutoh, N.; Otani, T.; Kishi, S. Progressive enlargement of scattered photocoagulation scars in diabetic retinopathy. Retina 2004, 24, 507–511. [Google Scholar] [CrossRef]
- Higaki, M.; Nozaki, M.; Yoshida, M.; Ogura, Y. Less Expansion of Short-Pulse Laser Scars in Panretinal Photocoagulation for Diabetic Retinopathy. J. Ophthalmol. 2018, 2018, 9371895. [Google Scholar] [CrossRef]
- Hirano, T.; Toriyama, Y.; Iesato, Y.; Imai, A.; Hirabayashi, K.; Nagaoka, T.; Takamura, Y.; Sugimoto, M.; Murata, T. Effect of leaking perifoveal microaneurysms on resolution of diabetic macular edema treated by combination therapy using anti-vascular endothelial growth factor and short pulse focal/grid laser photocoagulation. Jpn. J. Ophthalmol. 2017, 61, 51–60. [Google Scholar] [CrossRef]
- Datlinger, F.; Datlinger, A.; Pollreisz, A.; Sacu, S.; Schmidt-Erfurth, U.; Datlinger, P. Intraprocedural OCT monitoring of the immediate treatment response during indocyanine green angiography-guided laser therapy of teleangiectatic capillaries in diabetic macular edema. Sci. Rep. 2022, 12, 2315. [Google Scholar] [CrossRef]
- Ikegami, Y.; Shiraya, T.; Araki, F.; Ueta, T.; Toyama, T.; Yanagita, T.; Numaga, J.; Shoji, N.; Kato, S. Microperimetric analysis of diabetic macular edema after navigated direct photocoagulation with short-pulse laser for microaneurysms. Int. J. Retin. Vitr. 2023, 9, 1–7. [Google Scholar] [CrossRef]
- Lavinsky, D.; Wang, J.; Huie, P.; Dalal, R.; Lee, S.J.; Lee, D.Y.; Palanker, D. Nondamaging Retinal Laser Therapy: Rationale and Applications to the Macula. Investig. Opthalmol. Vis. Sci. 2016, 57, 2488–2500. [Google Scholar] [CrossRef]
- Hamada, M.; Ohkoshi, K.; Inagaki, K.; Ebihara, N.; Murakami, A. Subthreshold Photocoagulation Using Endpoint Management in the PASCAL® System for Diffuse Diabetic Macular Edema. J. Ophthalmol. 2018, 2018, 7465794. [Google Scholar] [CrossRef]
- Nozaki, M.; Wong, I.Y.; Kawasaki, R.; Lee, J.E.; Takamura, Y.; Lee, J.E.; Yoshida, S.; Shin, J.P.; Kida, T.; Chang, W.; et al. Anti-VEGF Monotherapy versus Combined Anti-VEGF and Endpoint Management Laser for Diabetic Macular Edema (END-DME Study). J. Retin. 2022, 7, 65–74. [Google Scholar] [CrossRef]
- Tatsumi, T.; Takatsuna, Y.; Oshitari, T.; Kaiho, T.; Kawasaki, Y.; Shiko, Y.; Sugawara, T.; Baba, T.; Yamamoto, S. Randomized clinical trial comparing intravitreal aflibercept combined with subthreshold laser to intravitreal aflibercept monotherapy for diabetic macular edema. Sci. Rep. 2022, 12, 10672. [Google Scholar] [CrossRef]
- Al-Barki, A.; Al-Hijji, L.; High, R.; Schatz, P.; Do, D.; Nguyen, Q.D.; Luttrull, J.K.; Kozak, I. Comparison of short-pulse subthreshold (532 nm) and infrared micropulse (810 nm) macular laser for diabetic macular edema. Sci. Rep. 2021, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Ogura, S.; Yasukawa, T.; Kato, A.; Kuwayama, S.; Hamada, S.; Hirano, Y.; Uemura, A.; Yoshida, M.; Ogura, Y. Indocyanine Green Angiography-Guided Focal Laser Photocoagulation for Diabetic Macular Edema. Ophthalmologica 2015, 234, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Paques, M.; Philippakis, E.; Bonnet, C.; Falah, S.; Ayello-Scheer, S.; Zwillinger, S.; Girmens, J.-F.; Dupas, B. Indocyanine-green-guided targeted laser photocoagulation of capillary macroaneurysms in macular oedema: A pilot study. Br. J. Ophthalmol. 2017, 101, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.J. Binding of Sulfobromophthalein (BSP) Sodium and Indocyanine Green (ICG) by Plasma 1 Lipoproteins. Exp. Biol. Med. 1966, 122, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Farías, D.C.; Serrano, R.M.; Gancharov, J.B.; Cuadras, U.D.D.; Sahel, J.; Wiechers, F.G.; Dupas, B.; Paques, M. Indocyanine green angiography for identifying telangiectatic capillaries in diabetic macular oedema. Br. J. Ophthalmol. 2020, 104, 509–513. [Google Scholar] [CrossRef]
- Mori, K.; Yoshida, S.; Kobayashi, Y.; Ishikawa, K.; Nakao, S.; Hisatomi, T.; Haruta, M.; Isihibashi, T.; Sonoda, K.-H. Decrease in the number of microaneurysms in diabetic macular edema after anti-vascular endothelial growth factor therapy: Implications for indocyanine green angiography-guided detection of refractory microaneurysms. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 735–741. [Google Scholar] [CrossRef]
- Chaperon, M.; Kodjikian, L.; Agard, E.; Mathis, T.; Billant, J.; El-Chehab, H.; Pradat, P.; Dot, C. Screening of telangiectatic capillaries in chronic macular edema based on multimodal imaging: A study of 101 eyes. LyoMAC1 study. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 2501–2508. [Google Scholar] [CrossRef]
- Itou, J.; Furushima, K.; Haruta, M.; Kato, N.; Arai, R.; Mori, K.; Ishikawa, K.; Yoshida, S. Reduced size of telangiectatic capillaries after intravitreal injection of anti-vascular endothelial growth factor agents in diabetic macular edema. Clin. Ophthalmol. 2023, 17, 239–245. [Google Scholar] [CrossRef]
- Séjournet, L.; Kodjikian, L.; Elbany, S.; Allignet, B.; Agard, E.; Chaperon, M.; Billant, J.; Denis, P.; Mathis, T.; Burillon, C.; et al. Focal Photocoagulation as an Adjunctive Therapy to Reduce the Burden of Intravitreal Injections in Macula Edema Patients, the LyoMAC2 Study. Pharmaceutics 2023, 15, 308. [Google Scholar] [CrossRef]
- Diaz-Llopis, M.; Gallego-Pinazo, R.; Cogollos, S.; Marco, D.; Arevalo, J.F.; Garcia-Delpech, S.; Mullor, J. Macular laser photocoagulation guided by spectral-domain optical coherence tomography versus fluorescein angiography for diabetic macular edema. Clin. Ophthalmol. 2011, 5, 613–617. [Google Scholar] [CrossRef]
- Hirano, T.; Iesato, Y.; Toriyama, Y.; Imai, A.; Murata, T. Detection of Fovea-Threatening Diabetic Macular Edema by Optical Coherence Tomography to Maintain Good Vision by Prophylactic Treatment. Ophthalmic Res. 2014, 52, 65–73. [Google Scholar] [CrossRef]
- Kozak, I.; El-Emam, S.Y.; Cheng, L.; Bartsch, D.-U.; Chhablani, J.; Freeman, W.R.; Arevalo, J.F.M. Fluorescein angiography versus optical coherence tomography-guided planning for macular laser photocoagulation in diabetic macular edema. Retina 2014, 34, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Takamura, Y.; Matsumura, T.; Arimura, S.; Gozawa, M.; Morioka, M.; Inatani, M.; Yamada, Y. Direct Photocoagulation Guided by Merged Retinal Images for the Treatment of Focal Diabetic Macular Edema. Int. J. Endocrinol. 2018, 2018, 2401094. [Google Scholar] [CrossRef]
- Hasegawa, N.; Nozaki, M.; Takase, N.; Yoshida, M.; Ogura, Y. New Insights into Microaneurysms in the Deep Capillary Plexus Detected by Optical Coherence Tomography Angiography in Diabetic Macular Edema. Investig. Opthalmol. Vis. Sci. 2016, 57, OCT348–OCT355. [Google Scholar] [CrossRef]
- Kaizu, Y.; Nakao, S.; Wada, I.; Arima, M.; Yamaguchi, M.; Ishikawa, K.; Akiyama, M.; Kishimoto, J.; Hisatomi, T.; Sonoda, K.-H. Microaneurysm Imaging Using Multiple En Face OCT Angiography Image Averaging: Morphology and Visualization. Ophthalmol. Retin. 2020, 4, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Parrulli, S.; Corvi, F.; Cozzi, M.; Monteduro, D.; Zicarelli, F.; Staurenghi, G. Microaneurysms visualisation using five different optical coherence tomography angiography devices compared to fluorescein angiography. Br. J. Ophthalmol. 2020, 105, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Horii, T.; Murakami, T.; Nishijima, K.; Sakamoto, A.; Ota, M.; Yoshimura, N. Optical Coherence Tomographic Characteristics of Microaneurysms in Diabetic Retinopathy. Am. J. Ophthalmol. 2010, 150, 840–848.e1. [Google Scholar] [CrossRef]
- Byeon, S.H.; Chu, Y.K.; Hong, Y.T.; Kim, M.; Kang, H.M.; Kwon, O.W. New insights into the pathoanatomy of diabetic macular edema: Angiographic patterns and optical coherence tomography. Retina 2012, 32, 1087–1099. [Google Scholar] [CrossRef]
- Maltsev, D.S.; Kulikov, A.N.; Burnasheva, M.A.; Kazak, A.A.; Chhablani, J. Structural en face optical coherence tomography imaging for identification of leaky microaneurysms in diabetic macular edema. Int. Ophthalmol. 2020, 40, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Byeon, S.H.; Kwon, O.W. Optical coherence tomography-guided selective focal laser photocoagulation: A novel laser protocol for diabetic macular edema. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, D.S.; Kulikov, A.N.; Burnasheva, M.A.; Kazak, A.A.; Chhablani, J. Efficacy of navigated focal laser photocoagulation in diabetic macular edema planned with en face optical coherence tomography versus fluorescein angiography. Int. Ophthalmol. 2020, 40, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, K.; Kim, J.; Tysinger, B.; Blim, J.; Emerson, G.; Ferrone, P.J.; Kim, J.E.; Seabury, S.; Hahn, P. The Broader Economic Value of Treatment for Diabetic Macular Edema. Diabetes Care 2023, 46, dc222527. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, H.; Qiu, W. Intravitreal anti-vascular endothelial growth factor, laser photocoagulation, or combined therapy for diabetic macular edema: A systematic review and network meta-analysis. Front. Endocrinol. 2023, 14, 1096105. [Google Scholar] [CrossRef]
- Everett, L.A.; Paulus, Y.M. Laser Therapy in the Treatment of Diabetic Retinopathy and Diabetic Macular Edema. Curr. Diabetes Rep. 2021, 21, 35. [Google Scholar] [CrossRef]
- Bressler, S.B.; Ayala, A.R.; Bressler, N.M.; Melia, M.; Qin, H.; Ferris, F.; Flaxel, C.J.; Friedman, S.M.; Glassman, A.R.; Jampol, L.M.; et al. Persistent Macular Thickening After Ranibizumab Treatment for Diabetic Macular Edema with Vision Impairment. JAMA Ophthalmol. 2016, 134, 278–285. [Google Scholar] [CrossRef]
- Dugel, P.U.; Campbell, J.H.; Kiss, S.; Loewenstein, A.; Shih, V.; Xu, X.; Holekamp, N.M.; Augustin, A.J.; Ho, A.C.; Gonzalez, V.H.; et al. Association between early anatomic response to anti–vascular endothelial growth factor therapy and long-term outcome in diabetic macular edema. Retina 2019, 39, 88–97. [Google Scholar] [CrossRef]
- Kuroiwa, D.A.K.; Malerbi, F.K.; Regatieri, C.V.S. New Insights in Resistant Diabetic Macular Edema. Ophthalmologica 2021, 244, 485–494. [Google Scholar] [CrossRef]
- Lee, J.; Gil Moon, B.; Cho, A.R.; Yoon, Y.H. Optical Coherence Tomography Angiography of DME and Its Association with Anti-VEGF Treatment Response. Ophthalmology 2016, 123, 2368–2375. [Google Scholar] [CrossRef]
- Sugimoto, M.; Ichio, A.; Mochida, D.; Tenma, Y.; Miyata, R.; Matsubara, H.; Kondo, M. Multiple Effects of Intravitreal Aflibercept on Microvascular Regression in Eyes with Diabetic Macular Edema. Ophthalmol. Retin. 2019, 3, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Takamura, Y.; Yamada, Y.; Inatani, M. Role of Microaneurysms in the Pathogenesis and Therapy of Diabetic Macular Edema: A Descriptive Review. Medicina 2023, 59, 435. [Google Scholar] [CrossRef]
- Kato, F.; Nozaki, M.; Kato, A.; Yasukawa, T. Retinal Microvascular Changes after Intravitreal Triamcinolone Acetonide in Diabetic Macular Edema. J. Clin. Med. 2023, 12, 3475. [Google Scholar] [CrossRef]
- Yoshida, S.; Murakami, T.; Nozaki, M.; Suzuma, K.; Baba, T.; Hirano, T.; Sawada, O.; Sugimoto, M.; Takamura, Y.; Tsuiki, E. Review of clinical studies and recommendation for a therapeutic flow chart for diabetic macular edema. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Takamura, Y.; Morioka, M.; Gozawa, M.; Matsumura, T.; Inatani, M. Microaneurysm density in residual oedema after anti-vascular endothelial growth factor therapy for diabetic macular oedema. Acta Ophthalmol. 2021, 99, e876–e883. [Google Scholar] [CrossRef] [PubMed]
- Gelfman, C.M.; Grishanin, R.; Bender, K.O.; Nguyen, A.; Greengard, J.; Sharma, P.; Nieves, J.; Kiss, S.; Gasmi, M. Comprehensive Preclinical Assessment of ADVM-022, an Intravitreal Anti-VEGF Gene Therapy for the Treatment of Neovascular AMD and Diabetic Macular Edema. J. Ocul. Pharmacol. Ther. 2021, 37, 181–190. [Google Scholar] [CrossRef] [PubMed]
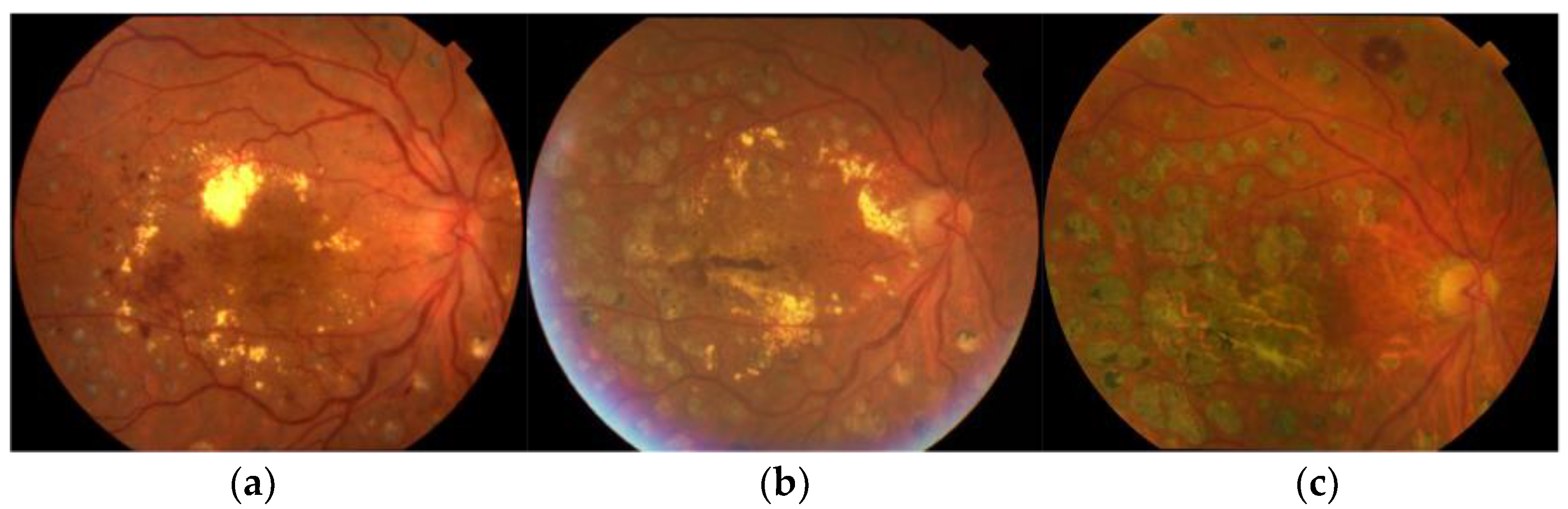
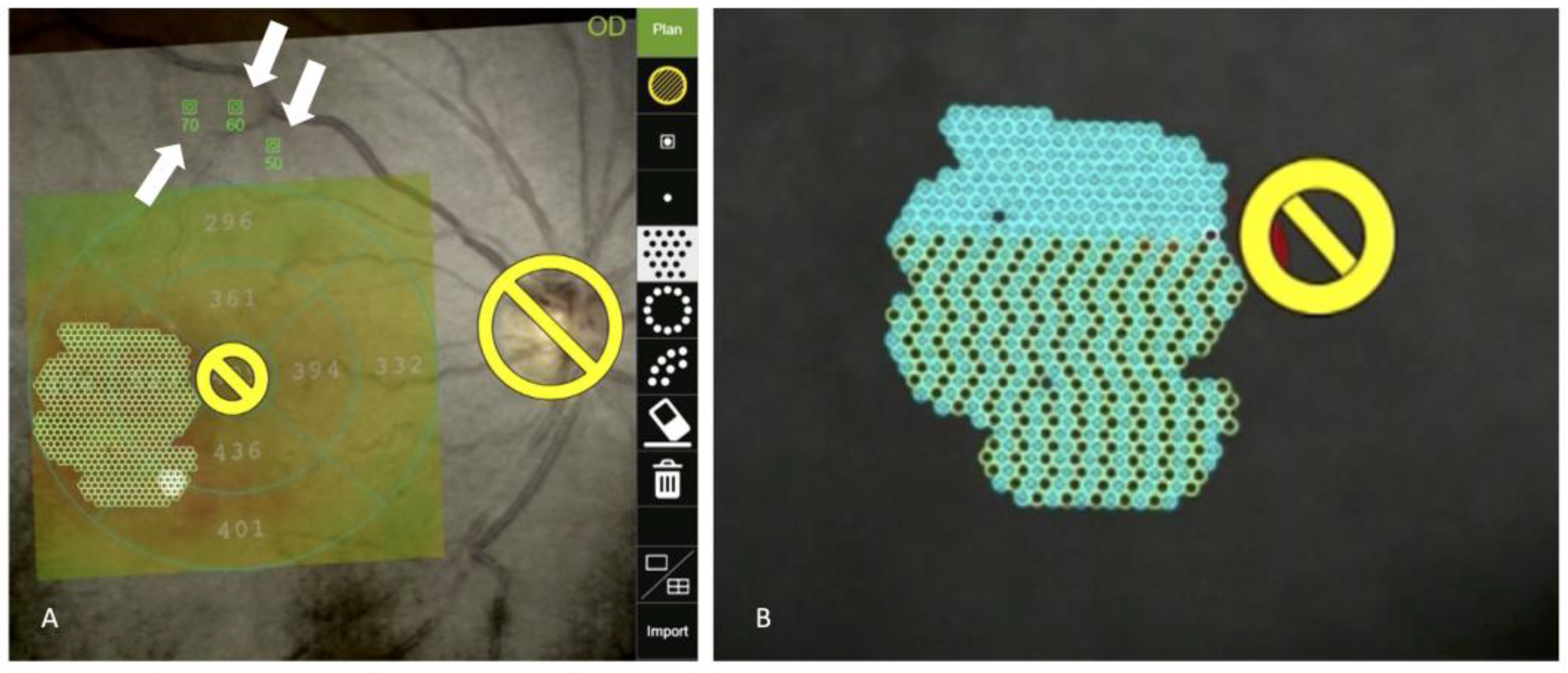
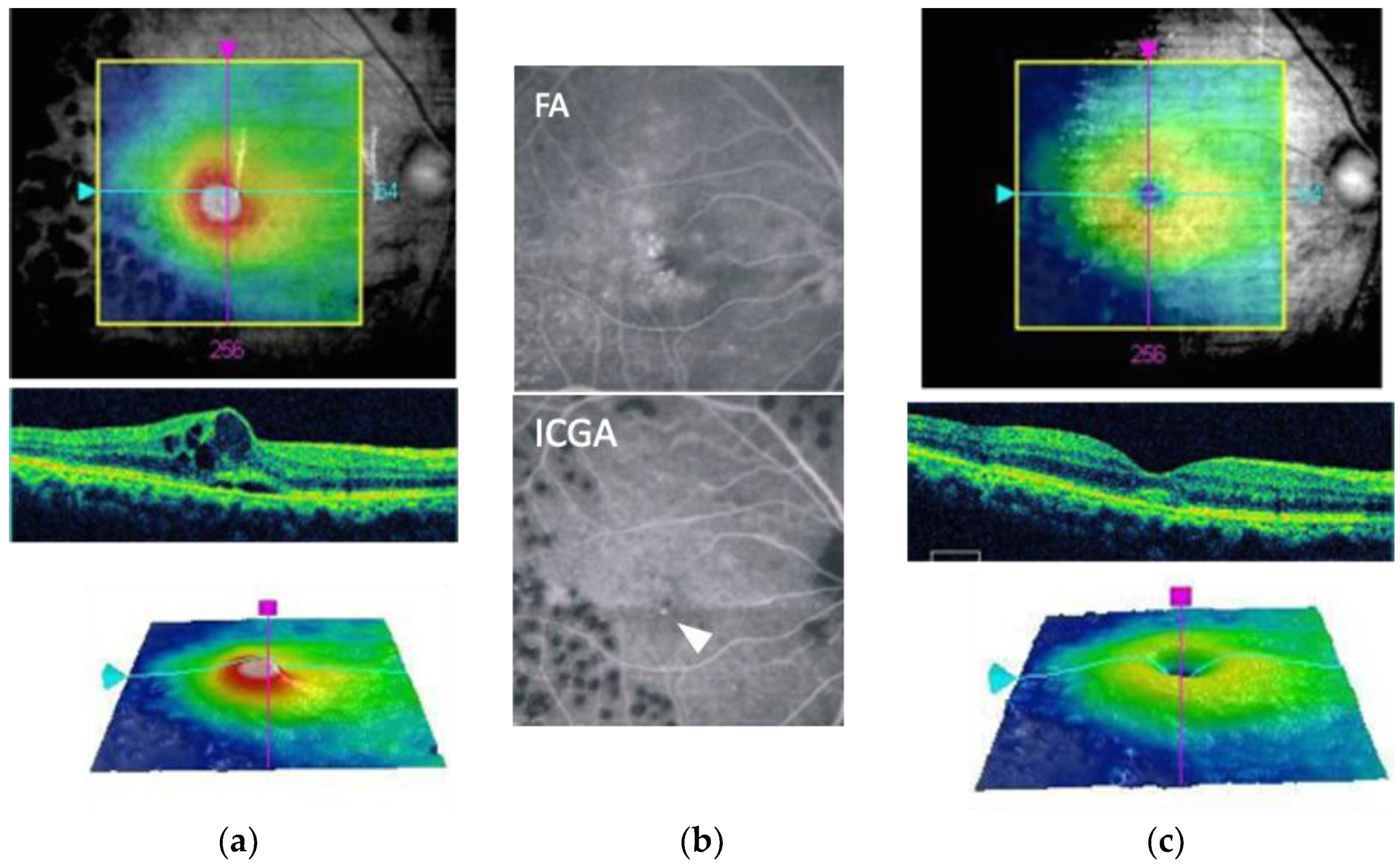
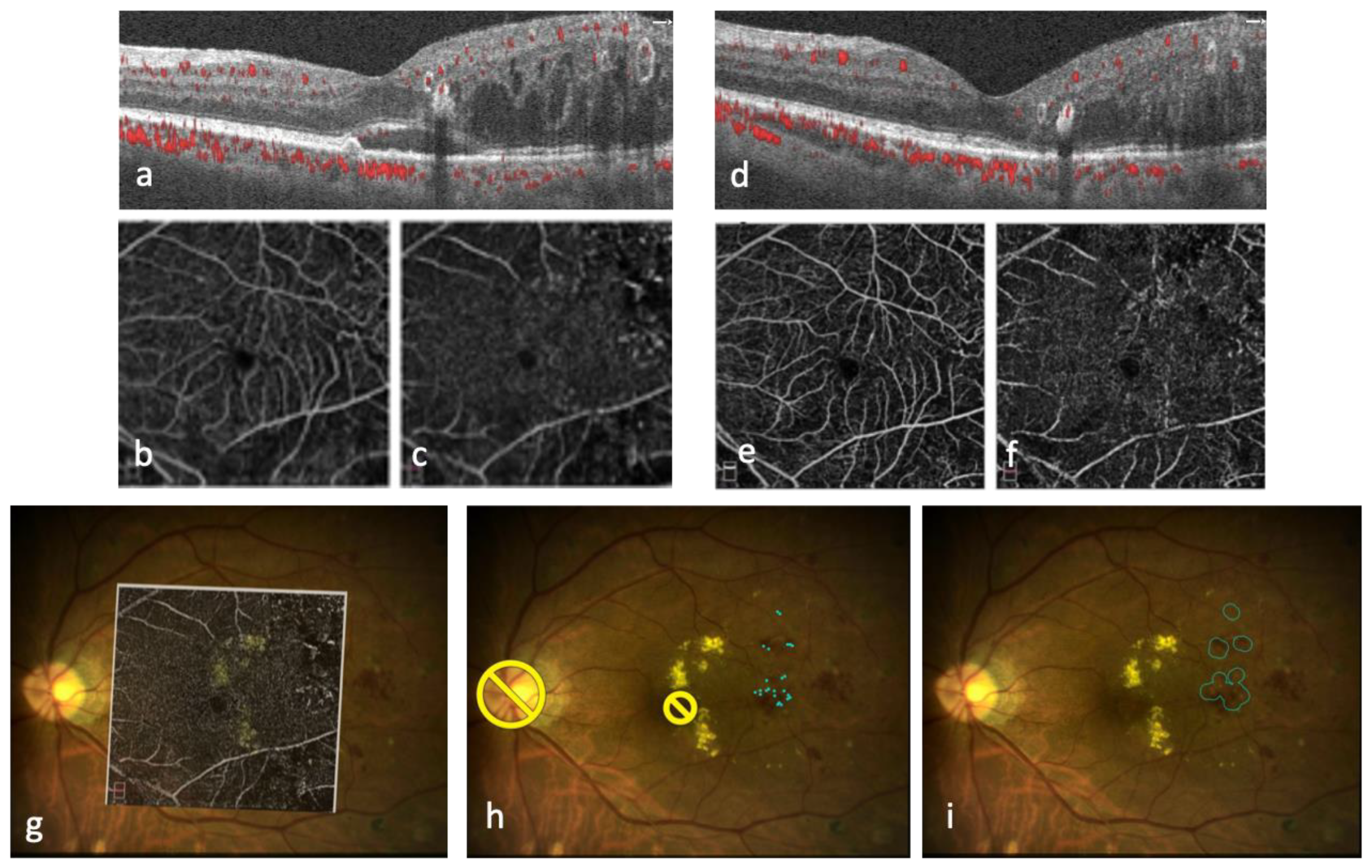
| Type of Laser System | Type of Treatment | Advantage | Disadvantage | Device |
|---|---|---|---|---|
| Navigation laser | Focal laser Microsecond pulsing laser | Accurate image-guided laser with eye tracking | Evaluate laser only via live monitor | Navilas (OD-OS) |
| Micropulse laser | Micropulse laser | Non-damaging laser with pattern Numerous clinical studies have been reported | Central retinal thickness >400 μm cannot respond | IQ 577, IQ 532 (IRIDEX) |
| Selective Retina Laser (SRT) | Microsecond laser | Real-time feedback by detecting the pressure wave of microbubbles generated after irradiation | Distribution of the laser device is limited | R: GEN® (Lutronic vision) |
| Nanosecond laser | Nanosecond laser | Non-damaging laser | One study report in DME | 2RT (Ellex) |
| Pattern Scan (multisport) laser | Focal laser Endpoint management (EpM) laser (PASCAL) SubLuminal laser (EasyRet) Micropulse laser (Supra Scan 577) SmartPulse (Lumenis) | Short-pulse laser shows less expansion of spots | No eye-tracking system Ideal setting has not yet been determined (EpM laser) | PASCAL (IRIDEX/Topcon) EasyRet (Quantel) Supra Scan 577 (Quantel) Smart532 (Lumenis) |
| First Author, Study Name and Year | Total Eyes | Follow-Up | Intervention | Clinical Results | Number of Injections |
|---|---|---|---|---|---|
| Nguyen et al., READ-2, 2010 [9] | IVR = 33, Laser = 33, IVR + laser = 34 | 24 months | IVR at baseline and months 1, 3, and 5. Focal/grid laser at baseline and month 3 (if needed) IVR and focal/grid laser at baseline and month 3 Starting at month 6, IVR could be given to all groups. | There were no statistically significant differences in BCVA and CMT among 3 groups. However, the monotherapy group did not include patients with resolved or controlled edema who had poor visual acuity, whereas this was the case in 22% of combination therapy patients. | 9.3 (IVR monotherapy) 4.4 (laser) 2.9 (combination therapy) |
| Mitchell et al., RESTORE, 2011 [10] | IVR = 116, IVR + laser = 118, sham + laser = 111 | 12 months | 3 monthly injections followed by as needed. Laser at baseline and as needed. | IVR monotherapy and IVR + laser showed significantly better clinical outcome compared to sham + laser. However, there was no difference between IVR monotherapy and IVR + laser therapy. | 7.0 (IVR monotherapy) 6.8 (IVR + laser) 7.3 (laser + sham) |
| Elman et al., Protocol I 2015 [23] | IVR + deferred laser = 111, IVR + prompt laser = 124 | 5 years | 3 monthly injections followed by as needed. Prompt laser given 7–10 days after initial IVR. Deferred laser given if needed after 6 months | +9.8 letters in IVR + deferred laser and +7.2 letters in IVR + prompt laser (p = 0.09) 38% in IVR + deferred laser and 27% in IVR + prompt laser gained at least a 15-letter improvement (p = 0.03) | 17 (IVR + deferred laser) 14 (IVR + prompt laser) |
| Liegl et al. 2014 [32] | IVR monotherapy = 32 IVR + Navilas laser = 34 | 12 months | 3 monthly injections followed by as needed. Navilas laser given after 3 loading doses. | Navigated laser combination therapy and IVR monotherapy similarly improved mean BCVA letter score (+8.41 vs. +6.31 letters, p = 0.258) | 6.88 (IVR) 3.88 (IVR + Navilas laser) (p < 0.001) |
| Payne et al., TREX-DME, 2021 [34] | IVR (0.3 mg) monthly = 24 IVR (0.3 mg) TAE = 40 IVR (0.3 mg) TAE + Navilas laser = 45 | 3 years | 4 monthly injections followed by TAE regimen. Navilas laser given at week 4 and again every 3 months if microaneurysm leakage was present on fluorescein angiography For 3rd year, IVR given as needed. | There were no significant differences among 3 groups. | Third year 3.0 (monthly) 3.1 (IVR TAE) 2.4 (IVR TAE + Navilas laser) |
| Khattab et al., 2019 [44] | IVA = 27 IVA + SMPL = 27 | 18 months | 3 monthly injections followed by as needed 3 IVAs followed by SPML (within 1 week after the 3rd injection) | Comparable anatomical and visual outcomes between 2 groups. | 7.3 (IVA monotherapy) 4.1 (IVA + SMPL) (p < 0.005) |
| Koushan et al., DAM, 2022 [47] | IVA = 15 IVA + SMPL = 15 | 48 weeks | 1 injection followed by as needed SMPL given on the day of the first injection | Eyes that received SMPL showed a numerically greater improvement in BCVA, although this was not statistically significant. | 8.5 (IVA monotherapy) 7.9 (IVA + SMPL) (p = 0.61) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nozaki, M.; Ando, R.; Kimura, T.; Kato, F.; Yasukawa, T. The Role of Laser Photocoagulation in Treating Diabetic Macular Edema in the Era of Intravitreal Drug Administration: A Descriptive Review. Medicina 2023, 59, 1319. https://doi.org/10.3390/medicina59071319
Nozaki M, Ando R, Kimura T, Kato F, Yasukawa T. The Role of Laser Photocoagulation in Treating Diabetic Macular Edema in the Era of Intravitreal Drug Administration: A Descriptive Review. Medicina. 2023; 59(7):1319. https://doi.org/10.3390/medicina59071319
Chicago/Turabian StyleNozaki, Miho, Ryota Ando, Toshiya Kimura, Fusae Kato, and Tsutomu Yasukawa. 2023. "The Role of Laser Photocoagulation in Treating Diabetic Macular Edema in the Era of Intravitreal Drug Administration: A Descriptive Review" Medicina 59, no. 7: 1319. https://doi.org/10.3390/medicina59071319
APA StyleNozaki, M., Ando, R., Kimura, T., Kato, F., & Yasukawa, T. (2023). The Role of Laser Photocoagulation in Treating Diabetic Macular Edema in the Era of Intravitreal Drug Administration: A Descriptive Review. Medicina, 59(7), 1319. https://doi.org/10.3390/medicina59071319






