An Analysis of Respiratory Muscle Paralysis of Adult Patients in Guillain–Barré Syndrome: A Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Grouping Criteria
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Basic Clinical Data
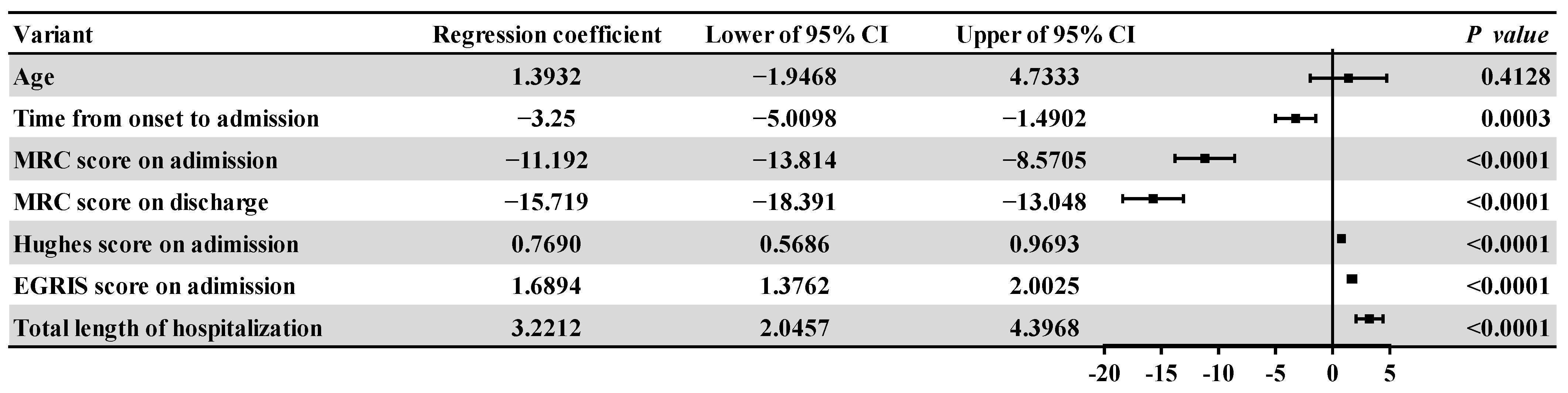
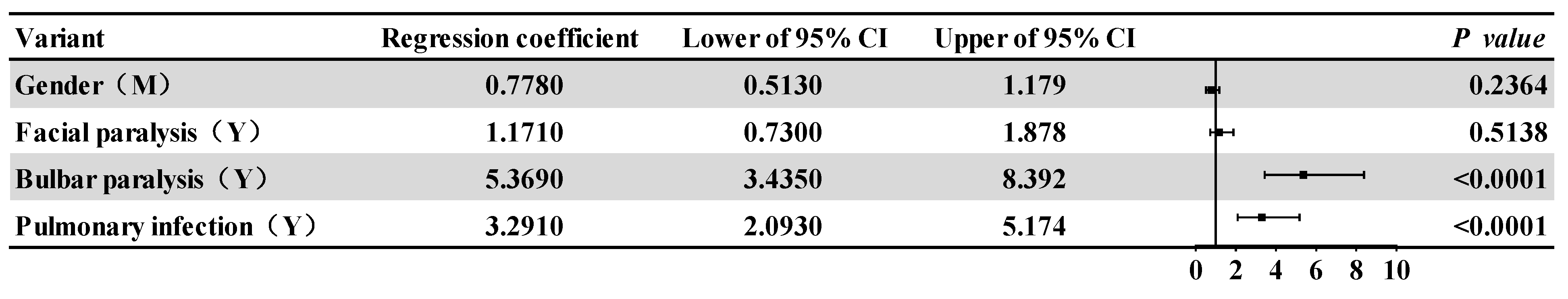
3.2. Comparisons between the Affected Group and the Control Group
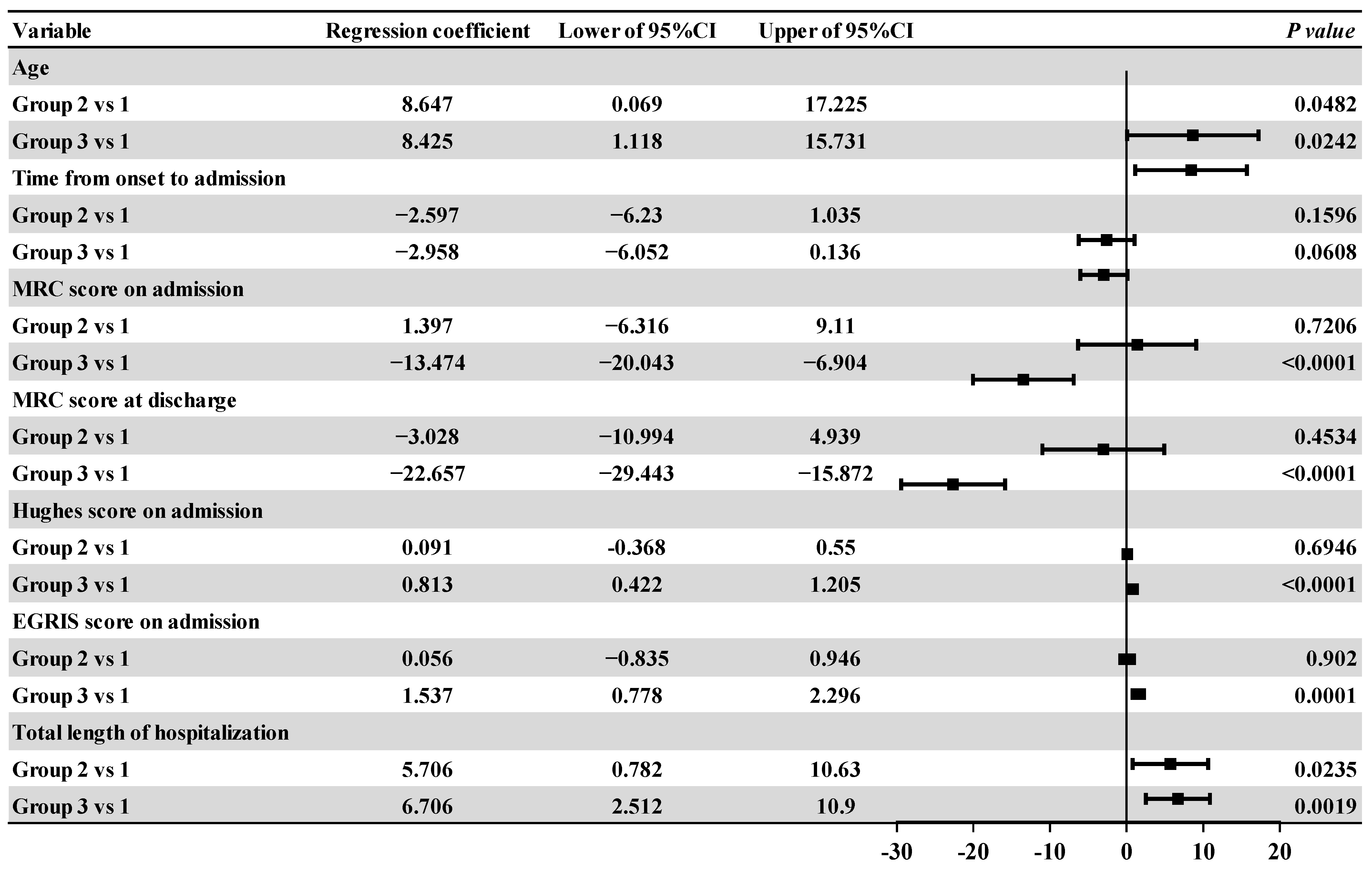
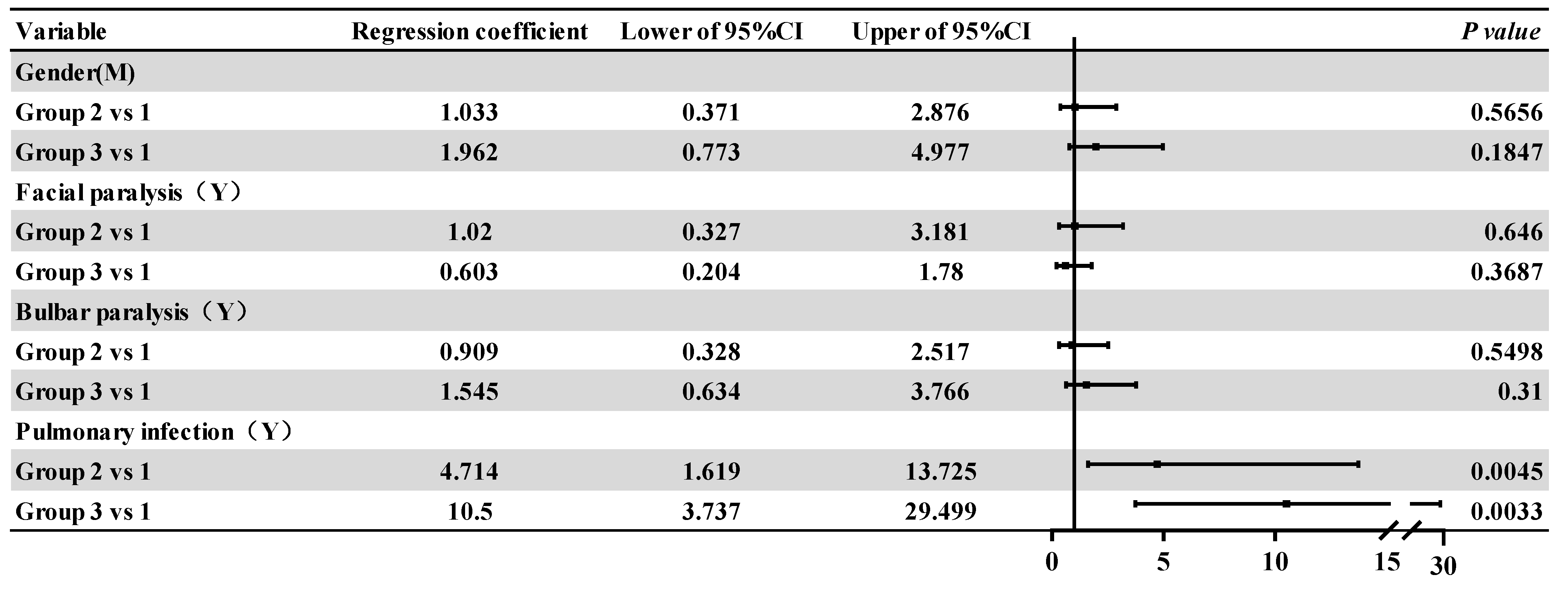
3.3. Intra-Group Comparative Analysis of the Affected Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finsterer, J. Diagnose SARS-CoV-2 associated Guillain-Barre syndrome upon appropriate criteria and after exclusion of differentials. J. Med. Virol. 2021, 93, 5687–5688. [Google Scholar] [CrossRef]
- Olsen, B.; Lundkvist, Å. Zika virus—Ancient virus gets new life in a new ecosystem. Microcephaly and Guillain-Barre syndrome are possible consequences when there is no background herd immunity in the population. Lakartidningen 2016, 113, DX9X. [Google Scholar] [PubMed]
- Shahrizaila, N.; Lehmann, H.C.; Kuwabara, S. Guillain-Barré syndrome. Lancet 2021, 397, 1214–1228. [Google Scholar] [CrossRef]
- van den Berg, B.; Walgaard, C.; Drenthen, J.; Fokke, C.; Jacobs, B.C.; Van Doorn, P.A. Guillain-Barré syndrome: Pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol. 2014, 10, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, J.A.; Willison, H.J. Guillain-Barré syndrome: A century of progress. Nat. Rev. Neurol. 2016, 12, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Asbury, A.K.; Cornblath, D.R. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann. Neurol. 1990, 27 (Suppl. S1), S21–S24. [Google Scholar] [CrossRef] [PubMed]
- Willison, H.J.; Jacobs, B.C.; van Doorn, P.A. Guillain-Barré syndrome. Lancet 2016, 388, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Krarup, C.; Vorstrup, S. Guillain-Barre syndrome. Ugeskr. Laeger. 2000, 162, 4781. [Google Scholar] [PubMed]
- van den Berg, B.; Bunschoten, C.; van Doorn, P.A.; Jacobs, B.C. Mortality in Guillain-Barre syndrome. Neurology 2013, 80, 1650–1654. [Google Scholar] [CrossRef]
- Durand, M.C.; Porcher, R.; Orlikowski, D.; Aboab, J.; Devaux, C.; Clair, B.; Annane, D.; Gaillard, J.L.; Lofaso, F.; Raphael, J.C.; et al. Clinical and electrophysiological predictors of respiratory failure in Guillain-Barré syndrome: A prospective study. Lancet Neurol. 2006, 5, 1021–1028. [Google Scholar] [CrossRef]
- Walgaard, C.; Lingsma, H.F.; Ruts, L.; Drenthen, J.; van Koningsveld, R.; Garssen, M.J.; van Doorn, P.A.; Steyerberg, E.W.; Jacobs, B.C. Prediction of respiratory insufficiency in Guillain-Barré syndrome. Ann. Neurol. 2010, 67, 781–787. [Google Scholar] [CrossRef]
- Lawn, N.D.; Fletcher, D.D.; Henderson, R.D.; Wolter, T.D.; Wijdicks, E.F. Anticipating mechanical ventilation in Guillain-Barré syndrome. Arch. Neurol. 2001, 58, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Polkey, M.I. Respiratory Muscle Assessment in Clinical Practice. Clin. Chest Med. 2019, 40, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.S. Respiratory failure because of neuromuscular disease. Curr. Opin Neurol. 2016, 29, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Rabinstein, A.A. Noninvasive ventilation for neuromuscular respiratory failure: When to use and when to avoid. Curr. Opin. Crit. Care 2016, 22, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Hadden, R.D.; Cornblath, D.R.; Hughes, R.A.C.; Zielasek, J.; Hartung, H.P.; Toyka, K.V.; Swan, A.V. Electrophysiological classification of Guillain-Barré syndrome: Clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Ann. Neurol. 1998, 44, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Kleyweg, R.P.; van der Meché, F.G.; Schmitz, P.I. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 1991, 14, 1103–1109. [Google Scholar] [CrossRef]
- Toamad, U.; Kongkamol, C.; Setthawatcharawanich, S.; Limapichat, K.; Phabphal, K.; Sathirapanya, P. Clinical presentations as predictors of prolonged mechanical ventilation in Guillain-Barré syndrome in an institution with limited medical resources. Singap. Med. J. 2015, 56, 558–561. [Google Scholar] [CrossRef]
- Albawaliz, A.; Abdallah, D.; Seehra, G.K.; Elkafrawy, A.; Al-Shyoukh, A. Asymmetric Lower Extremity Involvement and Facial Palsy: An Atypical Case of Guillain-Barre Syndrome. Cureus 2020, 12, e8912. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Suzuki, H.; Sonoo, M.; Kuwabara, S.; Yokota, T.; Nomura, K.; Chiba, A.; Kaji, R.; Kanda, T.; Kaida, K.; et al. Markers for Guillain-Barré syndrome with poor prognosis: A multi-center study. J. Peripher. Nerv. Syst. 2017, 22, 433–439. [Google Scholar] [CrossRef]
- Kumar, M.; Kalita, J.; Misra, U.K.; Dhar, N. Prediction models for mechanical ventilation and outcome in Guillain-Barré syndrome. J. Clin. Neurosci. 2021, 92, 131–135. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, K.; Li, C.; Zhang, W.; Wu, X.; Fang, S. Risk Factors for Mechanical Ventilation in Patients with Guillain-Barré Syndrome. Neurocrit. Care 2022, 37, 121–128. [Google Scholar] [CrossRef]
- Roodbol, J.; Korinthenberg, R.; Venema, E.; de Wit, M.C.Y.; Lingsma, H.F.; Catsman-Berrevoets, C.E.; Jacobs, B.C.; Korinthenberg, R.; Roodbol, J.; de Wit, M.C.Y.; et al. Predicting respiratory failure and outcome in pediatric Guillain-Barré syndrome. Eur. J. Paediatr. Neurol. 2023, 44, 18–24. [Google Scholar] [CrossRef]
- Wijdicks, E.F.; Roy, T.K. BiPAP in early guillain-barré syndrome may fail. Can. J. Neurol. Sci. 2006, 33, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Green, C.; Baker, T.; Subramaniam, A. Predictors of respiratory failure in patients with Guillain-Barré syndrome: A systematic review and meta-analysis. Med. J. Aust. 2018, 208, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.C.; Lofaso, F.; Lefaucheur, J.P.; Chevret, S.; Gajdos, P.; Raphael, J.C.; Sharshar, T. Electrophysiology to predict mechanical ventilation in Guillain-Barré syndrome. Eur. J. Neurol. 2003, 10, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, S.; Yuki, N. Axonal Guillain-Barré syndrome: Concepts and controversies. Lancet Neurol. 2013, 12, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Wang, L.; Liu, H.; Gong, L.; Ji, H.; Wu, H.; Chu, W. Risk factors for the severity of Guillain-Barré syndrome and predictors of short-term prognosis of severe Guillain-Barré syndrome. Sci. Rep. 2021, 11, 11578. [Google Scholar] [CrossRef]
- Kokubun, N.; Nishibayashi, M.; Uncini, A.; Odaka, M.; Hirata, K.; Yuki, N. Conduction block in acute motor axonal neuropathy. Brain 2010, 133, 2897–2908. [Google Scholar] [CrossRef]
- Ali, M.I.; Fernández-Pérez, E.R.; Pendem, S.; Brown, D.R.; Wijdicks, E.F.; Gajic, O. Mechanical ventilation in patients with Guillain-Barré syndrome. Respir. Care 2006, 51, 1403–1407. [Google Scholar]
- Witsch, J.; Lawn, N.D.; Wolter, T.D.; Wijdicks, E.F. Long-term outcome in patients with Guillain-Barré syndrome requiring mechanical ventilation. J. Neurol. 2013, 260, 1367–1374. [Google Scholar] [CrossRef] [PubMed]

| Measure | Categories | Score |
|---|---|---|
| Time from onset to admission | >7days | 0 |
| 4–7 days | 1 | |
| ≤3 days | 2 | |
| Facial or bulbar palsy on admission | Absence | 0 |
| Presence | 1 | |
| MRC score on admission | 60–51 | 0 |
| 50–41 | 1 | |
| 40–31 | 2 | |
| 30–21 | 3 | |
| ≤20 | 4 | |
| Total score | 0–7 |
| Item | Affected (n = 129) | Non-Affected (n = 326) | X2/t/Z | p | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 75 (58.1) | 209 (64.1) | 1.405 | 0.236 | |
| Female | 54 (41.9) | 117 (35.9) | |||
| Age A | 55 (40.5, 66) | 53 (40, 62) | −0.948 | 0.343 | |
| EMG type | |||||
| Demyelinating | 9 (7.0) | 51 (15.6) | |||
| Axon | 44 (34.1) | 113 (34.7) | 10.715 | 0.013 | |
| Both have | 71 (55.0) | 137 (42.0) | |||
| Both have not | 5 (3.9) | 25 (7.7) | |||
| Time from onset to admission | 4 (2, 7) | 7 (4, 12) | −5.132 | <0.001 | |
| Facial paralysis | 33 (25.6) | 74 (22.7) | 0.427 | 0.514 | |
| bulbar palsy | 70 (54.3) | 59 (18.1) | 59.511 | <0.001 | |
| MRC score A | |||||
| Admission | 39 (28, 52) | 52.5 (45, 59) | −7.228 | <0.001 | |
| Discharge | 36 (24, 51) | 54 (48, 60) | −9.209 | <0.001 | |
| Hughes score on admission A | 4 (3, 4) | 3 (2, 4) | −7.106 | <0.001 | |
| EGRIS score on admission A | 3 (2.00, 5.00) | 2 (1, 3) | −8.982 | <0.001 | |
| Total length of hospitalization A | 10 (6, 12.5) | 8 (6.75, 10) | −5.132 | 0.002 | |
| Pulmonary infection | 53 (41.1) | 57 (17.5) | 28.084 | <0.001 | |
| Infection | |||||
| URI | 36 (27.9) | 80 (24.5) | 7.010 | 0.200 | |
| Diarrhea | 18 (14.0) | 46 (14.1) | |||
| URI and diarrhea | 5 (3.9) | 6 (1.8) | |||
| Other | 11 (8.5) | 14 (4.3) | |||
| Non | 59 (45.7) | 180 (55.2) | |||
| Patients with Respiratory Muscle Paralysis (n = 129) | ||||||
|---|---|---|---|---|---|---|
| Items | Group 1 (n = 84) | Group 2 (n = 18) | Group 3 (n = 27) | X2/F/Z | p (α = 0.05) | |
| Gender | ||||||
| Male | 46 (54.8) | 10 (55.6) | 19 (70.4) | 2.103 | 0.350 | |
| Female | 38 (45.2) | 8 (44.4) | 8 (29.6) | |||
| Age | 49.80 ± 17.103 | 58.44 ± 16.992 | 58.22 ± 15.067 | 3.814 | 0.025 a | |
| EMG type | ||||||
| Demyelinating | 8 (9.5) | 0 (0.00) | 1 (3.7) | 7.787 | 0.194 | |
| Axon | 28 (33.3) | 3 (16.7) | 13 (48.1) | |||
| Both have | 44 (52.4) | 14 (77.8) | 13 (48.1) | |||
| Both have not | 4 (4.8) | 1 (5.6) | 0 (0.0) | |||
| Time from onset to admission | 5 (3,8) | 3 (2, 5) | 3 (2, 5) | 9.426 | 0.009 b | |
| Facial paralysis | 23 (27.4) | 5 (27.8) | 5 (18.5) | 0.896 | 0.639 | |
| bulbar palsy | 44 (52.4) | 9 (50.0) | 17 (63.0) | 1.075 | 0.584 | |
| MRC score | ||||||
| Admission | 41.5 (30, 54) | 44 (34, 54) | 28 (12, 42) | 12.636 | 0.002 c | |
| Discharge | 44.5 (30, 54) | 37.56 ± 14.513 | 12 (0, 30) | 27.227 | <0.001 d | |
| Hughes score on admission | 4 (2, 4) | 4 (3, 4) | 4 (4, 5) | 15.021 | 0.001 e | |
| EGRIS score on admission | 3 (2, 4) | 3.22 ± 1.833 | 5 (3, 6) | 13.675 | 0.001 f | |
| Total length of hospitalization | 9 (6, 11) | 10.5 (7,14) | 12 (5, 23) | 4.151 | 0.125 | |
| Pulmonary infection | 21 (25.0) | 11 (61.1) | 21 (77.8) | 26.979 | <0.001 | |
| Infection | ||||||
| URI | 25 (29.8) | 4 (22.2) | 7 (25.9) | 6.970 | 0.505 | |
| Diarrhea | 11 (13.1) | 2 (11.1) | 5 (18.5) | |||
| URI and diarrhea | 5 (6.0) | 0 (0.0) | 0 (0.0) | |||
| Other | 4 (4.8) | 3 (16.7) | 4 (14.8) | |||
| Non | 39 (46.4) | 9 (50.0) | 11 (40.7) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Wang, X.; Wang, X.; Li, G.; Zhong, D. An Analysis of Respiratory Muscle Paralysis of Adult Patients in Guillain–Barré Syndrome: A Retrospective Analysis. Medicina 2023, 59, 1267. https://doi.org/10.3390/medicina59071267
Wang A, Wang X, Wang X, Li G, Zhong D. An Analysis of Respiratory Muscle Paralysis of Adult Patients in Guillain–Barré Syndrome: A Retrospective Analysis. Medicina. 2023; 59(7):1267. https://doi.org/10.3390/medicina59071267
Chicago/Turabian StyleWang, Anqi, Xiaojing Wang, Xinrui Wang, Guozhong Li, and Di Zhong. 2023. "An Analysis of Respiratory Muscle Paralysis of Adult Patients in Guillain–Barré Syndrome: A Retrospective Analysis" Medicina 59, no. 7: 1267. https://doi.org/10.3390/medicina59071267
APA StyleWang, A., Wang, X., Wang, X., Li, G., & Zhong, D. (2023). An Analysis of Respiratory Muscle Paralysis of Adult Patients in Guillain–Barré Syndrome: A Retrospective Analysis. Medicina, 59(7), 1267. https://doi.org/10.3390/medicina59071267





