Higher Human Cytomegalovirus (HCMV) Specific IgG Antibody Levels in Plasma Samples from Patients with Metastatic Brain Tumors Are Associated with Longer Survival
Abstract
1. Introduction
2. Materials and Methods
Clinical Samples
3. HCMV Serology
Statistical Analysis
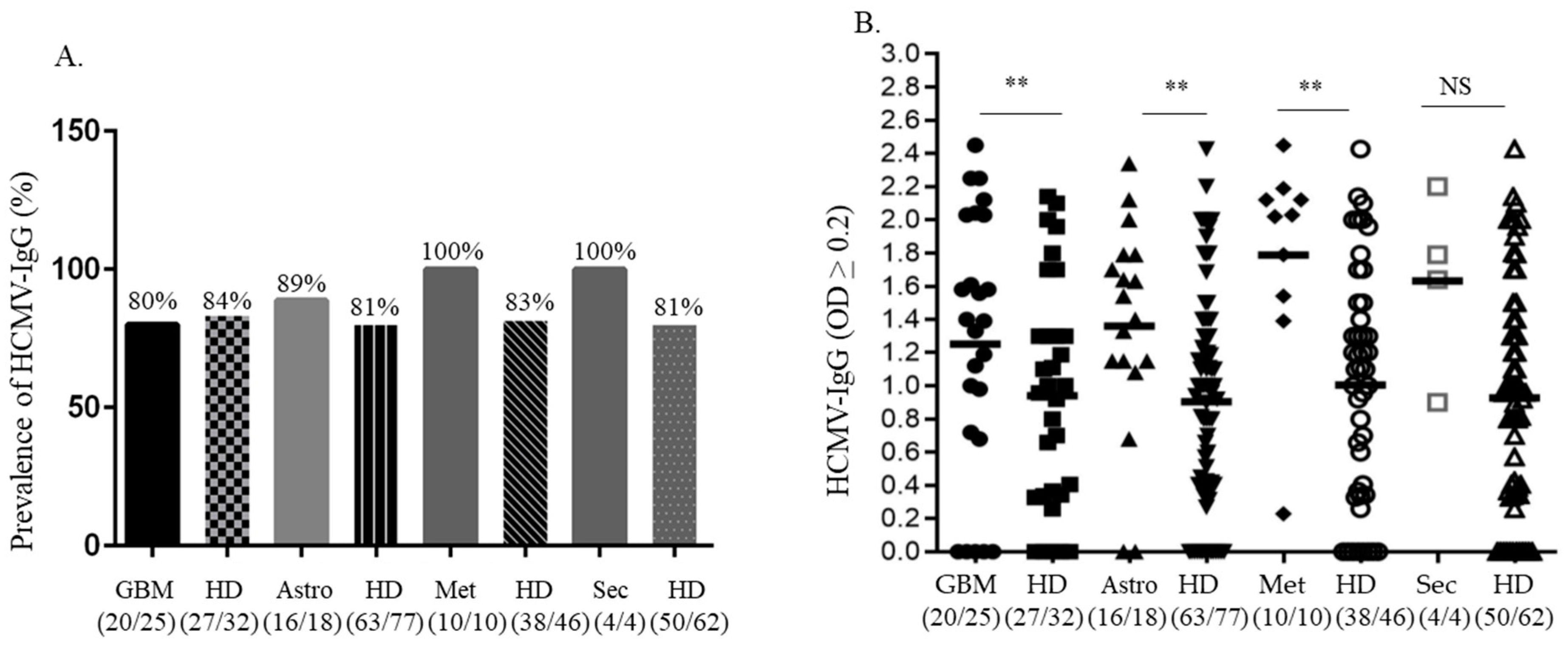
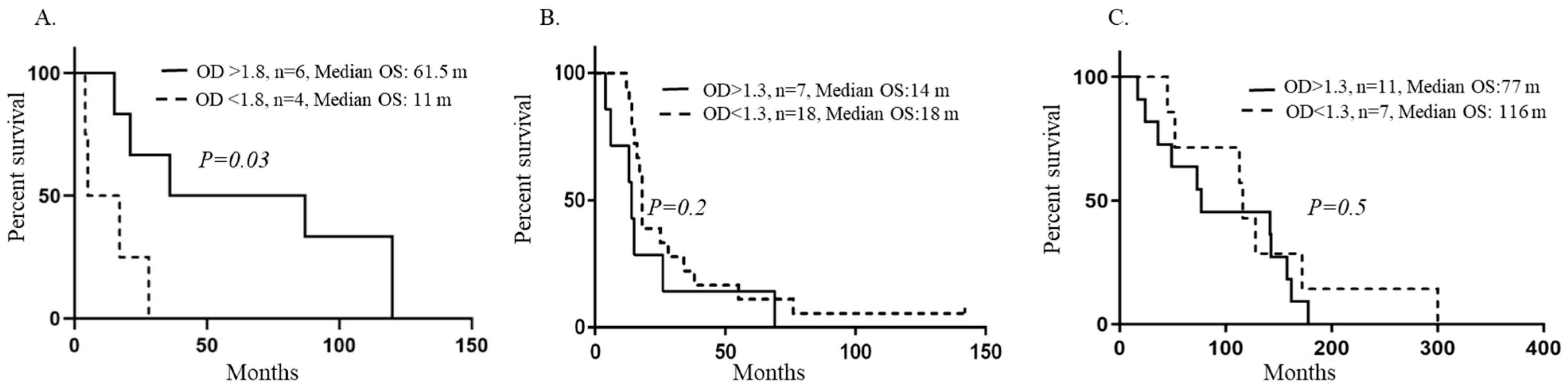
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cinatl, J.; Scholz, M.; Kotchetkov, R.; Vogel, J.U.; Doerr, H.W. Molecular mechanisms of the modulatory effects of HCMV infection in tumor cell biology. Trends Mol. Med. 2004, 10, 19–23. [Google Scholar] [CrossRef]
- Cobbs, C.S.; Harkins, L.; Samanta, M.; Gillespie, G.Y.; Bharara, S.; King, P.H.; Nabors, L.B.; Cobbs, C.G.; Britt, W.J. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002, 62, 3347–3350. [Google Scholar]
- Dziurzynski, K.; Chang, S.M.; Heimberger, A.B.; Kalejta, R.F.; Dallas, S.R.M.; Smit, M.; Soroceanu, L.; Cobbs, C.S. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro-Oncology 2012, 14, 246–255. [Google Scholar] [CrossRef]
- Michaelis, M.; Doerr, H.W.; Cinatl, J., Jr. Oncomodulation by human cytomegalovirus: Evidence becomes stronger. Med. Microbiol. Immunol. 2009, 198, 79–81. [Google Scholar] [CrossRef]
- Söderberg-Nauclér, C.; Johnsen, J.I. Cytomegalovirus infection in brain tumors: A potential new target for therapy? Oncoimmunology 2012, 1, 739–740. [Google Scholar] [CrossRef]
- Batich, K.A.; Reap, E.A.; Archer, G.E.; Sanchez-Perez, L.; Nair, S.K.; Schmittling, R.J.; Norberg, P.; Xie, W.; Herndon, J.E., 2nd; Healy, P.; et al. Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clin. Cancer Res. 2017, 23, 1898–1909. [Google Scholar] [CrossRef]
- Stragliotto, G.; Pantalone, M.R.; Rahbar, A.; Bartek, J.; Soderberg-Naucler, C. Valganciclovir as Add-on to Standard Therapy in Glioblastoma Patients. Clin. Cancer Res. 2020, 26, 4031–4039. [Google Scholar] [CrossRef]
- Pantalone, M.R.; Rahbar, A.; Soderberg-Naucler, C.; Stragliotto, G. Valganciclovir as Add-on to Second-Line Therapy in Patients with Recurrent Glioblastoma. Cancers 2022, 14, 1958. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Renzette, N.; Kowalik, T.F. Genetic analysis of cytomegalovirus in malignant gliomas. J. Virol. 2012, 86, 6815–6824. [Google Scholar] [CrossRef]
- Bianchi, E.; Roncarati, P.; Hougrand, O.; Guerin-El Khourouj, V.; Boreux, R.; Kroonen, J.; Martin, D.; Robe, P.; Rogister, B.; Delvenne, P.; et al. Human cytomegalovirus and primary intracranial tumours: Frequency of tumour infection and lack of correlation with systemic immune anti-viral responses. Neuropathol. Appl. Neurobiol. 2015, 41, e29–e40. [Google Scholar] [CrossRef]
- dos Santos, C.J.; Stangherlin, L.M.; Figueiredo, E.G.; Correa, C.; Teixeira, M.J.; da Silva, M.C.C. High prevalence of HCMV and viral load in tumor tissues and peripheral blood of glioblastoma multiforme patients. J. Med. Virol. 2014, 86, 1953–1961. [Google Scholar] [CrossRef]
- Libard, S.; Popova, S.N.; Amini, R.-M.; Karja, V.; Pietilainen, T.; Hamalainen, K.M.; Sundstrom, C.; Hesselager, G.; Bergqvist, M.; Ekman, S.; et al. Human cytomegalovirus tegument protein pp65 is detected in all intra- and extra-axial brain tumours independent of the tumour type or grade. PLoS ONE 2014, 9, e108861. [Google Scholar] [CrossRef]
- Lucas, K.G.; Bao, L.; Bruggeman, R.; Dunham, K.; Specht, C. The detection of CMV pp65 and IE1 in glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 231–238. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Xie, W.; Schmittling, R.; Learn, C.; Friedman, A.; McLendon, R.E.; Sampson, J.H. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-Oncology 2008, 10, 10–18. [Google Scholar] [CrossRef]
- Rahbar, A.; Orrego, A.; Peredo, I.; Dzabic, M.; Wolmer-Solberg, N.; Straat, K.; Stragliotto, G.; Soderberg-Naucler, C. Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2013, 57, 36–42. [Google Scholar] [CrossRef]
- Rahbar, A.; Peredo, I.; Solberg, N.W.; Taher, C.; Dzabic, M.; Xu, X.; Skarman, P.; Fornara, O.; Tammik, C.; Yaiw, K.; et al. Discordant humoral and cellular immune responses to Cytomegalovirus (CMV) in glioblastoma patients whose tumors are positive for CMV. Oncoimmunology 2015, 4, e982391. [Google Scholar] [CrossRef]
- Ranganathan, P.; Clark, P.A.; Kuo, J.S.; Salamat, M.S.; Kalejta, R.F. Significant association of multiple human cytomegalovirus genomic Loci with glioblastoma multiforme samples. J. Virol. 2012, 86, 854–864. [Google Scholar] [CrossRef]
- Sjostrom, S.; Hjalmars, U.; Juto, P.; Wadell, G.; Hallmans, G.; Tjonneland, A.; Halkjaer, J.; Manjer, J.; Almquist, M.; Melin, B.S. Human immunoglobulin G levels of viruses and associated glioma risk. Cancer Causes Control CCC 2011, 22, 1259–1266. [Google Scholar] [CrossRef]
- Stangherlin, L.M.; Castro, F.L.F.; Medeiros, R.S.S.; Guerra, J.M.; Kimura, L.M.; Shirata, N.K.; Nonogaki, S.; Dos Santos, C.J.; Silva, M.C.C. Human Cytomegalovirus DNA Quantification and Gene Expression in Gliomas of Different Grades. PLoS ONE 2016, 11, e0159604. [Google Scholar] [CrossRef]
- Baumgarten, P.; Michaelis, M.; Rothweiler, F.; Starzetz, T.; Rabenau, H.F.; Berger, A.; Jennewein, L.; Braczynski, A.K.; Franz, K.; Seifert, V.; et al. Human cytomegalovirus infection in tumor cells of the nervous system is not detectable with standardized pathologico-virological diagnostics. Neuro-Oncology 2014, 16, 1469–1477. [Google Scholar] [CrossRef]
- Cosset, E.; Petty, T.J.; Dutoit, V.; Cordey, S.; Padioleau, I.; Otten-Hernandez, P.; Farinelli, L.; Kaiser, L.; Bruyere-Cerdan, P.; Tirefort, D.; et al. Comprehensive metagenomic analysis of glioblastoma reveals absence of known virus despite antiviral-like type I interferon gene response. Int. J. Cancer 2014, 135, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, A.; Alenda, C.; Irles, E.; Ochoa, E.; Quintanar, T.; Rodriguez-Lescure, A.; Soto, J.L.; Barbera, V.M. Lack of cytomegalovirus detection in human glioma. Virol. J. 2017, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Holdhoff, M.; Guner, G.; Rodriguez, F.J.; Hicks, J.L.; Zheng, Q.; Forman, M.S.; Ye, X.; Grossman, S.A.; Meeker, A.K.; Heaphy, C.M.; et al. Absence of Cytomegalovirus in Glioblastoma and Other High-grade Gliomas by Real-time PCR, Immunohistochemistry, and in Situ Hybridization. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 3150–3157. [Google Scholar] [CrossRef]
- Lehrer, S.; Green, S.; Rosenzweig, K.E.; Rendo, A. No circulating human cytomegalovirus in 14 cases of glioblastoma. Neuro-Oncology 2015, 17, 320. [Google Scholar] [CrossRef]
- Poltermann, S.; Schlehofer, B.; Steindorf, K.; Schnitzler, P.; Geletneky, K.; Schlehofer, J.R. Lack of association of herpesviruses with brain tumors. J. Neurovirol. 2006, 12, 90–99. [Google Scholar] [CrossRef]
- Priel, E.; Wohl, A.; Teperberg, M.; Nass, D.; Cohen, Z.R. Human cytomegalovirus viral load in tumor and peripheral blood samples of patients with malignant gliomas. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2015, 22, 326–330. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ito, Y.; Isomura, H.; Takemura, N.; Okamoto, A.; Motomura, K.; Tsujiuchi, T.; Natsume, A.; Wakabayashi, T.; Toyokuni, S.; et al. Lack of presence of the human cytomegalovirus in human glioblastoma. Mod. Pathol. Off. J. United States Can. Acad. Pathol. 2014, 27, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Peredo-Harvey, I.; Rahbar, A.; Soderberg-Naucler, C. Presence of the Human Cytomegalovirus in Glioblastomas—A Systematic Review. Cancers 2021, 13, 5051. [Google Scholar] [CrossRef]
- Garcia-Martinez, A.; Irles, E.; Barbera, V.M.; Egoavil, C.; Alenda, C.; Castillejo, A.; Castillejo, M.I.; Ochoa, E.; Barea, M.C.; Quintanar, T.; et al. Lack of human cytomegalovirus in gliomas. A viral load analysis by quantitative real time-PCR. Lab. Investig. 2013, 93, 416A. [Google Scholar] [CrossRef]
- Hashida, Y.; Taniguchi, A.; Yawata, T.; Hosokawa, S.; Murakami, M.; Hiroi, M.; Ueba, T.; Daibata, M. Prevalence of human cytomegalovirus, polyomaviruses, and oncogenic viruses in glioblastoma among Japanese subjects. Infect. Agents Cancer 2015, 10, 3. [Google Scholar] [CrossRef]
- Matlaf, L.A.; Harkins, L.E.; Bezrookove, V.; Cobbs, C.S.; Soroceanu, L. Cytomegalovirus pp71 protein is expressed in human glioblastoma and promotes pro-angiogenic signaling by activation of stem cell factor. PLoS ONE 2013, 8, e68176. [Google Scholar] [CrossRef] [PubMed]
- El Baba, R.; Pasquereau, S.; Ahmad, S.H.; Monnien, F.; Abad, M.; Bibeau, F.; Herbein, G. EZH2-Myc driven glioblastoma elicited by cytomegalovirus infection of human astrocytes. Oncogene 2023, 42, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Herbein, G. High-Risk Oncogenic Human Cytomegalovirus. Viruses 2022, 14, 2462. [Google Scholar] [CrossRef] [PubMed]
- Ahlfors, K.; Forsgren, M.; Ivarsson, S.A.; Harris, S.; Svanberg, L. Congenital cytomegalovirus infection: On the relation between type and time of maternal infection and infant’s symptoms. Scand. J. Infect. Dis. 1983, 15, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Söderberg-Nauclér, C.; Fish, K.N.; Nelson, J.A. Reactivation of Latent Human Cytomegalovirus by Allogeneic Stimulation of Blood Cells from Healthy Donors. Cell 1997, 91, 119–126. [Google Scholar] [CrossRef]
- Reeves, M.B.; MacAry, P.A.; Lehner, P.J.; Sissons, J.G.; Sinclair, J.H. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc. Natl. Acad. Sci. USA 2005, 102, 4140–4145. [Google Scholar] [CrossRef]
- Farias, K.; Moreli, M.L.; Floriano, V.G.; da Costa, V.G. Evidence based on a meta-analysis of human cytomegalovirus infection in glioma. Arch. Virol. 2019, 164, 1249–1257. [Google Scholar] [CrossRef]
- Harkins, L.; Volk, A.L.; Samanta, M.; Mikolaenko, I.; Britt, W.J.; Bland, K.I.; Cobbs, C.S. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 2002, 360, 1557–1563. [Google Scholar] [CrossRef]
- Samanta, M.; Harkins, L.; Klemm, K.; Britt, W.J.; Cobbs, C.S. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J. Urol. 2003, 170, 998–1002. [Google Scholar] [CrossRef]
- Touma, J.; Liu, Y.; Rahbar, A.; Pantalone, M.R.; Almazan, N.M.; Vetvik, K.; Soderberg-Naucler, C.; Geisler, J.; Sauer, T. Detection of Human Cytomegalovirus Proteins in Paraffin-Embedded Breast Cancer Tissue Specimens—A Novel, Automated Immunohistochemical Staining Protocol. Microorganisms 2021, 9, 1059. [Google Scholar] [CrossRef]
- Radestad, A.F.; Estekizadeh, A.; Cui, H.L.; Kostopoulou, O.N.; Davoudi, B.; Hirschberg, A.L.; Carlson, J.; Rahbar, A.; Soderberg-Naucler, C. Impact of Human Cytomegalovirus Infection and its Immune Response on Survival of Patients with Ovarian Cancer. Transl. Oncol. 2018, 11, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Taher, C.; Frisk, G.; Fuentes, S.; Religa, P.; Costa, H.; Assinger, A.; Vetvik, K.K.; Bukholm, I.R.; Yaiw, K.C.; Smedby, K.E.; et al. High prevalence of human cytomegalovirus in brain metastases of patients with primary breast and colorectal cancers. Transl. Oncol. 2014, 7, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Krenzlin, H.; Zdioruk, M.; Nowicki, M.O.; Finkelberg, T.; Keric, N.; Lemmermann, N.; Skubal, M.; Chiocca, E.A.; Cook, C.H.; Lawler, S.E. Cytomegalovirus infection of glioblastoma cells leads to NF-kappaB dependent upregulation of the c-MET oncogenic tyrosine kinase. Cancer Lett. 2021, 513, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, L.; Matlaf, L.; Khan, S.; Akhavan, A.; Singer, E.; Bezrookove, V.; Decker, S.; Ghanny, S.; Hadaczek, P.; Bengtsson, H.; et al. Cytomegalovirus Immediate-Early Proteins Promote Stemness Properties in Glioblastoma. Cancer Res. 2015, 75, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Söderberg-Nauclér, C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J. Intern. Med. 2006, 259, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Goerig, N.L.; Frey, B.; Korn, K.; Fleckenstein, B.; Uberla, K.; Schmidt, M.A.; Dorfler, A.; Engelhorn, T.; Eyupoglu, I.; Ruhle, P.F.; et al. Early Mortality of Brain Cancer Patients and its Connection to Cytomegalovirus Reactivation During Radiochemotherapy. Clin. Cancer Res. 2020, 26, 3259–3270. [Google Scholar] [CrossRef]
- Fornara, O.; Bartek, J., Jr.; Rahbar, A.; Odeberg, J.; Khan, Z.; Peredo, I.; Hamerlik, P.; Bartek, J.; Stragliotto, G.; Landazuri, N.; et al. Cytomegalovirus infection induces a stem cell phenotype in human primary glioblastoma cells: Prognostic significance and biological impact. Cell Death Differ. 2016, 23, 261–269. [Google Scholar] [CrossRef]
- Krenzlin, H.; Behera, P.; Lorenz, V.; Passaro, C.; Zdioruk, M.; Nowicki, M.O.; Grauwet, K.; Zhang, H.; Skubal, M.; Ito, H.; et al. Cytomegalovirus promotes murine glioblastoma growth via pericyte recruitment and angiogenesis. J. Clin. Investig. 2019, 129, 1671–1683. [Google Scholar] [CrossRef]
- Foster, H.; Piper, K.; DePledge, L.; Li, H.F.; Scanlan, J.; Jae-Guen, Y.; Boeckh, M.; Cobbs, C. Human cytomegalovirus seropositivity is associated with decreased survival in glioblastoma patients. Neuro-Oncol. Adv. 2019, 1, vdz020. [Google Scholar] [CrossRef]
- Liu, Z.; Poiret, T.; Meng, Q.; Rao, M.; von Landenberg, A.; Schoutrop, E.; Valentini, D.; Dodoo, E.; Peredo-Harvey, I.; Maeurer, M. Epstein-Barr virus- and cytomegalovirus-specific immune response in patients with brain cancer. J. Transl. Med. 2018, 16, 182. [Google Scholar] [CrossRef]
- Baryawno, N.; Rahbar, A.; Wolmer-Solberg, N.; Taher, C.; Odeberg, J.; Darabi, A.; Khan, Z.; Sveinbjornsson, B.; FuskevAg, O.M.; Segerstrom, L.; et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J. Clin. Investig. 2011, 121, 4043–4055. [Google Scholar] [CrossRef] [PubMed]
- Wolmer-Solberg, N.; Baryawno, N.; Rahbar, A.; Fuchs, D.; Odeberg, J.; Taher, C.; Wilhelmi, V.; Milosevic, J.; Mohammad, A.A.; Martinsson, T.; et al. Frequent detection of human cytomegalovirus in neuroblastoma: A novel therapeutic target? Int. J. Cancer 2013, 133, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Heukers, R.; Fan, T.S.; de Wit, R.H.; van Senten, J.R.; De Groof, T.W.M.; Bebelman, M.P.; Lagerweij, T.; Vieira, J.; de Munnik, S.M.; Smits-de Vries, L.; et al. The constitutive activity of the virally encoded chemokine receptor US28 accelerates glioblastoma growth. Oncogene 2018, 37, 4110–4121. [Google Scholar] [CrossRef] [PubMed]
- Vanderbeek, A.M.; Rahman, R.; Fell, G.; Ventz, S.; Chen, T.; Redd, R.; Parmigiani, G.; Cloughesy, T.F.; Wen, P.Y.; Trippa, L.; et al. The clinical trials landscape for glioblastoma: Is it adequate to develop new treatments? Neuro-Oncology 2018, 20, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
| Diagnosis | Gender M F (%) | Age (Median, Y) (Interval) | MIB Index (Interval) | OS (Median) (m) | Alive (n) |
|---|---|---|---|---|---|
| Primary GBM (n = 25) | 20 5 (80) (20) | 61 (44–74) | 10–60 | 18 | 1 |
| Astrocytoma (Grades II–III, n = 18) | 11 7 (61) (39) | 44 (23–58) | 5–40 | 114.5 | 4 |
| Brain Metastasis * (n = 10) | 4 6 (40) (60) | 59 (27–70) | ND | 5 | 0 |
| Secondary GBM (n = 4) | 2 2 (50) (50) | 53.5 (29–73) | 15–100 | 76.5 | 0 |
| Ganglioglioma (Grade 1) (n = 1) | 1 - | 20 | ND | 127 | 1 |
| Meningioma (n = 1) | - 1 | 16 | ND | 146 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peredo-Harvey, I.; Bartek, J., Jr.; Ericsson, C.; Yaiw, K.-C.; Nistér, M.; Rahbar, A.; Söderberg-Naucler, C. Higher Human Cytomegalovirus (HCMV) Specific IgG Antibody Levels in Plasma Samples from Patients with Metastatic Brain Tumors Are Associated with Longer Survival. Medicina 2023, 59, 1248. https://doi.org/10.3390/medicina59071248
Peredo-Harvey I, Bartek J Jr., Ericsson C, Yaiw K-C, Nistér M, Rahbar A, Söderberg-Naucler C. Higher Human Cytomegalovirus (HCMV) Specific IgG Antibody Levels in Plasma Samples from Patients with Metastatic Brain Tumors Are Associated with Longer Survival. Medicina. 2023; 59(7):1248. https://doi.org/10.3390/medicina59071248
Chicago/Turabian StylePeredo-Harvey, Inti, Jiri Bartek, Jr., Christer Ericsson, Koon-Chu Yaiw, Monica Nistér, Afsar Rahbar, and Cecilia Söderberg-Naucler. 2023. "Higher Human Cytomegalovirus (HCMV) Specific IgG Antibody Levels in Plasma Samples from Patients with Metastatic Brain Tumors Are Associated with Longer Survival" Medicina 59, no. 7: 1248. https://doi.org/10.3390/medicina59071248
APA StylePeredo-Harvey, I., Bartek, J., Jr., Ericsson, C., Yaiw, K.-C., Nistér, M., Rahbar, A., & Söderberg-Naucler, C. (2023). Higher Human Cytomegalovirus (HCMV) Specific IgG Antibody Levels in Plasma Samples from Patients with Metastatic Brain Tumors Are Associated with Longer Survival. Medicina, 59(7), 1248. https://doi.org/10.3390/medicina59071248









