Monitoring the Coagulation Profile of COVID-19 Patients Using Standard and ClotPro® Hemostasis Tests
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Inflammation and Coagulation Parameters and Mortality
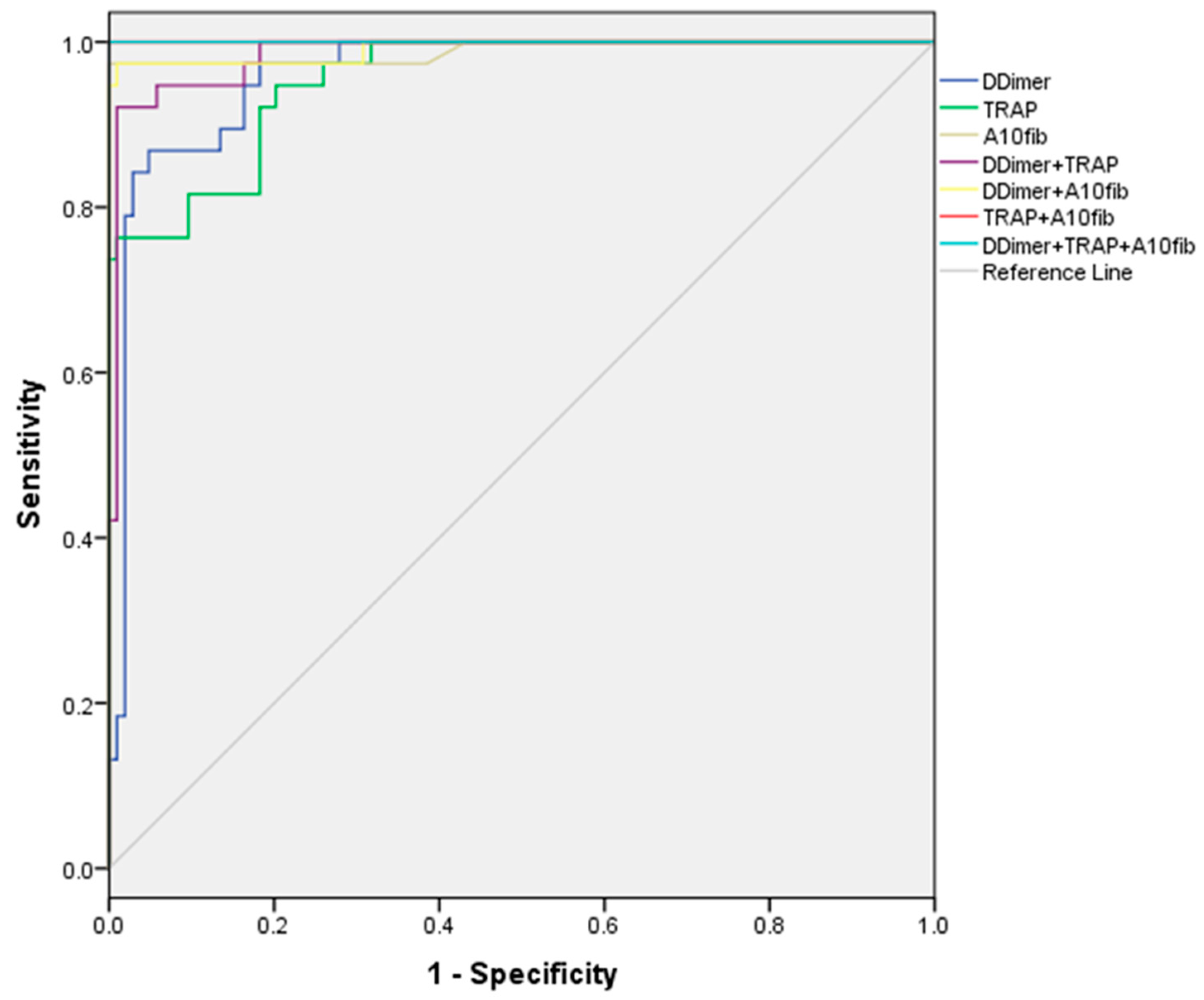
3.2. Cutoff Values of Tests to Discriminate Patient Mortality
3.3. Correlation between Analyzed Parameters
3.4. Predictors of Mortality
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481, Erratum in Lancet Respir. Med. 2020, 8, e26. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef]
- Huertas, A.; Montani, D.; Savale, L.; Pichon, J.; Tu, L.; Parent, F.; Humbert, M. Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020, 56, 2001634. [Google Scholar] [CrossRef]
- Ji, P.; Zhu, J.; Zhong, Z.; Li, H.; Pang, J.; Li, B.; Zhang, J. Association of elevated inflammatory markers and severe COVID-19: A meta-analysis. Medicine 2020, 99, e23315. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Semeraro, F.; Ammollo, C.T.; Caironi, P.; Masson, S.; Latini, R.; Panigada, M.; Pesenti, A.; Semeraro, N.; Gattinoni, L.; Colucci, M. D-dimer corrected for thrombin and plasmin generation is a strong predictor of mortality in patients with sepsis. Blood Transfus 2019, 18, 304–311. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leucoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef]
- Han, H.; Yang, L.; Liu, R.; Liu, F.; Wu, K.L.; Li, J.; Liu, X.H.; Zhu, C.L. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020, 58, 1116–1120. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Zátroch, I.; Smudla, A.; Babik, B.; Tánczos, K.; Kóbori, L.; Szabó, Z.; Fazakas, J. Procoagulation, hypercoagulation and fibrinolytic “shut down” detected with ClotPro® viscoelastic tests in COVID-19 patients. Orv. Hetil. 2020, 161, 899–907. [Google Scholar] [CrossRef]
- Bachler, M.; Bösch, J.; Stürzel, D.P.; Hell, T.; Giebl, A.; Ströhle, M.; Klein, S.J.; Schäfer, V.; Lehner, G.F.; Joannidis, M.; et al. Impaired fibrinolysis in critically ill COVID-19 patients. Br. J. Anaesth 2020, 126, 590–598. [Google Scholar] [CrossRef]
- Kruse, J.M.; Magomedov, A.; Kurreck, A.; Münch, F.H.; Koerner, R.; Kamhieh-Milz, J.; Kahl, A.; Gotthardt, I.; Piper, S.K.; Eckardt, K.U.; et al. Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. Crit Care. 2020, 24, 676. [Google Scholar] [CrossRef] [PubMed]
- Tescione, M.; Vadalà, E.; Marano, G.; Bruni, A.; Garofalo, E.; Lavalle, O.; Longhini, F.; Battaglia, E.; Polimeni, N.; Labate, D.; et al. Aggregometry and thromboelastography to identify the timing to trach a COVID-19 patient receiving both antiplatelet therapy and enoxaparin. Clin. Case Rep. 2021, 9, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Clinical Management of COVID-19: Interim Guidance, 27 May 2020. No. WHO/2019-nCoV/clinical/2020.5; World Health Organization: Geneva, Switzerland, 2020.
- Pantic, N.; Pantic, I.; Jevtic, D.; Mogulla, V.; Oluic, S.; Durdevic, M.; Nordin, T.; Jecmenica, M.; Milovanovic, T.; Gavrancic, T.; et al. Celiac Disease and Thrombotic Events: Systematic Review of Published Cases. Nutrients 2022, 14, 2162. [Google Scholar] [CrossRef]
- Esmon, C.T. The protein C pathway. Chest 2003, 124 (Suppl. S3), 26S–32S. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Gross-Cohen, M.; Weissmann, M.; Ilan, N.; Sanderson, R.D. Opposing Functions of Heparanase-1 and Heparanase-2 in Cancer Progression. Trends Biochem. Sci. 2018, 43, 18–31. [Google Scholar] [CrossRef]
- Vandevelde, A.; Devreese, K.M.J. Laboratory Diagnosis of Antiphospholipid Syndrome: Insights and Hindrances. J. Clin. Med. 2022, 11, 2164. [Google Scholar] [CrossRef]
- Sabina, S. COVID19 biomarkers: What did we learn from systematic reviews? Front. Cell. Infect. Microbiol. 2022, 12, 1844. [Google Scholar]
- Gralinski, L.E.; Bankhead, A., 3rd; Jeng, S.; Menachery, V.D.; Proll, S.; Belisle, S.E.; Matzke, M.; Webb-Robertson, B.-J.; Luna, M.L.; Shukla, A.K.; et al. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. MBio 2013, 4.4, e00271-13. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.; Clark, C.; Iba, T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef]
- Zou, K.H.; O’Malley, A.J.; Mauri, L. Receiver-Operating Characteristic Analysis for Evaluating Diagnostic Tests and Predictive Models. Circulation 2007, 115, 654–657. [Google Scholar] [CrossRef]
- Perić, V.S.; Golubović, M.D.; Lazarević, M.V.; Kostić, T.L.; Stokanović, D.S.; Đorđević, M.N.; Marjanović, V.G.; Stošić, M.D.; Milić, D.J. Predictive potential of biomarkers and risk scores for major adverse cardiac events in elderly patients undergoing major elective vascular surgery. Rev. Cardiovasc. Med. 2021, 22, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Baroiu, L.; Lese, A.C.; Stefanopol, I.A.; Iancu, A.; Dumitru, C.; Ciubara, A.B.; Bujoreanu, F.C.; Baroiu, N.; Ciubara, A.; Nechifor, A.; et al. The role of D-Dimers in the initial evaluation of COVID-19. Ther. Clin. Risk Manag. 2022, 18, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Almskog, L.M.; Wikman, A.; Svensson, J.; Wanecek, M.; Bottai, M.; van der Linden, J.; Ågren, A. Rotational thromboelastometry predicts care level in COVID-19. MedRxiv 2020, 2020-06. [Google Scholar]
- Ibañez, C.; Perdomo, J.; Calvo, A.; Ferrando, C.; Reverter, J.C.; Tassies, D.; Blasi, A. High D dimers and low global fibrinolysis coexist in COVID19 patients: What is going on in there? J. Thromb. Thrombolysis 2020, 15, 1–5. [Google Scholar] [CrossRef]
- Reed, M.J.; Nimmo, A.F.; McGee, D.; Manson, L.; Neffendorf, A.E.; Moir, L.; Donaldson, L.S. Rotational thrombolelas-tometry produces potentially clinical useful results within10 min in bleeding Emergency Department patients: TheDEUCE study. Eur. J. Emerg. Med. 2013, 20, 160–166. [Google Scholar] [CrossRef]
- Kelly, J.M.; Rizoli, S.; Veigas, P.; Hollands, S.; Min, A. Using rotational thromboelastometry clot firmness at 5 minutes (ROTEM®EXTEM A5) to predict massive transfusion and in-hospital mortality in trauma: A retrospective analysis of 1146 patients. Anaesthesia 2018, 73, 1103–1109. [Google Scholar] [CrossRef]
- Heubner, L.; Greiner, M.; Vicent, O.; Beyer-Westendorf, J.; Tiebel, O.; Scholz, U.; Güldner, A.; Mirus, M.; Fries, D.; Koch, T.; et al. Predictive ability of viscoelastic testing using ClotPro® for short-term outcome in patients with severe COVID-19 ARDS with or without ECMO therapy: A retrospective study. Thromb. J. 2022, 20, 48. [Google Scholar] [CrossRef]
- Herrmann, J.; Notz, Q.; Schlesinger, T.; Stumpner, J.; Kredel, M.; Sitter, M.; Schmid, B.; Kranke, P.; Schulze, H.; Meybohm, P.; et al. Point of care diagnostic of hypercoagulability and platelet function in COVID-19 induced acute respiratory distress syndrome: A retrospective observational study. Thromb. J. 2021, 19, 39. [Google Scholar] [CrossRef]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef]
- Hottz, E.D.; Azevedo-Quintanilha, I.G.; Palhinha, L.; Teixeira, L.; Barreto, E.A.; Pão, C.R.; Righy, C.; Franco, S.; Souza, T.M.; Kurtz, P.; et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 2020, 36, 1330–1341. [Google Scholar] [CrossRef]
- Ellinor IB Peerschke Donna DCastellone, A.K. Stroobants, John Francis. Reference Range Determination for Whole-Blood Platelet Aggregation Using the Multiplate Analyzer. Am. J. Clin. Pathol. 2014, 142, 647–656. [Google Scholar]
- Guarino, M.; Perna, B.; Maritati, M.; Remelli, F.; Trevisan, C.; Spampinato, M.D.; Costanzini, A.; Volpato, S.; Contini, C.; De Giorgio, R. Presepsin levels and COVID-19 severity: A systematic review and meta-analysis. Clin. Exp. Med. 2022, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Assal, H.H.; Abdelrahman, S.M.; Abdelbasset, M.A.; Abdelaziz, M.; Sabry, I.M.; Shaban, M.M. Presepsin as a Novel Biomarker in predicting In-hospital Mortality in Patients With COVID-19 Pneumonia. Int. J. Infect. Dis. 2022, 118, 155–163. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Dong, X.; Liu, G.-H.; Gao, Y.-D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allerg. Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef]
| Survival (N = 104) | Mortality (N = 38) | t * or Z ** or χ2 *** (p) | ||
|---|---|---|---|---|
| Age (years), mean ± SD | 62.67 ± 12.10 | 66.71 ± 8.44 | 2.229 (0.028) * | |
| Fibrinogen concentration (2–4 g/L), mean ± SD | 7.62 ± 1.54 | 8.56 ± 2.29 | 2.344 (0.023) * | |
| Fibrinogen concentration (>4 g/L), no. of patients (%) | 103 (99.0%) | 34 (89.5%) | 4.944 (0.018) *** | |
| Anti-Xa values (0.7–1), mean ± SD | 0.38 ± 0.14 | 0.37 ± 0.15 | 0.323 (0.747) * | |
| INR (0.85–1.25), mean ± SD | 1.26 ± 0.16 | 1.48 ± 0.23 | 5.500 (˂0.0001) * | |
| INR (>1), no. of patients (%) | 102 (98.1%) | 38 (100.0%) | 0.003 (1.000) *** | |
| D-Dimer (−230 ng/mL), mean ± SD | 444.0 (407.0–737.5) | 1285.0 (970.0–1542.0) | 8.393 (˂0.0001) ** | |
| D-Dimer (>230 ng/mL), no. of patients (%) | 89 (85.6%) | 38 (100.0%) | 4.697 (0.011) *** | |
| D-Dimer (>1000 ng/mL), no. of patients (%) | 2 (1.9%) | 27 (71.1%) | 77.640 (˂0.0001) *** | |
| aPTT (29–34 s), mean ± SD | 37.53 ± 8.92 | 36.38 ± 7.14 | 0.712 (0.478) * | |
| aPTT (s), no. of patients (%) | <34 | 45 (43.3%) | 20 (52.6%) | 1.348 (0.510) *** |
| 34–38 | 14 (13.5%) | 3 (7.9%) | ||
| >38 | 45 (43.3%) | 15 (39.5%) | ||
| ADP test (590–1130 AU/min), mean ± SD | 365.14 ± 150.88 | 670.21 ± 224.77 | 7.753 (˂0.0001) * | |
| ADP test (AU/min), no. of patients (%) | <406 | 61 (58.7%) | 3 (7.9%) | 37.248 (˂0.0001) *** |
| 406–992 | 43 (41.3%) | 30 (78.9%) | ||
| >992 | 0 (0.0%) | 5 (13.2%) | ||
| ADP test (AU/min) (>590), no. of patients (%) | 5 (4.8%) | 24 (63.2%) | 54.771 (˂0.0001) *** | |
| ASPI test (790–1490 AU/min), mean ± SD | 486.61 ± 229.59 | 843.84 ± 217.04 | 8.326 (˂0.0001) * | |
| ASPI test (<790 AU/min), no. of patients (%) | 103 (99.0%) | 15 (39.5%) | 66.129 (˂0.0001) *** | |
| ASPI test (>800 AU/min), no. of patients (%) | 0 (0.0%) | 22 (57.9%) | 66.896 (˂0.0001) *** | |
| TRAP test (923–1509 AU/min), mean ± SD | 548.25 ± 293.04 | 1375.90 ± 367.61 | 13.884 (˂0.0001) * | |
| TRAP test (AU/min), | <923 | 94 (90.4%) | 7 (18.4%) | 78.997 (˂0.0001) *** |
| 923–1509 | 10 (9.6%) | 14 (36.8%) | ||
| >1509 | 0 (0.0%) | 17 (44.7%) | ||
| TRAP test (>1500 AU/min), no. of patients (%) | 0 (0.0%) | 20 (52.6%) | 59.435 (˂0.0001) *** | |
| PSEP (˂337 pg/mL), mean | 293.0 (239.0–395.5) | 593.0 (446.2–743.8) | 5.074 (˂0.0001) ** | |
| PSEP (>337 pg/mL), no. of patients (%) | 27 (26.0%) | 33 (86.8%) | 39.818 (˂0.0001) *** | |
| PSEP (>1000 pg/mL), no. of patients (%) | 0 (0.0%) | 3 (7.9%) | 5.005 (0.018) *** | |
| CT ex-test (s), mean ± SD | 65.38 ± 16.38 | 57.58 ± 15.77 | 2.535 (0.012) * | |
| CT EX-test (38–65 s), no. of patients (%) | <38 | 1 (1.0%) | 3 (7.9%) | 8.958 (0.011) *** |
| 38–65 | 47 (45.2%) | 23 (60.5%) | ||
| >65 | 56 (53.8%) | 12 (31.6%) | ||
| A5 EX-test (39–58 mm), mean | 53.0 (47.0–56.0) | 56.5 (51.0–60.0) | 2.628 (0.009) ** | |
| A5 EX-test (<39 mm), no. of patients (%) | 7 (6.7%) | 0 (0.0%) | 1.446 (0.190) *** | |
| A10 EX-test (47–64 mm), mean | 59.5 (55.0–63.0) | 63.5 (58.5–66.0) | 3.174 (0.002) ** | |
| A10 EX-test (mm), no. of patients (%) | <38 | 10 (9.6%) | 0 (0.0%) | 6.415 (0.040) *** |
| 38–64 | 89 (85.6%) | 33 (86.8%) | ||
| >64 | 5 (4.8%) | 5 (13.2%) | ||
| MCF EX-test (53–67 mm), mean | 61.0 (58.0–64.0) | 63.0 (60.0–67.2) | 2.405 (0.016) ** | |
| MCF EX-test (mm), no. of patients (%) | <53 | 4 (3.8%) | 0 (0.0%) | 2.099 (0.350) *** |
| 53–68 | 89 (85.6%) | 32 (84.2%) | ||
| >67 | 11 (10.6%) | 6 (15.8%) | ||
| CT FIB-test (55–87 s), mean | 67.5 (45.2–86.5) | 63.0 (47.8–73.5) | 0.332 (0.740) ** | |
| CT FIB-test (>70 s), no. of patients (%) | 46 (44.2%) | 12 (31.6%) | 1.357 (0.185) *** | |
| A5 FIB-test (6–21 mm), mean ± SD | 22.62 ± 6.43 | 27.45 ± 6.58 | 3.939 (˂ 0.0001) * | |
| A5 FIB-test (>9 mm), no. of patients (%) | 103 (99.0%) | 38 (100.0%) | 0.000 (1.000) *** | |
| A10 FIB-test (7–23 mm), mean ± SD | 17.48 ± 4.67 | 36.26 ± 4.46 | 21.472 (˂0.0001) * | |
| A10 FIB-test (>23 mm), no. of patients (%) | 13 (12.5%) | 37 (97.4%) | 84.189 (˂0.0001) *** | |
| MCF FIB-test (9–27 mm), mean ± SD | 24.62 ± 7.63 | 30.90 ± 7.91 | 4.294 (˂0.0001) * | |
| MCF FIB-test (>25 mm), no. of patients (%) | 48 (46.2%) | 28 (73.7%) | 7.409 (0.004) *** | |
| AUC (95% CI for AUC) | p | Cutoff | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| Age | 0.637 (0.536–737) | 0.013 | 68 | 71.1 | 64.4 |
| Fibrinogen (g/L) | 0.702 (0.591–0813) | 0.000 | 9.14 | 71.1 | 74.0 |
| Anti-Xa | 0.487 (0.378–0.596) | 0.816 | 0.4 | 55.3 | 47.1 |
| INR | 0.790 (0.696–0.884) | 0.000 | 1.38 | 76.3 | 75.0 |
| D-Dimer (ng/mL) | 0.961 (0.930–0.991) | 0.000 | 860 | 86.8 | 95.2 |
| aPTT (s) | 0.474 (0.373–0.575) | 0.637 | 31.2 | 73.7 | 37.5 |
| ADP (AU/min) | 0.878 (0.816–0.940) | 0.000 | 591 | 63.2 | 95.2 |
| ASPI (AU/min) | 0.848 (0.772–0.924) | 0.000 | 728 | 65.8 | 97.1 |
| TRAP (AU/min) | 0.955 (0.923–0.987) | 0.000 | 1180 | 76.3 | 99.0 |
| PSEP (ng/mL) | 0.779 (0.701–0.856) | 0.000 | 335 | 89.5 | 74.0 |
| CT EX-test (s) | 0.364 (0.261–0.468) | 0.013 | 37 | 100.0 | 1.0 |
| A5 EX-test (mm) | 0.644 (0.544–0.744) | 0.009 | 57 | 50.0 | 77.9 |
| A10 EX-test (mm) | 0.674 (0.574–0.774) | 0.002 | 64 | 50.0 | 82.7 |
| MCF EX-test (mm) | 0.632 (0.533–0.730) | 0.016 | 59 | 97.4 | 27.9 |
| CT FIB-test (s) | 0.482 (0.381–0.583) | 0.740 | 43 | 97.4 | 22.1 |
| A5 FIB-test (mm) | 0.704 (0.597–0.810) | 0.000 | 28 | 68.4 | 78.8 |
| A10 FIB-test (mm) | 0.989 (0.968–1.000) | 0.000 | 30 | 97.4 | 100.0 |
| MCF FIB-test (mm) | 0.703 (0.607–0.798) | 0.000 | 36 | 42.1 | 94.2 |
| FI | Anti-Xa | INR | DD | aPTT | ADP | ASPI | TRAP | PSEP | CT EX-Test | A5 EX-Test | A10 EX-Test | MCF EX-Test | CFT EX-Test | CT FIB-Test | A5 FIB-Test | A10 FIB-Test | MCF FIB-Test | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | −0.058 0.497 | 0.226 0.007 | 0.284 0.001 | 0.176 0.036 | 0.274 0.001 | −0.010 0.910 | −0.104 0.219 | 0.092 0.276 | 0.114 0.175 | 0.273 0.001 | 0.066 0.438 | 0.098 0.244 | 0.117 0.165 | −0.029 0.731 | 0.390 0.000 | 0.208 0.013 | 0.338 0.000 | 0.152 0.071 |
| Fibrinogen (g/L) | 0.046 0.584 | 0.011 0.899 | 0.261 0.002 | 0.296 0.000 | 0.078 0.354 | 0.156 0.064 | 0.181 0.031 | 0.073 0.391 | −0.274 0.001 | 0.432 0.000 | 0.405 0.000 | 0.396 0.000 | −0.177 0.035 | −0.390 0.000 | 0.680 0.000 | 0.110 0.195 | 0.613 0.000 | |
| Anti-Xa | 0.042 0.622 | −0.026 0.757 | 0.290 0.000 | 0.038 0.653 | −0.043 0.612 | −0.006 0.940 | −0.033 0.696 | 0.062 0.467 | 0.006 0.942 | 0.011 0.897 | −0.018 0.834 | 0.042 0.621 | 0.076 0.366 | 0.143 0.089 | 0.029 0.731 | 0.089 0.290 | ||
| INR | 0.351 0.000 | 0.093 0.272 | 0.249 0.003 | 0.192 0.022 | 0.218 0.009 | 0.144 0.088 | −0.158 0.061 | 0.184 0.029 | 0.145 0.086 | 0.090 0.288 | −0.167 0.047 | −0.107 0.207 | 0.305 0.000 | 0.500 0.000 | 0.334 0.000 | |||
| D-Dimer | 0.146 0.083 | 0.487 0.000 | 0.513 0.000 | 0.555 0.000 | 0.196 0.019 | 0.037 0.660 | 0.341 0.000 | 0.362 0.000 | 0.319 0.000 | −0.150 0.075 | −0.007 0.933 | 0.357 0.000 | 0.572 0.000 | 0.371 0.000 | ||||
| aPTT (s) | −0.031 0.711 | 0.087 0.305 | −0.012 0.883 | −0.090 0.288 | 0.283 0.001 | 0.157 0.062 | 0.160 0.057 | 0.227 0.006 | −0.090 0.286 | 0.069 0.411 | 0.421 0.000 | −0.048 0.572 | 0.461 0.000 | |||||
| ADP (AU/min) | 0.838 0.000 | 0.768 0.000 | 0.175 0.038 | −0.135 0.106–9 | 0.392 0.000 | 0.444 0.000 | 0.346 0.000 | −0.291 0.000 | −0.178 0.034 | 0.147 0.081 | 0.532 0.000 | 0.312 0.000 | ||||||
| ASPI (AU/min) | 0.794 0.000 | 0.159 0.058 | −0.266 0.001 | 0.490 0.000 | 0.574 0.000 | 0.490 0.000 | −0.359 0.000 | −0.362 0.000 | 0.306 0.000 | 0.424 0.000 | 0.414 0.000 | |||||||
| TRAP (AU/min) | 0.184 0.029 | −0.247 0.003 | 0.426 0.000 | 0.422 0.000 | 0.440 0.000 | −0.286 0.001 | 0.206 0.014 | 0.259 0.002 | 0.627 0.000 | 0.313 0.000 | ||||||||
| PSEP (pg/mL) | −0.080 0.344 | −0.022 0.793 | −0.001 0.992 | −0.021 0.801 | 0.024 0.778 | 0.069 0.415 | 0.042 0.620 | 0.351 0.000 | 0.034 0.690 | |||||||||
| CT EX-test (s) | −0.399 0.000 | −0.355 0.000 | −0.277 0.001 | 0.315 0.000 | 0.722 0.000 | −0.351 0.000 | −0.127 0.131 | −0.319 0.000 | ||||||||||
| A5 EX-test (mm) | 0.907 0.000 | 0.933 0.000 | −0.770 0.000 | −0.562 0.000 | 0.663 0.000 | 0.265 0.001 | 0.703 0.000 | |||||||||||
| A10 EX-test (mm) | 0.883 0.000 | −0.728 0.000 | −0.558 0.000 | 0.624 0.000 | 0.269 0.000 | 0.691 0.000 | ||||||||||||
| MCF EX-test (mm) | −0.772 0.000 | −0.463 0.000 | 0.633 0.000 | 0.279 0.001 | 0.665 0.000 | |||||||||||||
| CT FIB-test (s) | −0.429 0.000 | 0.089 0.294 | −0.421 0.142 | |||||||||||||||
| A5 FIB-test (mm) | 0.294 0.000 | 0.917 0.000 | ||||||||||||||||
| A10 FIB-test (mm) | −0.329 0.000 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI for OR) | p | OR (95% CI for OR) | p | |
| Age (≥68) | 4.445 (1.981–9.970) | 0.000 | ||
| Fibrinogen (g/L) | 1.392 (1.093–1.773) | 0.007 | ||
| Fibrinogen (>4 g/L) | 0.083 (0.009–0.764) | 0.028 | ||
| Fibrinogen (≥9.14 g/L) | 7.000 (3.062–16.003) | 0.000 | ||
| INR | 486.244 (41.870–5646.800) | 0.000 | ||
| INR (≥1.38) | 9.667 (4.051–23.065) | 0.000 | ||
| D-Dimer (ng/mL) | 1.006 (1.004–1.008) | 0.000 | ||
| D-Dimer (>1000 ng/mL) | 125.182 (26.168–598.831) | 0.000 | ||
| D-Dimer (≥860 ng/mL) | 130.680 (35.590–479.835) | 0.000 | 23.735 (2.824–199.461) | 0.004 |
| ADP test (AU/min) | 1.011 (1.007–1.016) | 0.000 | ||
| ADP test (<normal range>) | 17.007 (5.085–56.873) | 0.000 | ||
| ADP test (>590 AU/min) | 33.943 (11.139–103.433) | 0.000 | ||
| ASPI test (AU/min) | 1.011 (1.007–1.016) | 0.000 | ||
| ASPI test (<normal range>) | 157.933 (19.847–1256.730) | 0.000 | ||
| ASPI (≥728 AU/min) | 64.744 (17.131–244.686) | 0.000 | ||
| TRAP test (AU/min) | 1.006 (1.004–1.009) | 0.000 | ||
| TRAP test (<normal range>) | 23.421 (8.669–63.278) | 0.000 | 21.983 (2.365–204.311) | 0.001 |
| TRAP test (≥1180 AU/min) | 331.889 (40.372–2728.392) | 0.000 | ||
| PSEP (pg/mL) | 1.003 (1.001–1.004) | 0.000 | ||
| PSEP (>337 pg/mL) | 18.822 (6.668–53.131) | 0.000 | ||
| PSEP (≥335 pg/mL) | 24.241 (7.870–74.663) | 0.000 | ||
| CT EX-test (s) | 0.970 (0.947–0.994) | 0.014 | ||
| CT EX-test (<normal range>) | 0.376 (0.186–0.763) | 0.007 | ||
| A5 EX-test (mm) | 1.100 (1.032–1.173) | 0.003 | ||
| A5 EX-test (≥57 mm) | 3.522 (1.604–7.734) | 0.002 | ||
| A10 EX-test (mm) | 1.121 (1.035–1.215) | 0.005 | ||
| A10 EX-test (<normal range>) | 3.985 (1.300–12.211) | 0.016 | ||
| A10 EX-test (≥64 mm) | 4.778 (2.117–10.782) | 0.000 | ||
| MCF EX-test (mm) | 1.107 (1.026–1.195) | 0.009 | ||
| MCF EX-test (≥59 mm) | 14.307 (1.875–109.149) | 0.010 | ||
| A5 FIB-test (mm) | 1.128 (1.056–1.204) | 0.000 | ||
| A5 FIB-test (≥28 mm) | 8.076 (3.521–18.525) | 0.000 | ||
| A10 FIB-test (mm) | 1.895 (1.410–2.546) | 0.000 | ||
| A10 FIB-test (>23 mm) | 259.000 (32.697–2051.584) | 0.000 | 289.509 (3.438–24378.575) | 0.012 |
| MCF FIB-test (mm) | 1.111 (1.053–1.172) | 0.000 | ||
| MCF FIB-test (>25 mm) | 3.267 (1.441–7.406) | 0.005 | ||
| MCF FIB-test (≥36 mm) | 11.879 (4.173–33.810) | 0.000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milić, D.; Lazarević, M.; Vuković, N.; Kamenov, A.; Perić, V.; Golubović, M.; Stošić, M.; Spasić, D.; Stojiljković, V.; Stokanović, D. Monitoring the Coagulation Profile of COVID-19 Patients Using Standard and ClotPro® Hemostasis Tests. Medicina 2023, 59, 1202. https://doi.org/10.3390/medicina59071202
Milić D, Lazarević M, Vuković N, Kamenov A, Perić V, Golubović M, Stošić M, Spasić D, Stojiljković V, Stokanović D. Monitoring the Coagulation Profile of COVID-19 Patients Using Standard and ClotPro® Hemostasis Tests. Medicina. 2023; 59(7):1202. https://doi.org/10.3390/medicina59071202
Chicago/Turabian StyleMilić, Dragan, Milan Lazarević, Natalija Vuković, Aleksandar Kamenov, Velimir Perić, Mlađan Golubović, Marija Stošić, Dimitrije Spasić, Vladimir Stojiljković, and Dragana Stokanović. 2023. "Monitoring the Coagulation Profile of COVID-19 Patients Using Standard and ClotPro® Hemostasis Tests" Medicina 59, no. 7: 1202. https://doi.org/10.3390/medicina59071202
APA StyleMilić, D., Lazarević, M., Vuković, N., Kamenov, A., Perić, V., Golubović, M., Stošić, M., Spasić, D., Stojiljković, V., & Stokanović, D. (2023). Monitoring the Coagulation Profile of COVID-19 Patients Using Standard and ClotPro® Hemostasis Tests. Medicina, 59(7), 1202. https://doi.org/10.3390/medicina59071202







