Tertiary Cytoreduction for Isolated Lymphnode Recurrence (ILNR) Ovarian Cancer in a BRCA2 Mutated Patient: Our Experience and Prevalence of BRCA 1 or 2 Genes Mutational Status in ILNR
Abstract
1. Introduction
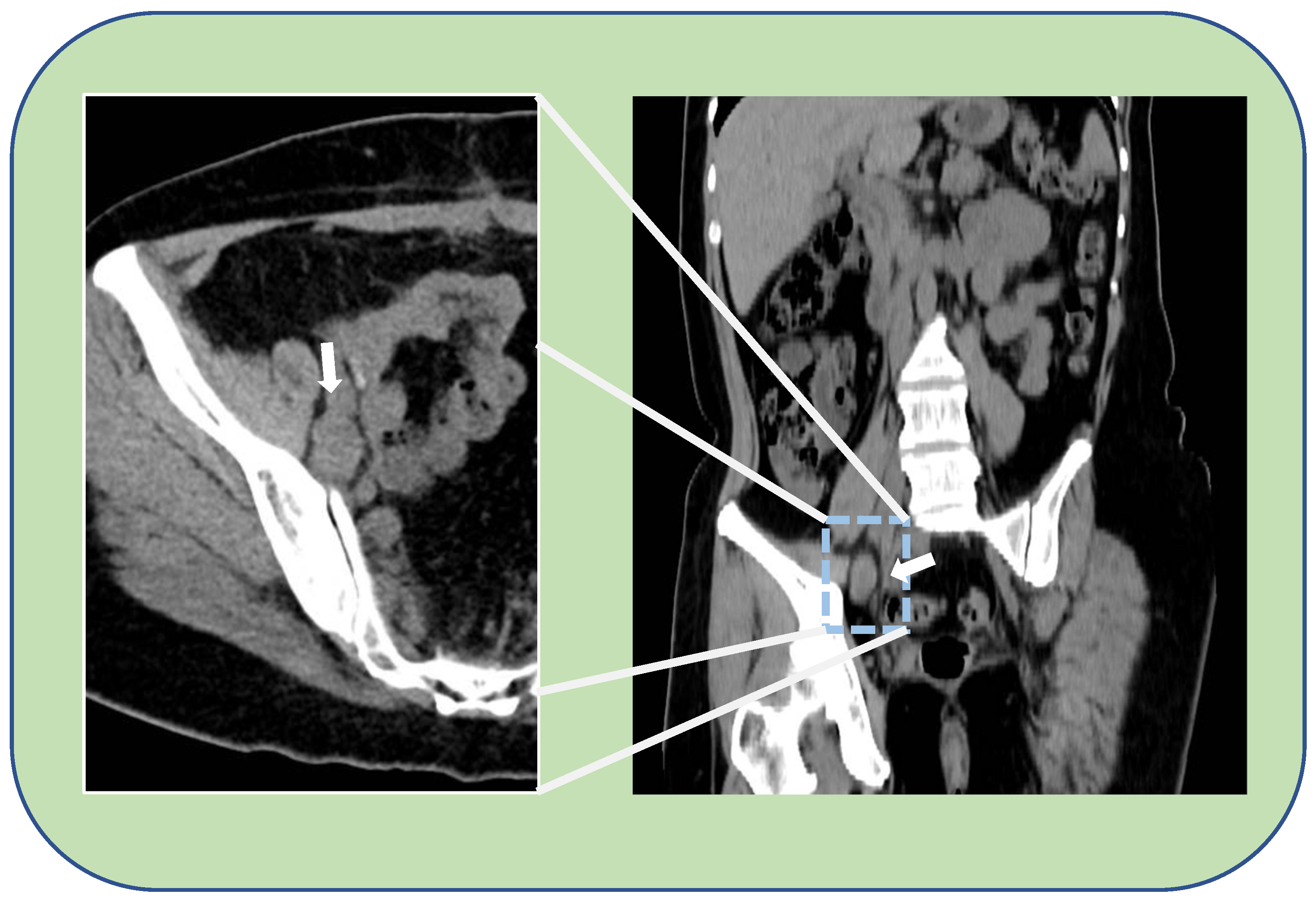
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, D.S.; McCaughty, K.; Diaz, J.P.; Huh, J.; Schwabenbauer, S.; Hummer, A.J.; Venkatraman, E.S.; Aghajanian, C.; Sonoda, Y.; Abu-Rustum, N.R.; et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer 2006, 106, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.K.; Pujade-Lauraine, E.; Aoki, D.; Mirza, M.R.; Lorusso, D.; Oza, A.M.; du Bois, A.; Vergote, I.; Reuss, A.; Bacon, M. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: Recurrent disease. Ann Oncol. 2017, 28, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Hollis, R.L.; Churchman, M.; Gourley, C. Distinct implications of different BRCA mutations: Efficacy of cytotoxic chemotherapy, PARP inhibition and clinical outcome in ovarian cancer. Onco. Targets Ther. 2017, 10, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Previs, R.A.; Secord, A.A. Ovarian cancer: Clinical trial breakthroughs and impact on management. Obstet. Gynecol. Clin. N. Am. 2019, 46, 67–88. [Google Scholar] [CrossRef]
- Marchetti, C.; De Leo, R.; Musella, A.; D’Indinosante, M.; Capoluongo, E.; Minucci, A.; Benedetti Panici, P.; Scambia, G.; Fagotti, A. BRCA Mutation Status to personalize management of recurrent ovarian cancer: A multicenter Study. Ann. Surg. Oncol. 2018, 25, 3701–3708. [Google Scholar] [CrossRef]
- DuBois, A.S.J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Jensen, P.T.; Selle, F.; Guyon, F.; Pomel, C.; et al. Randomized phase III study to evaluate the impact of secondary cytoreductive surgery in recurrent ovarian cancer: Final analysis of AGO DESKTOP III/ENGTO-ov20. J. Clin. Oncol. 2020, 38. [Google Scholar]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.-W.; Park, S.-Y.; Kim, B.-G.; Nam, J.-H.; et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef]
- Shi, T.; Zhu, J.; Feng, Y.; Tu, D.; Zhang, Y.; Zhang, P.; Jia, H.; Huang, X.; Cai, Y.; Yin, S.; et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive re- lapsed ovarian cancer (SOC-1): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 439–449. [Google Scholar] [CrossRef]
- Guida, F.; Dioun, S.; Fagotti, A.; Melamed, A.; Grossi, A.; Scambia, G.; Wright, J.D.; Tergas, A.I. Role of tertiary cytoreductive surgery in recurrent epithelial ovarian cancer: Systematic review and meta-analysis. Gynecol. Oncol. 2022, 166, 181–187. [Google Scholar] [CrossRef]
- Falcone, F.; Scambia, G.; Benedetti Panici, P.; Signorelli, M.; Cormio, G.; Giorda, G.; Bogliolo, S.; Marinaccio, M.; Ghezzi, F.; Rabaiotti, E.; et al. Tertiary cytoreductive surgery in recurrent epithelial ovarian cancer: A multicentre MITO retrospective study. Gynecol. Oncol. 2017, 147, 66–72. [Google Scholar] [CrossRef]
- Manning-Geist, B.L.; Chi, D.S.; Long Roche, K.; Zivanovic, O.; Sonoda, Y.; Gardner, G.J.; O’Cearbhaill, R.E.; Abu-Rustum, N.R.; Leitao, M.M., Jr. Tertiary cytoreduction for recurrent ovarian carcinoma: An updated and expanded analysis. Gynecol. Oncol. 2021, 162, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.; Plantade, A.; Pages, C.; Afchain, P.; Louvet, C.; Tournigand, G.; de Gramont, A. Isolated lymph node relapse of epithelial ovarian carcinoma: Outcomes and prognostic factors. Gynecol. Oncol. 2007, 104, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Benedetti Panici, P.; Perniola, G.; Angioli, R.; Zullo, M.A.; Manci, N.; Palaia, I.; Bellati, F.; Plotti, F.; Calcagno, M.; Basile, S. Bulky lymph node resection in patients with recurrent epithelial ovarian cancer: Impact of surgery. Int. J. Gynecol. Cancer 2007, 17, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Fotiou, S.; Aliki, T.; Petros, Z.; Ioanna, S.; Konstantinos, V.; Vasiliki, M.; George, C. Secondary cytoreductive surgery in patients presenting with isolated nodal recurrence of epithelial ovarian cancer. Gynecol. Oncol. 2009, 114, 178–182. [Google Scholar] [CrossRef]
- Ferrero, A.; Ditto, A.; Giorda, G.; Gadducci, A.; Greggi, S.; Daniele, A.; Fuso, L.; Panuccio, E.; Scaffa, C.; Raspagliesi, F.; et al. Secondary cytoreductive surgery for isolated lymph node recurrence of epithelial ovarian cancer: A multicenter study. Eur. J. Surg. Oncol. 2014, 40, 891–898. [Google Scholar] [CrossRef]
- Sapienza, L.G.; Gomes, M.J.L.; Calsavara, V.F.; Leitao, M.M., Jr.; Baiocchi, G. Does para-aortic irradiation reduce the risk of distant metastasis in advanced cervical cancer? A systematic review and meta-analysis of randomized clinical trials. Gynecol. Oncol. 2017, 144, 312–317. [Google Scholar] [CrossRef]
- Delangle, R.; Rossard, L.; Cirier, J.; Delvallée, J.; Bendifallah, S.; Touboul, C.; Collinet, P.; Coutant, C.; Akladios, C.; Lavoué, V.; et al. Isolated lymph node recurrence in epithelial ovarian cancer: Recurrence with better prognosis? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 249, 64–69. [Google Scholar] [CrossRef]
- Kimball, R.E.; Schlaerth, J.B.; Kute, T.E.; Schlaerth, A.C.; Santoso, J.; Ballon, S.C.; Spirtos, N.M. Flow cytometric analysis of lymph node metastases in advanced ovarian cancer: Clinical and biologic significance. Am. J. Obstet. Gynecol. 1997, 176, 1319–1327. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.W.; Lee, S.H.; Paek, J.; Yim, G.W.; Kim, G.E.; Kim, S.; Kim, J.H.; Kim, Y.T.; Nam, E.J. Comparison of the efficacy and toxicity between radiotherapy and chemotherapy in nodal and isolated nonnodal recurrence of ovarian cancer. Int. J. Gynecol. Cancer 2011, 21, 1032–1039. [Google Scholar] [CrossRef]
- Hollis, R.L.; Carmichael, J.; Meynert, A.M.; Churchman, M.; Hallas-Potts, A.; Rye, T.; MacKean, M.; Nussey, F.; Semple, C.A.; Herrington, C.S.; et al. Clinical and molecular characterization of ovarian carcinoma displaying isolated lymph node relapse. Am. J. Obstet. Gynecol. 2019, 221, 245.e1–245.e15. [Google Scholar] [CrossRef]
- Gallotta, V.; Conte, C.; D’Indinosante, M.; Capoluongo, E.; Minucci, A.; De Rose, A.M.; Ardito, F.; Giuliante, F.; Di Giorgio, A.; Zannoni, G.F.; et al. Prognostic factors value of germline and somatic brca in patients undergoing surgery for recurrent ovarian cancer with liver metastases. Eur. J. Surg. Oncol. 2019, 45, 2096–2102. [Google Scholar] [CrossRef]
- Gallotta, V.; Bruno, M.; Conte, C.; Giudice, M.T.; Davià, F.; Moro, F.; Zannoni, G.F.; Fagotti, A.; De Bonis, M.; Capoluongo, E.; et al. Salvage lymphadenectomy in recurrent ovarian cancer patients: Analysis of clinical outcome and BRCA1/2 gene mutational status. Eur. J. Surg. Oncol. 2020, 46, 1327–1333. [Google Scholar] [CrossRef]
- Petrillo, M.; Fagotti, A.; Ferrandina, G.; Fanfani, F.; Costantini, B.; Vizzielli, G.; Pedone Anchora, L.; Nero, C.; Margariti, P.A.; Scambia, G. Ovarian cancer patients with localized relapse: Clinical outcome and prognostic factors. Gynecol. Oncol. 2013, 131, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Huang, H.; Huang, Q.D.; Li, Z.; Feng, Y.L.; Liu, J.H. Treatment and prognostic analysis of ovarian cancer patients with isolated region of lymph node recurrence. Zhonghua Fu Chan Ke Za Zhi 2012, 47, 928–933. [Google Scholar]
- Pergialiotis, V.; Androutsou, A.; Papoutsi, E.; Bellos, I.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A. Survival outcomes of ovarian cancer patients treated with secondary cytoreductive surgery for isolated lymph node recurrence: A systematic review of the literature. Int. J. Surg. 2019, 69, 61–66. [Google Scholar] [CrossRef]
- Conte, C.; Marchetti, C.; Loverro, M.; Giudice, M.T.; Rosati, A.; Gallotta, V.; Scambia, G.; Fagotti, A. Role of minimally invasive secondary cytoreduction in patients with recurrent ovarian cancer. Int. J. Gynecol. Cancer 2023, 33, 137–144. [Google Scholar] [CrossRef]
- Morice, P.; Joulie, F.; Rey, A.; Atallah, D.; Camatte, S.; Pautier, P.; Thoury, A.; Lhommé, C.; Duvillard, P.; Castaigne, D. Are nodal metastases in ovarian cancer chemoresistant lesions? Analysis of nodal involvement in 105 patients treated with preoperative chemotherapy. Eur. J. Gynaecol. Oncol. 2004, 25, 169–174. [Google Scholar] [PubMed]
- Santillan, A.; Karam, A.K.; Li, A.J.; Karam, A.K.; Li, A.J.; Giuntoli, R.; Gardner, G.J.; Caa, I.; Karlan, B.Y.; Bristow, R.E. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol. Oncol. 2007, 104, 686–690. [Google Scholar] [CrossRef]
- Stabile, G.; Zinicola, G.; Romano, F.; Laganà, A.S.; Dal Pozzolo, C.; Ricci, G. Pelvic mass, ascites, hydrothorax: A malignant or benign condition? Meigs syndrome with high levels of CA 125. Prz Menopauzalny 2021, 20, 103–107. [Google Scholar] [CrossRef]
- Mascilini, F.; Quagliozzi, L.; Moro, F.; Moruzzi, M.C.; Gallotta, V.; Alletti, S.G.; Scambia, G.; Testa, A.C.; Fagotti, A. Role of Intraoperative Ultrasound to Extend the Application of Minimally Invasive Surgery for Treatment of Recurrent Gynecologic Cancer. J. Minim Invasive Gynecol. 2018, 25, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, G.; Cappuccio, S.; Parente, E.; Fagotti, A.; Gallotta, V.; Conte, C.; Costantini, B.; Gueli Alletti, S.; Scambia, G.; Vizzielli, G. Resectability and Vascular Management of Retroperitoneal Gynecological Malignancies: A Large Single-institution Case-Series. Anticancer Res. 2017, 37, 6899–6906. [Google Scholar] [PubMed]
- Bruno, M.; Legge, F.; Gentile, C.; Carone, V.; Stabile, G.; Di Leo, F.; Ludovisi, M.; Di Florio, C.; Guido, M. Risk Assessment Model for Complications in Minimally Invasive Hysterectomy: A Pilot Study. Int. J. Environ. Res. Public Health 2023, 20, 234. [Google Scholar] [CrossRef] [PubMed]
- Stabile, G.; Mordeglia, D.; Romano, F.; Carlucci, S.; Mangino, F.P.; Nappi, L.; Sorrentino, F.; De Manzini, N.; Ricci, G. Postcoital Vaginal Perforation and Evisceration in Women with No Prior Pelvic Surgery: Laparoscopic Management and Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 9746. [Google Scholar] [CrossRef] [PubMed]
- Tew, W.P.; Lacchetti, C.; Ellis, A.; Maxian, K.; Banerjee, S.; Bookman, M.; Jones, M.B.; Lee, J.M.; Lheureux, S.; Liu, J.F.; et al. PARP Inhibitors in the Management of Ovarian Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3468–3493. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, M.; Ludovisi, M.; Ronsini, C.; Capanna, G.; Stabile, G.; Guido, M. Tertiary Cytoreduction for Isolated Lymphnode Recurrence (ILNR) Ovarian Cancer in a BRCA2 Mutated Patient: Our Experience and Prevalence of BRCA 1 or 2 Genes Mutational Status in ILNR. Medicina 2023, 59, 606. https://doi.org/10.3390/medicina59030606
Bruno M, Ludovisi M, Ronsini C, Capanna G, Stabile G, Guido M. Tertiary Cytoreduction for Isolated Lymphnode Recurrence (ILNR) Ovarian Cancer in a BRCA2 Mutated Patient: Our Experience and Prevalence of BRCA 1 or 2 Genes Mutational Status in ILNR. Medicina. 2023; 59(3):606. https://doi.org/10.3390/medicina59030606
Chicago/Turabian StyleBruno, Matteo, Manuela Ludovisi, Carlo Ronsini, Giulia Capanna, Guglielmo Stabile, and Maurizio Guido. 2023. "Tertiary Cytoreduction for Isolated Lymphnode Recurrence (ILNR) Ovarian Cancer in a BRCA2 Mutated Patient: Our Experience and Prevalence of BRCA 1 or 2 Genes Mutational Status in ILNR" Medicina 59, no. 3: 606. https://doi.org/10.3390/medicina59030606
APA StyleBruno, M., Ludovisi, M., Ronsini, C., Capanna, G., Stabile, G., & Guido, M. (2023). Tertiary Cytoreduction for Isolated Lymphnode Recurrence (ILNR) Ovarian Cancer in a BRCA2 Mutated Patient: Our Experience and Prevalence of BRCA 1 or 2 Genes Mutational Status in ILNR. Medicina, 59(3), 606. https://doi.org/10.3390/medicina59030606







