Nephrotic Syndrome and Statin Therapy: An Outcome Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
- kidney replacement therapy initiation (dialysis initiation or kidney transplantation).
- major cardiovascular event (MACE) defined as cardiovascular death, myocardial infarction, or ischemic stroke.
- thrombotic complications including deep vein thrombosis, renal vein thrombosis, and pulmonary embolism.
- complete remission defined as proteinuria under 0.5 g per 24 h, serum albumin of at least 3.5 g per deciliter, and stable eGFR (eGFR remaining unchanged or declining by <15% during follow-up).
2.2. Covariates (Measurements) and Treatment
2.3. Statistical Analysis
2.4. Ethics Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaziri, N.D. Disorders of lipid metabolism in nephrotic syndrome: Mechanisms and consequences. Kidney Int. 2016, 90, 41–52. [Google Scholar] [CrossRef]
- Joven, J.; Villabona, C.; Vilella, E.; Masana, L.; Alberti, R.; Valles, M. Abnormalities of lipoprotein metabolism in patients with the nephrotic syndrome. N. Engl. J. Med. 1990, 323, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Warwick, G.L.; Packard, C.J.; Demant, T.; Bedford, D.K.; Boulton-Jones, J.M.; Shepherd, J. Metabolism of apolipoprotein B-containing lipoproteins in subjects with nephrotic-range proteinuria. Kidney Int. 1991, 40, 129–138. [Google Scholar] [CrossRef]
- Vega, G.L.; Toto, R.D.; Grundy, S.M. Metabolism of low density lipoproteins in nephrotic dyslipidemia: Comparison of hypercholesterolemia alone and combined hyperlipidemia. Kidney Int. 1995, 47, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, J.D.; Hiatt, R.A.; Killebrew, E.J.; Fireman, B.H. The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int. 1993, 44, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Busuioc, R.M.; Covic, A.; Kanbay, M.; Banach, M.; Burlacu, A.; Mircescu, G. Protein convertase subtilisin/kexin type 9 biology in nephrotic syndrome: Implications for use as therapy. Nephrol. Dial. Transplant. 2020, 35, 1663–1674. [Google Scholar] [CrossRef]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, 753–779. [Google Scholar] [CrossRef]
- Thomas, M.E.; Harris, K.P.; Ramaswamy, C.; Hattersley, J.M.; Wheeler, D.C.; Varghese, Z.; Williams, J.D.; Walls, J.; Moorhead, J.F. Simvastatin therapy for hypercholesterolemic patients with nephrotic syndrome or significant proteinuria. Kidney Int. 1993, 44, 1124–1129. [Google Scholar] [CrossRef]
- Rabelink, A.J.; Hene, R.J.; Erkelens, D.W.; Joles, J.A.; Koomans, H.A. Effects of simvastatin and cholestyramine on lipoprotein profile in hyperlipidaemia of nephrotic syndrome. Lancet 1988, 2, 1335–1338. [Google Scholar] [CrossRef]
- Gheith, O.A.; Sobh, M.A.; Mohamed Kel, S.; El-Baz, M.A.; El-Husseini, F.; Gazarin, S.S.; Ahmed, H.A.; Rasem, M.W.; Amer, G.M. Impact of treatment of dyslipidemia on renal function, fat deposits and scarring in patients with persistent nephrotic syndrome. Nephron 2002, 91, 612–619. [Google Scholar] [CrossRef]
- Olbricht, C.J.; Wanner, C.; Thiery, J.; Basten, A. Simvastatin in nephrotic syndrome. Simvastatin in Nephrotic Syndrome Study Group. Kidney Int. Suppl. 1999, 71, S113–S116. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M. Statins for slowing kidney disease progression: An as yet unproven indication. Am. J. Kidney Dis. 2008, 52, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Parlakpinar, H.; Gunata, M. Transplantation and immunosuppression: A review of novel transplant-related immunosuppressant drugs. Immunopharmacol. Immunotoxicol. 2021, 43, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Dogra, G.K.; Watts, G.F.; Herrmann, S.; Thomas, M.A.; Irish, A.B. Statin therapy improves brachial artery endothelial function in nephrotic syndrome. Kidney Int. 2002, 62, 550–557. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Appel, A.S.; Valeri, A.; Appel, G.B. The nephrotic syndrome, lipids, and risk factors for cardiovascular disease. Am. J. Kidney Dis. 1993, 22, 135–142. [Google Scholar] [CrossRef]
- Mahmoodi, B.K.; ten Kate, M.K.; Waanders, F.; Veeger, N.J.; Brouwer, J.L.; Vogt, L.; Navis, G.; van der Meer, J. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: Results from a large retrospective cohort study. Circulation 2008, 117, 224–230. [Google Scholar] [CrossRef]
- Loscalzo, J. Venous thrombosis in the nephrotic syndrome. N. Engl. J. Med. 2013, 368, 956–958. [Google Scholar] [CrossRef]
- Singhal, R.; Brimble, K.S. Thromboembolic complications in the nephrotic syndrome: Pathophysiology and clinical management. Thromb. Res. 2006, 118, 397–407. [Google Scholar] [CrossRef]
- Lopez-Alemany, R.; Longstaff, C.; Hawley, S.; Mirshahi, M.; Fabregas, P.; Jardi, M.; Merton, E.; Miles, L.A.; Felez, J. Inhibition of cell surface mediated plasminogen activation by a monoclonal antibody against alpha-Enolase. Am. J. Hematol. 2003, 72, 234–242. [Google Scholar] [CrossRef]
- Stefan, G.; Stancu, S.; Zugravu, A.; Popa, O.; Zubidat, D.; Petre, N.; Mircescu, G. Negative anti-phospholipase A2 receptor antibody status at three months predicts remission in primary membranous nephropathy. Ren. Fail. 2022, 44, 258–268. [Google Scholar] [CrossRef]
- DiMinno, G.; Silver, M.J.; Cerbone, A.M.; Rainone, A.; Postiglione, A.; Mancini, M. Increased fibrinogen binding to platelets from patients with familial hypercholesterolemia. Arteriosclerosis 1986, 6, 203–211. [Google Scholar] [CrossRef]
- Owens, A.P., 3rd; Byrnes, J.R.; Mackman, N. Hyperlipidemia, tissue factor, coagulation, and simvastatin. Trends Cardiovasc. Med. 2014, 24, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Kaba, N.K.; Francis, C.W.; Moss, A.J.; Zareba, W.; Oakes, D.; Knox, K.L.; Fernandez, I.D.; Rainwater, D.L.; Investigators, T. Effects of lipids and lipid-lowering therapy on hemostatic factors in patients with myocardial infarction. J. Thromb. Haemost. 2004, 2, 718–725. [Google Scholar] [CrossRef]
- Undas, A.; Brummel-Ziedins, K.E.; Mann, K.G. Statins and blood coagulation. Arter. Thromb. Vasc. Biol. 2005, 25, 287–294. [Google Scholar] [CrossRef]
- Zou, P.; Li, H.; Cai, J.; Chen, Z.; Li, C.; Li, X. Statins can benefit patients with primary membranous nephropathy on venous thromboembolism. Ren. Fail. 2021, 43, 302–306. [Google Scholar] [CrossRef] [PubMed]

| All (N = 154) | Treatment with Statins | p | ||
|---|---|---|---|---|
| Yes (n = 128) | No (n = 26) | |||
| Age (years) | 53 [39–64] | 53 [40–63] | 53 [36–65] | 0.6 |
| Male sex (%) | 64 | 64 | 65 | 0.8 |
| BMI (Kg/m2) | 27.0 [25.6–29.0] | 27.0 [26.0–29.2] | 27.0 [24.7–28.5] | 0.3 |
| Nephrotic syndrome cause (%) | 0.01 | |||
| Membranous nephropathy | 55 | 59 | 39 | |
| Minimal change disease | 31 | 28 | 42 | |
| FSGS | 11 | 12 | 8 | |
| MPGN | 3 | 2 | 12 | |
| Arterial hypertension (%) | 44 | 44 | 46 | 0.8 |
| Charlson score | 1 [0–2] | 1 [0–2] | 1 [0–2] | 0.6 |
| eGFR (mL/min) | 61.9 [45.2–81.0] | 61.9 [46.1–81.3] | 61.6 [41.0–80.0] | 0.7 |
| Serum albumin (g/dL) | 2.9 [2.5–3.4] | 3.0 [2.6–3.3] | 2.8 [2.4–3.0] | 0.07 |
| Proteinuria (g/g) | 6.4 [4.4–9.0] | 6.5 [4.3–9.1] | 6.2 [4.6–8.1] | 0.7 |
| Hematuria (RBC/mm3) | 34 [5–75] | 30 [5–73] | 38 [5–125] | 0.7 |
| Hemoglobin (g/dL) | 13.4 [11.9–14.8] | 13.7 [12.2–14.8] | 12.6 [10.1–15.1] | 0.1 |
| C-reactive protein (mg/L) | 2 [1–6] | 2 [1–6] | 2 [0–7] | 0.5 |
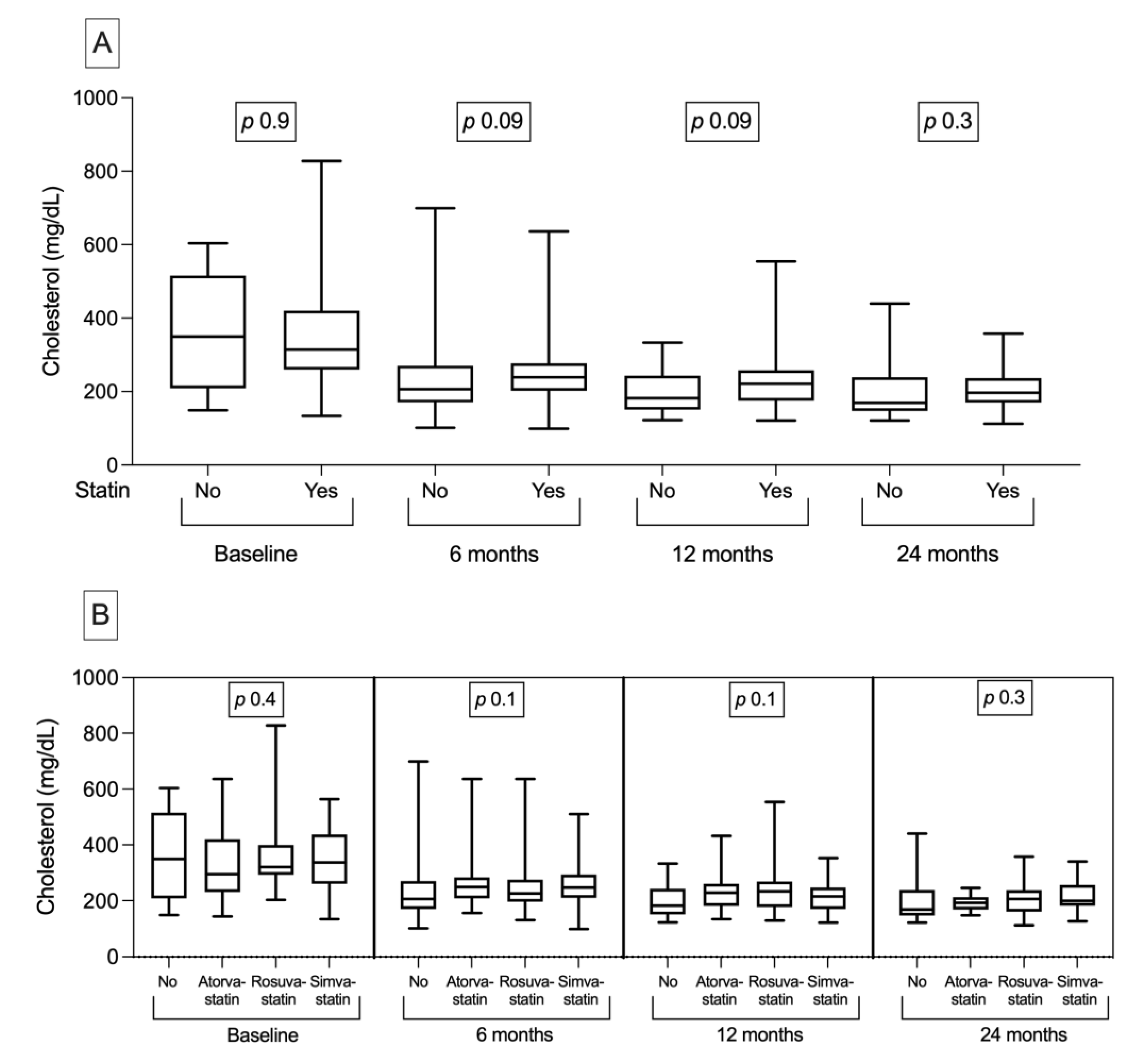
| Cholesterol (mg/dL) | 319 [255–420] | 314 [260–420] | 350 [213–511] | 0.9 |
| Triglycerides (mg/dL) | 215 [140–305] | 215 [140–301] | 203 [150–344] | 0.7 |
| Total lipids (mg/dL) | 1009 [881–1341] | 1009 [888–1326] | 1015 [713–1448] | 0.9 |
| Treatment | ||||
| Immunosuppression (%) | 100 | 100 | 100 | - |
| Type of immunosuppression (%) | ||||
| Corticotherapy only | 26 | 24 | 38 | 0.1 |
| Cyclophosphamide | 58 | 61 | 43 | 0.07 |
| Cyclosporine | 16 | 15 | 19 | 0.6 |
| RAAS blockade (%) | 60 | 60 | 62 | 0.8 |
| Outcome | ||||
| Response to therapy (%) | 0.4 | |||
| No remission | 21 | 22 | 12 | |
| Partial remission | 15 | 15 | 19 | |
| Complete remission | 64 | 63 | 69 | |
| KRT initiation (%) | 5 | 5 | 8 | 0.5 |
| Major cardiovascular event (%) | 8 | 9 | 4 | 0.4 |
| Thrombotic complications (%) | 11 | 9 | 23 | 0.03 |
| Endpoint (Model) | Variables | HR (95%CI) | p |
|---|---|---|---|
| Remission | eGFR (mL/min) Proteinuria (24 h) Serum albumin (g/dL) Statin versus no statin therapy | 1.00 (1.00, 1.01) 0.94 (0.89, 0.99) 1.16 (0.79, 1.69) 1.17 (0.70, 1.96) | 0.02 0.03 0.4 0.5 |
| ESKD | eGFR (mL/min) Proteinuria (24 h) Serum albumin (g/dL) Statin versus no statin therapy | 0.96 (0.93, 0.99) 1.03 (0.88, 1.22) 1.23 (0.33, 4.59) 1.57 (0.30, 8.10) | 0.03 0.6 0.7 0.5 |
| MACE | eGFR (mL/min) Proteinuria (24 h) Serum albumin (g/dL) Statin versus no statin therapy | 0.98 (0.95, 1.00) 0.77 (0.59, 0.98) 3.94 (1.20, 12.87) 0.44 (0.05, 3.59) | 0.09 0.04 0.02 0.4 |
| Thrombotic complication | eGFR (mL/min) Proteinuria (24 h) Serum albumin (g/dL) Statin versus no statin therapy | 0.99 (0.97, 1.00) 1.10 (1.00, 1.22) 0.67 (0.26, 1.75) 2.83 (1.02, 7.84) | 0.3 0.04 0.4 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busuioc, R.; Ștefan, G.; Stancu, S.; Zugravu, A.; Mircescu, G. Nephrotic Syndrome and Statin Therapy: An Outcome Analysis. Medicina 2023, 59, 512. https://doi.org/10.3390/medicina59030512
Busuioc R, Ștefan G, Stancu S, Zugravu A, Mircescu G. Nephrotic Syndrome and Statin Therapy: An Outcome Analysis. Medicina. 2023; 59(3):512. https://doi.org/10.3390/medicina59030512
Chicago/Turabian StyleBusuioc, Ruxandra, Gabriel Ștefan, Simona Stancu, Adrian Zugravu, and Gabriel Mircescu. 2023. "Nephrotic Syndrome and Statin Therapy: An Outcome Analysis" Medicina 59, no. 3: 512. https://doi.org/10.3390/medicina59030512
APA StyleBusuioc, R., Ștefan, G., Stancu, S., Zugravu, A., & Mircescu, G. (2023). Nephrotic Syndrome and Statin Therapy: An Outcome Analysis. Medicina, 59(3), 512. https://doi.org/10.3390/medicina59030512





