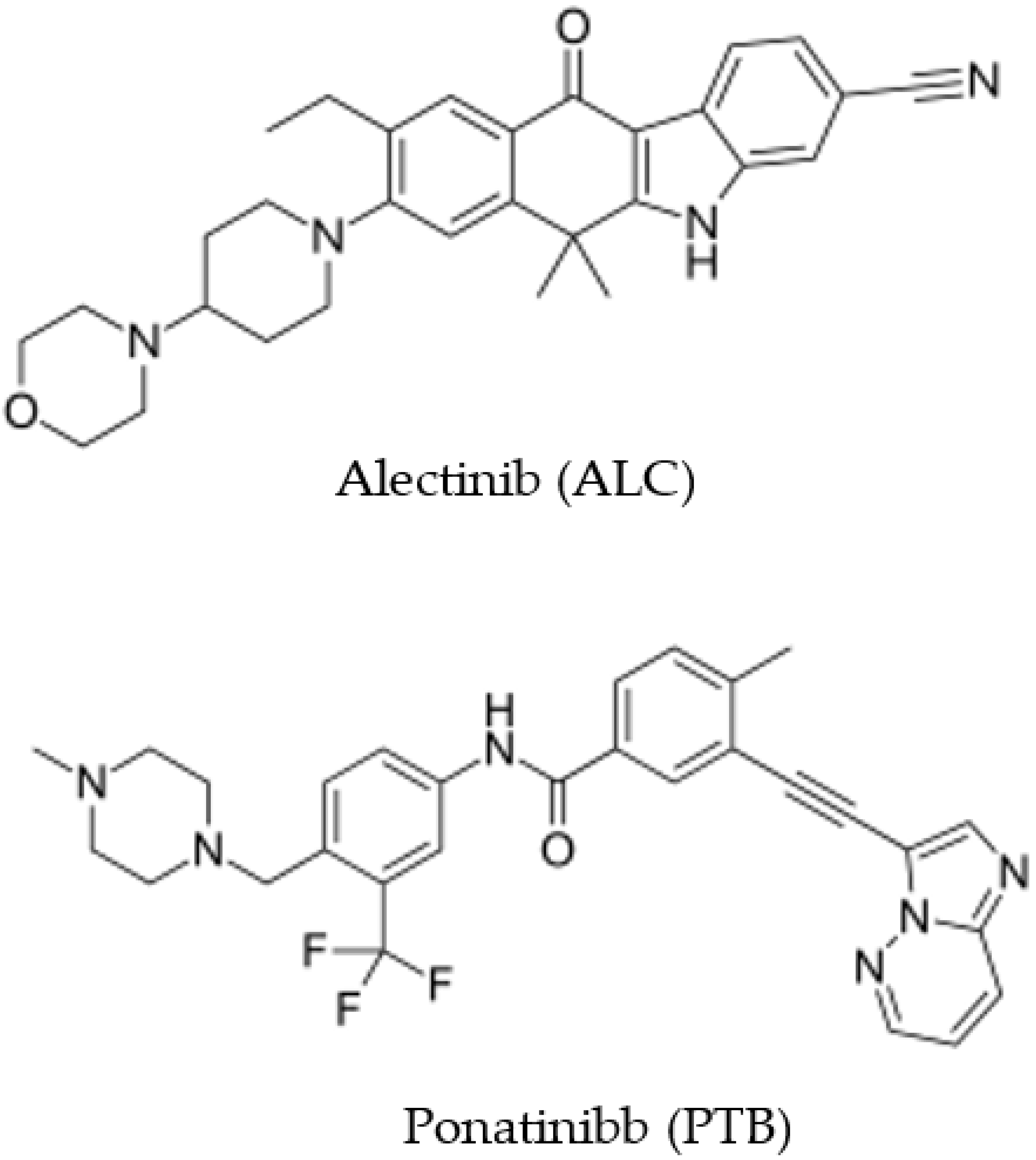
Development of Novel Microwell-Based Spectrofluorimetry and High-Performance Liquid Chromatography with Fluorescence Detection Methods and High Throughput for Quantitation of Alectinib in Bulk Powder and Urine Samples
Abstract
1. Introduction
2. Experimental
2.1. Instruments
2.2. Chemicals and Reagents
2.3. Preparation of Standard Solutions
2.4. Preparation of Urine Samples
2.5. General Analytical Procedure
2.5.1. MW-SFL
2.5.2. HPLC-FD
3. Results and Discussion
3.1. Development of the MW-SFL Method
The Strategy for Method Development and Its Design
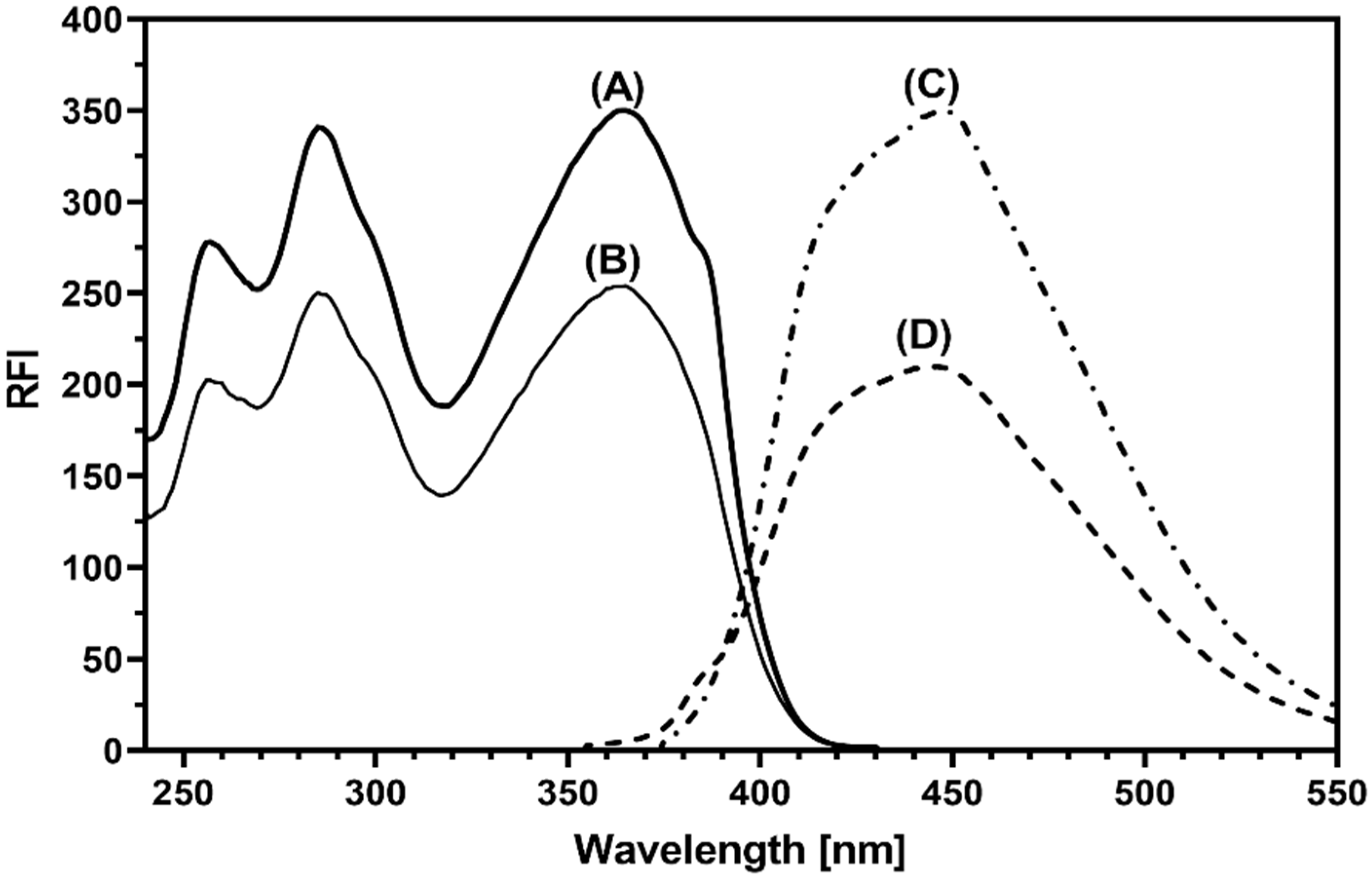
3.2. Spectral Featutres
3.3. The Optimization of the MW-SFL Method Variables
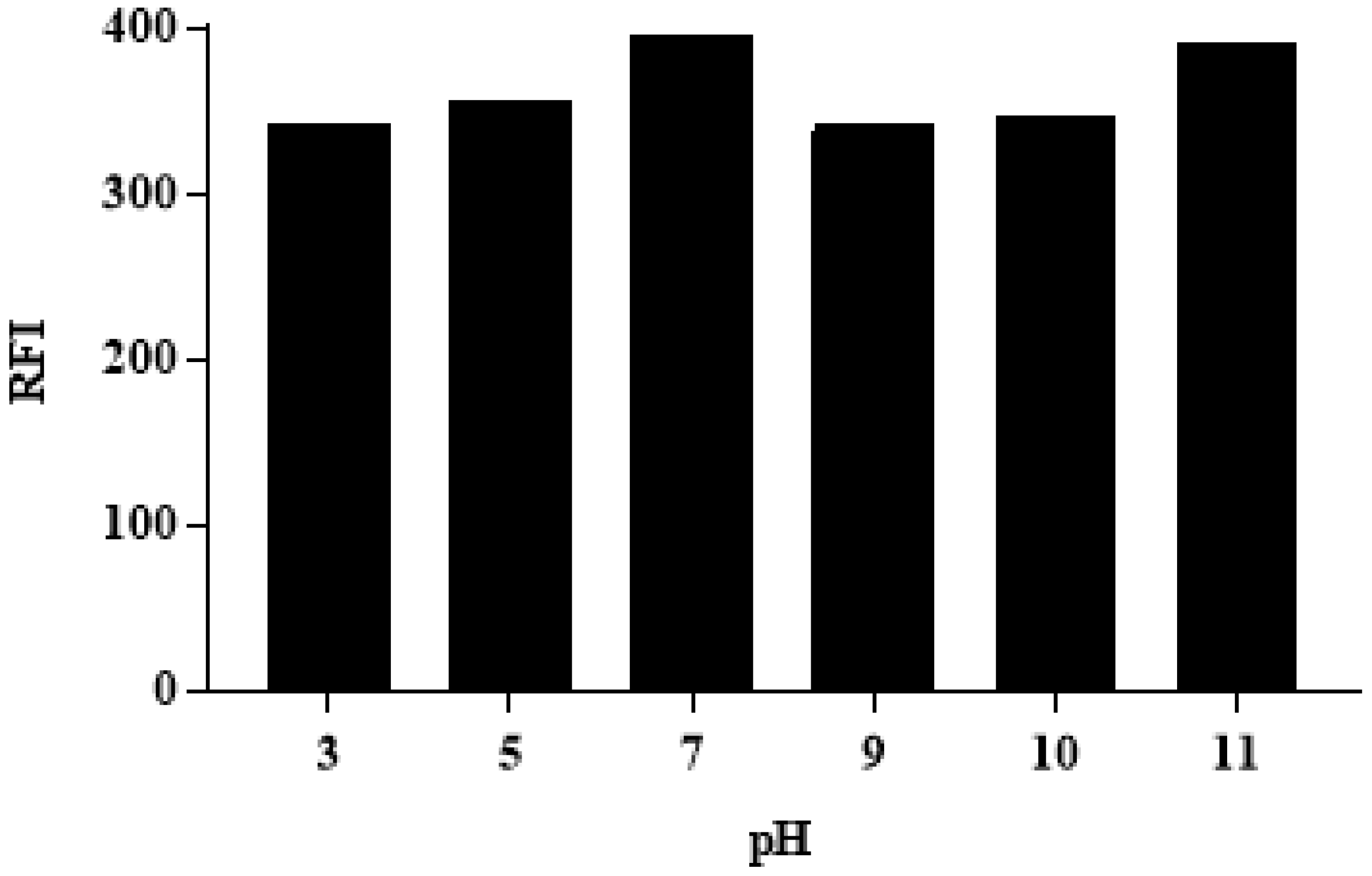
3.3.1. The effect of pH
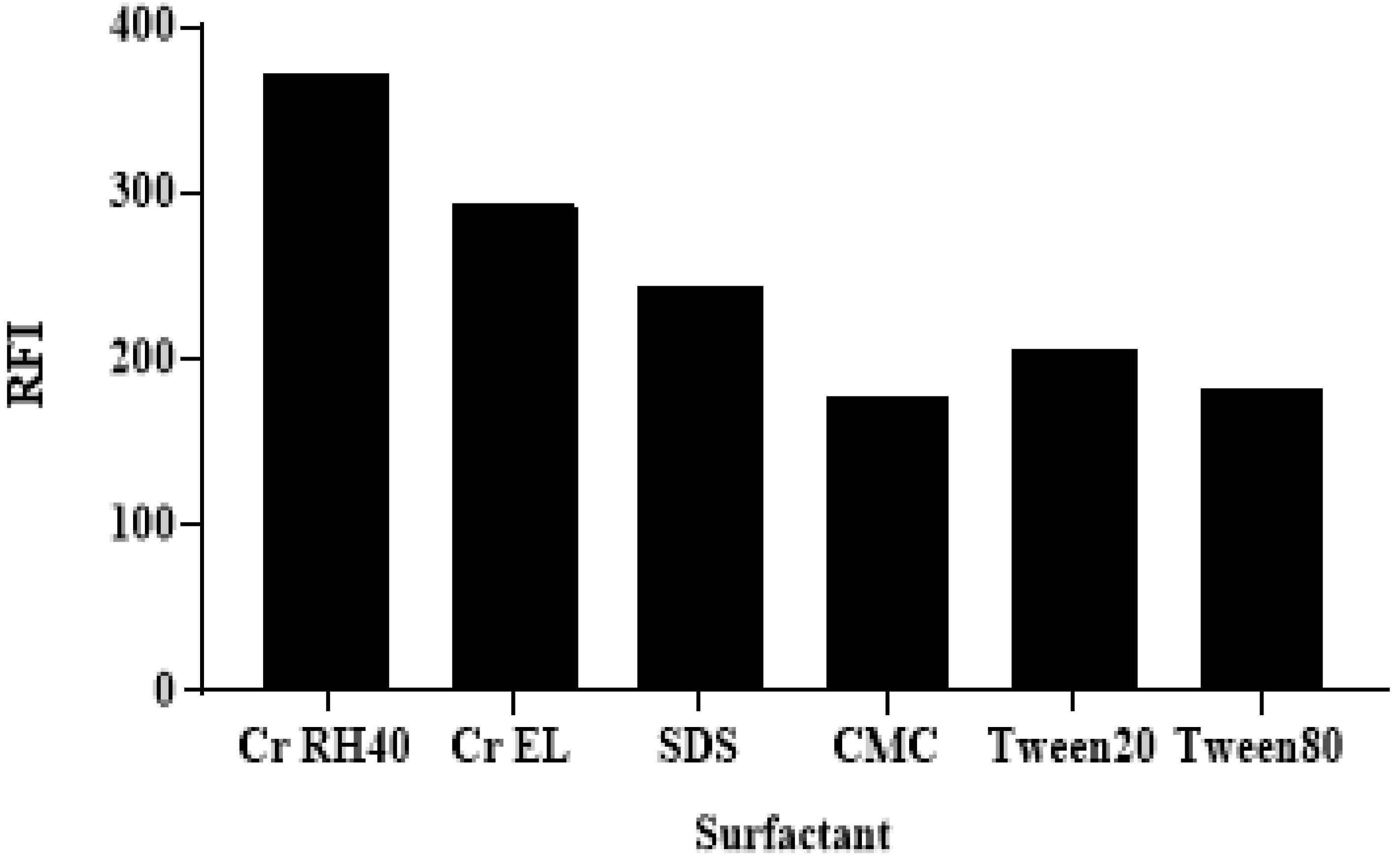
3.3.2. The Effects of Types of Surfactant
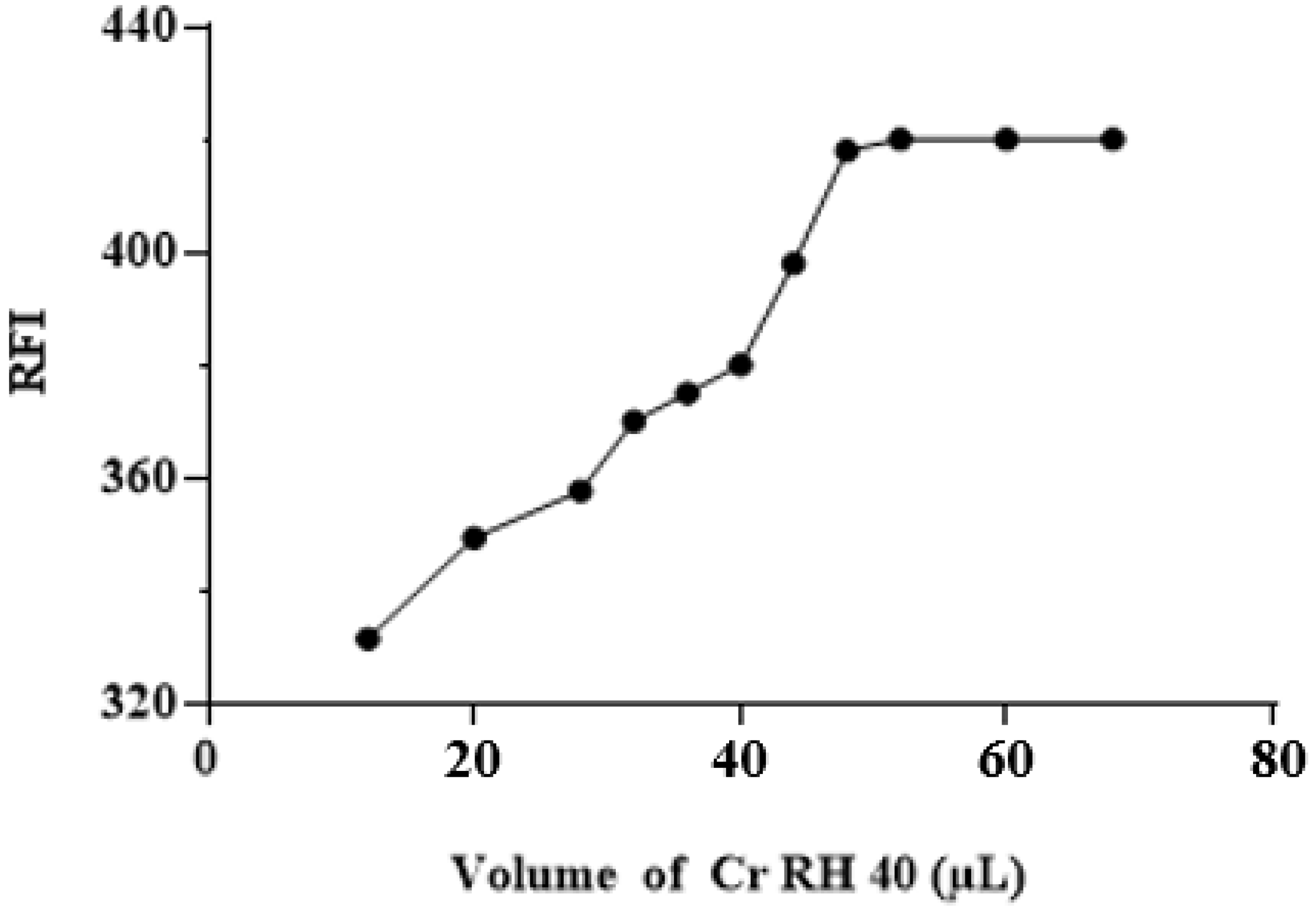
3.3.3. The effect of Cr RH 40 Volume
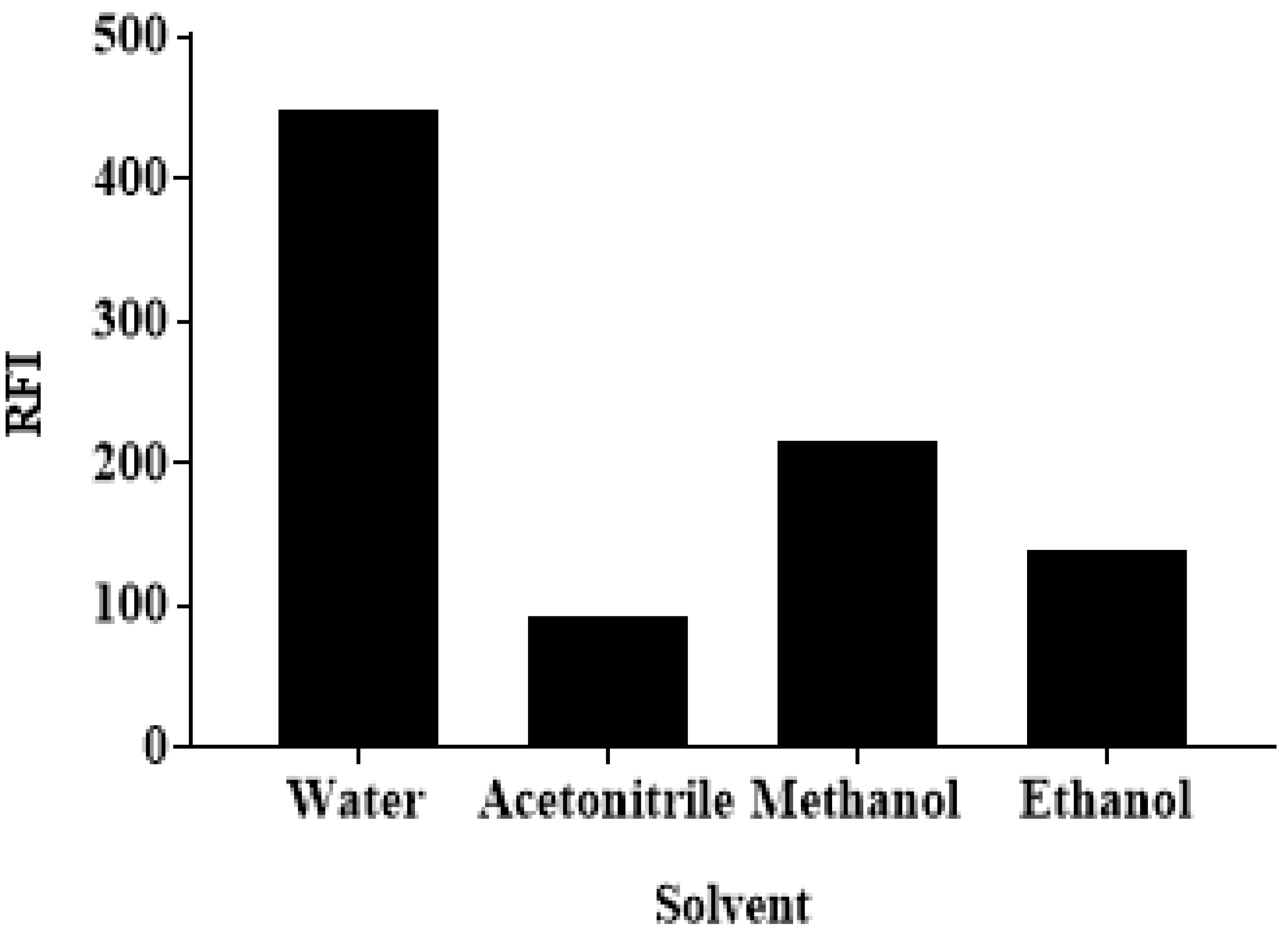
3.3.4. The Effect of the Solvent
3.4. The Mechanism of the Micellar Enhancement of ALC Fluorescence by Cr RH 40
3.5. The Development of the HPLC-FD Method
3.5.1. The Overview and Method Development Strategy
3.5.2. The Selection of Chromatographic Conditions
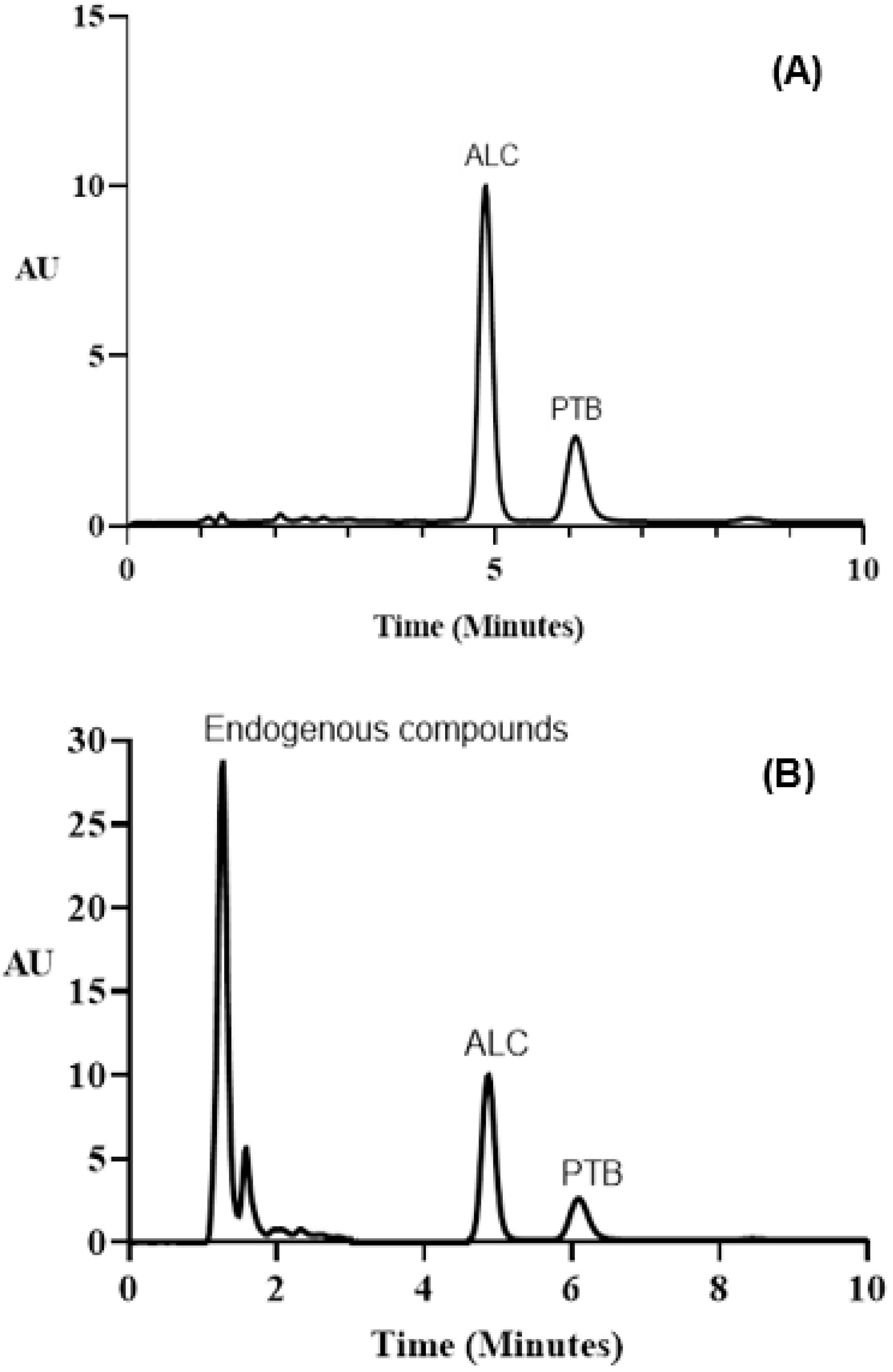
The Selection of Detection Wavelength and Internal Standard
Mobile Phase
Suitability Parameters
3.6. The Validation of the MW-SFL and HPLC-FD Methods
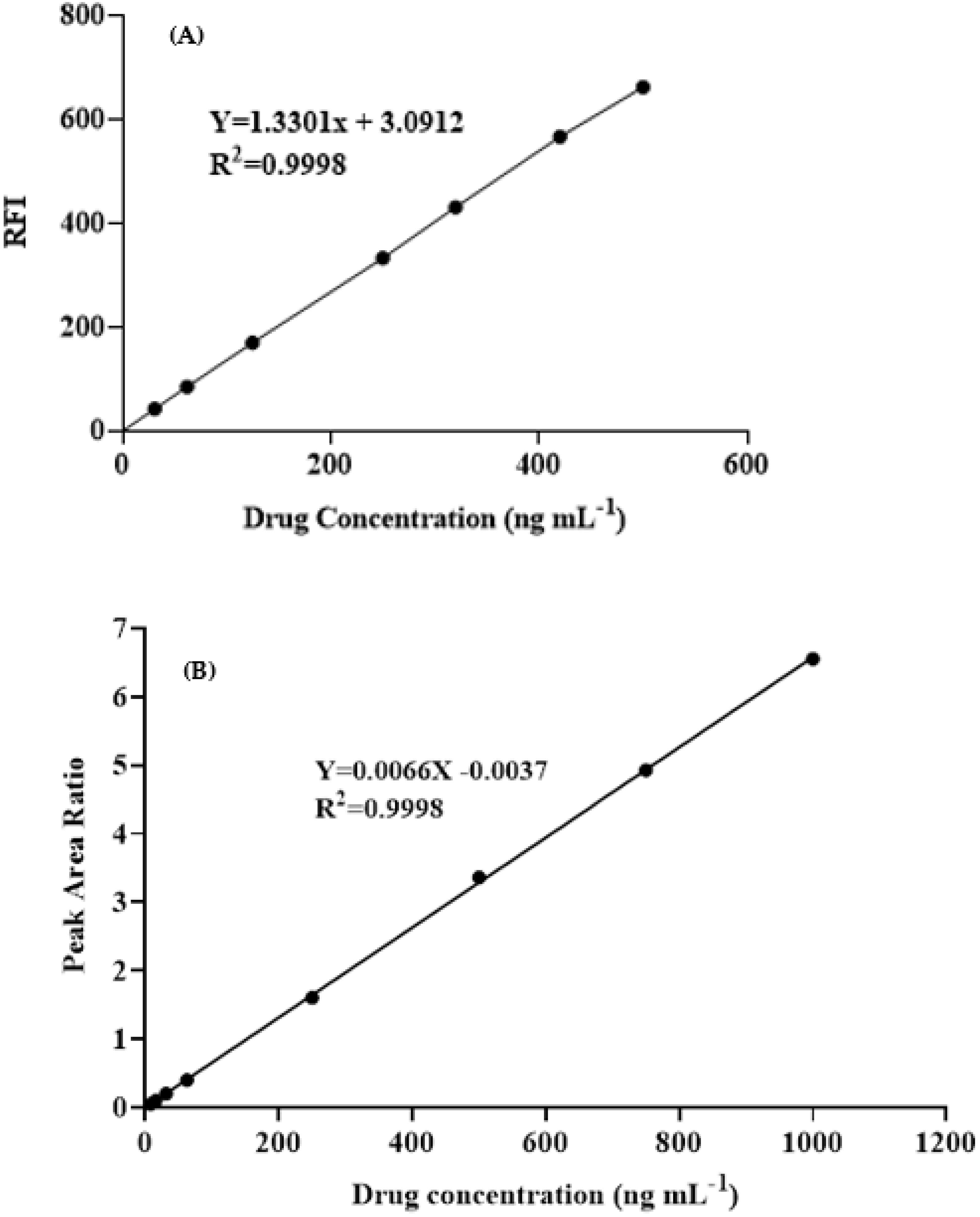
3.6.1. Linearity and Sensitivity
3.6.2. Precision and Accuracy
3.6.3. Robustness and Ruggedness
3.7. Urine Analysis by the Adopted MW-SFL and HPLC-FD Methods
3.8. A Comparison between the Proposed and Reported Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Medicines Agency. Alecensa (alectinib): Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/alecensa (accessed on 17 May 2019).
- Tomasini, P.; Egea, J.; Souquet-Bressand, M. Alectinib in the treatment of ALK-positive metastatic non-small cell lung cancer: Clinical trial evidence and experience with a focus on brain metastases. Ther. Adv. Respir. Dis. 2019, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- US.FDA. Alectinib Approved for (ALK) Positive Metastatic Non-Small Cell Lung Cancer (NSCLC). Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm584082.htm (accessed on 13 November 2019).
- Gadgeel, S.; Shaw, A.; Govindan, R. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non–small-cell lung cancer. J. Clin. Oncol. 2016, 34, 4079–4085. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.; Gandhi, L.; Gadgeel, S. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: A single-group, multicentre, phase 2 trial. Lancet Oncol. 2016, 17, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Shaw, A.; Gadgeel, S. A phase II, open-label, multicenter study of the ALK inhibitor alectinib in an ALK+ non-small-cell lung cancer (NSCLC) US/Canadian population who had progressed on crizotinib (NP28761). J. Clin. Oncol. 2015, 33, 8019. [Google Scholar] [CrossRef]
- McKeage, K. Alectinib: A review of its use in advanced ALK-rearranged non-small cell lung cancer. Drugs 2015, 75, 75–82. [Google Scholar] [CrossRef]
- Gadgeel, S.; Gandhi, L.; Riely, G.J. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): Results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014, 15, 1119–1128. [Google Scholar] [CrossRef]
- Morcos, P.N.; Yu, L.; Bogman, K. Absorption, distribution, metabolism and excretion (ADME) of the ALK inhibitor alectinib: Results from an absolute bioavailability and mass balance study in healthy subjects. Xenobiotica 2017, 47, 217–229. [Google Scholar] [CrossRef]
- Aydoğmuş, Z.; Sarı, F.; Ulu, S.T. Spectrofluorimetric determination of aliskiren in tablets and spiked human plasma through derivatization with dansyl chloride. J. Fluoresc. 2012, 22, 549–556. [Google Scholar] [CrossRef]
- Zhu, V.; Lu, Y.; Ou, S.I. Severe Acute Hepatitis in a Patient Receiving Alectinib for ALK-Positive Non–Small-Cell Lung Cancer: Histologic Analysis. Clin. Lung Cancer 2019, 20, e77–e80. [Google Scholar] [CrossRef]
- Ramachandran, P.; Morcus, R.; Tahir, M. Alectinib (Alecensa)-induced reversible grade IV nephrotoxicity: A case report and review of the literature. J. Med. Case Rep. 2018, 12, 303. [Google Scholar] [CrossRef]
- Balwani, G.; Joseph, E.; Reddi, S.; Nagpal, V.; Saha, R.N. Rapid, simple, and sensitive spectrofluorimetric method for the estimation of ganciclovir in bulk and pharmaceutical formulations. J. Spectrosc. 2013, 2013, 972806. [Google Scholar] [CrossRef]
- Rao, N.; Srinivas, Y. Method Development and Validation of Alectinib Drug by RP-HPLC in Bulk and Pharmaceutical Dosage Form. Asian J. Pharm. Anal. 2018, 8, 186–196. [Google Scholar] [CrossRef]
- Lee, S.; Nath, C.E.; Balzer, B.W.; Lewis, C.R.; Trahair, T.N.; Anazodo, A.C. An HPLC–PDA method for determination of alectinib concentrations in the plasma of an adolescent. Acta Chromatogr. 2019, 32, 166–169. [Google Scholar] [CrossRef]
- Veerman, M.; Lam, H.; Mathijssen, H. Quantification of afatinib, alectinib, crizotinib and osimertinib in human plasma by liquid chromatography/triple-quadrupole mass spectrometry; focusing on the stability of Osimertinib. J. Chromatogr. B Biomed. Appl. 2019, 1113, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, I.; Rani, A.P. Hplc-ms/ms method development and validation for determining stability of alectinib in human plasma samples. Int. J. Curr. Res. 2017, 9, 51506–51511. [Google Scholar]
- Heinig, K.; Miya, K.; Kamei, T. Bioanalysis of alectinib and metabolite M4 in human plasma, cross-validation and impact on PK assessment. Bioanalysis 2016, 8, 1465–1479. [Google Scholar] [CrossRef]
- Janssen, M.; de Vries, N.; Venekamp, N. Development and validation of a liquid chromatography-tandem mass spectrometry assay for nine oral anticancer drugs in human plasma. J. Pharm. Biomed. Anal. 2019, 174, 561–566. [Google Scholar] [CrossRef]
- Huang, X.-X.; Li, Y.-X.; Li, X.-Y. An UPLC–MS/MS method for the quantitation of alectinib in rat plasma. J. Pharm. Biomed. Anal. 2017, 132, 227–231. [Google Scholar] [CrossRef]
- Heinig, K.; Herzog, D.; Ferrari, L. LC–MS/MS determination of alectinib and its major human metabolite M4 in human urine: Prevention of nonspecific binding. Bioanalysis 2017, 9, 459–468. [Google Scholar] [CrossRef]
- Bose, A.; Thomas, I.; Kavitha, G.; Abraham, E. Fluorescence spectroscopy and its applications: A Review. Int. J. Adv. Pharm. Res. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Ali, M.F.B.; Atia, N.N. Sensitive spectrofluorimetric methods for determination of sitagliptin phosphate, dipeptidyl peptidase-4 inhibitor, in pharmaceutical tablets and spiked human urine. Curr. Pharm. Anal. 2018, 14, 483–490. [Google Scholar] [CrossRef]
- Syamittra, B.; Parasuraman, S.; Yeng, W.Y.; Ping, W.Y.; Thujithra, J.; Kumar, J.; Dhanaraj, S.A. A Review on adverse health effects of laboratory volatile solvents. Int. J. Clin. Ther. Diagn. 2014, 2, 59–63. [Google Scholar] [CrossRef]
- Khalil, N.Y.; Darwish, I.A.; Alanazi, M.; Hamidaddin, M.A. Development of 96-microwell plate assay with fluorescence reader and HPLC method with fluorescence detection for high-throughput analysis of linifanib in its bulk and dosage forms. Curr. Pharm. Anal. 2021, 17, 285–292. [Google Scholar] [CrossRef]
- Kaur, K.; Saini, S.; Malik, A.; Singh, B. Micelle enhanced and terbium sensitized spectrofluorimetric determination of danofloxacin in milk using molecularly imprinted solid phase extraction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 96, 790–795. [Google Scholar] [CrossRef]
- Tang, B.; Wang, X.; Jia, B. Simple, rapid, and sensitive spectrofluorimetric determination of Zaleplon in micellar medium. Anal. Lett. 2003, 36, 2985–2997. [Google Scholar] [CrossRef]
- Darwish, H.W.; Bakheit, A.H.; Darwish, I.A. Enhanced spectrofluorimetric determination of the multitargeted tyrosine kinase inhibitor, crizotinib, in human plasma via micelle-mediated approach. Trop. J. Pharm. Res. 2016, 15, 2209–2217. [Google Scholar] [CrossRef]
- Darwish, H.W.; Bakheit, A.H.; Al-Anazi, Z.S. Response surface methodology for optimization of micellar-enhanced spectrofluorimetric method for assay of foretinib in bulk powder and human urine. Spectrochim. Acta Part A. 2021, 257, 119811. [Google Scholar] [CrossRef]
- Rangel-Yagui, C.O.; Pessoa, A., Jr.; Tavares, L.C. Micellar solubilization of drugs. J. Pharm Sci. 2005, 8, 147–163. [Google Scholar] [PubMed]
- Leung, R.; Shah, D.O. Dynamic properties of micellar solutions: I. Effects of short-chain alcohols and polymers on micellar stability. J. Colloid Interface Sci. 1986, 113, 484–499. [Google Scholar] [CrossRef]
- Lakowicz, J. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline, Validation of Analytical Procedure: Q2(R2); ICH: Geneva, Switzerland, 2022. [Google Scholar]
| Method/Condition | Studied Range | Optimum Value |
|---|---|---|
| MW-SFL | ||
| Surfactants (1%, w/v) | Different a | Cr RH40 |
| Volume of Cr RH 40 (µL) | 15–70 | 60 |
| Solvent | Different b | Water |
| pH | 3–11 | 7 |
| Excitation wavelength (λex, nm) | 230–440 | 365 |
| Emission wavelength (λem, nm) | 350–550 | 450 |
| HPLC-FD | ||
| Organic solvent in a mobile phase | Acetonitrile, methanol | Methanol |
| Phosphate buffer:methanol ratio (%) | 60–90 | 80 |
| pH of buffer solution | 4–7 | 7 |
| Flaw rate (mL min–1) | 0.5–2 mL min–1 | 2 |
| Internal standard | Different c | Pontatinib |
| Excitation wavelength (nm) | 230–440 | 365 |
| Emission wavelength (nm) | 350–550 | 450 |
| Parameter | Value | Recommended Value |
|---|---|---|
| Retention time (min) | 4.86 | |
| Capacity factor (K`) | 3.5 | 1–10 |
| Separation factor (α) | 1.33 | >1 |
| Resolution factor (Rs) | 2.97 | ≥1.5 |
| Peak asymmetry factor | 1.10 | ≥1 |
| Number of theoretical plates (N) per meter | 1794 | Varied |
| Parameter | Value a | |
|---|---|---|
| MW-SFL | HPLC-FD | |
| Range (ng mL−1) | 30–500 | 5–1000 |
| Intercept | 3.09 | 0.0037 |
| Slope | 1.33 | 0.0066 |
| Determination coefficient (r2) | 0.9998 | 0.9998 |
| LOD b (ng mL−1) | 6.46 | 2.60 |
| LOQ c (ng mL−1) | 19.60 | 7.81 |
| ALC Concentration (ng mL−1) | Intra-Day a | Inter-Day b | ||
|---|---|---|---|---|
| Recovery (% ± RSD) | Error (%) | Recovery (% ± RSD) | Error (%) | |
| MW-SFL | ||||
| 125 | 99.8 ± 0.75 | −0.19 | 100.2 ± 1.52 | −0.21 |
| 250 | 98.5 ± 0.23 | −1.49 | 99.0 ± 0.81 | 0.97 |
| 420 | 96.3 ± 0.70 | −3.74 | 100.8 ± 0.88 | 0.81 |
| HPLC-FD | ||||
| 250 | 96.3 ± 0.58 | 3.70 | 97.3 ± 1.09 | 2.7 |
| 500 | 101.8 ± 0.70 | −1.80 | 98.4 ± 2.32 | 1.6 |
| 750 | 102.7 ± 0.23 | −2.68 | 102.9 ± 0.34 | 2.8 |
| Spiked Concentration (ng mL−1) | Measured Concentration (ng mL−1) | Recovery (% ± SD) a | Error (%) |
|---|---|---|---|
| MW-SFL | |||
| 60 | 54.54 | 91.20 ± 0.25 | 8.79 |
| 120 | 117.90 | 98.25 ± 1.55 | 3.09 |
| 240 | 231.87 | 96.61 ± 2.50 | 3.38 |
| 480 | 481.46 | 100.30 ± 1.02 | −0.30 |
| HPLC-FD | |||
| 120 | 116.94 | 95.45 ± 2.25 | 4.55 |
| 240 | 244.56 | 101.90 ± 1.35 | −1.90 |
| 480 | 478.46 | 99.68 ± 0.96 | 0.32 |
| Value | MW-SFL | LC-MS | HPLC-FD | LC-MS |
|---|---|---|---|---|
| Mean (%) | 99.40 | 98.84 | 97.87 | 99.40 |
| RSD (%) | 2.28 | 3.87 | 2.14 | 3.87 |
| N | 7 | 5 | 7 | 5 |
| t-test | 1.15 (2.77) a | 0.44 (2.77) a | ||
| F-test | 2.89 (4.53) b | 3.37 (4.53) b | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, H.S.; Alanazi, M.M.; Darwish, I.A.; Bakheit, A.H.; Alshehri, M.M.; Darwish, H.W. Development of Novel Microwell-Based Spectrofluorimetry and High-Performance Liquid Chromatography with Fluorescence Detection Methods and High Throughput for Quantitation of Alectinib in Bulk Powder and Urine Samples. Medicina 2023, 59, 441. https://doi.org/10.3390/medicina59030441
Almutairi HS, Alanazi MM, Darwish IA, Bakheit AH, Alshehri MM, Darwish HW. Development of Novel Microwell-Based Spectrofluorimetry and High-Performance Liquid Chromatography with Fluorescence Detection Methods and High Throughput for Quantitation of Alectinib in Bulk Powder and Urine Samples. Medicina. 2023; 59(3):441. https://doi.org/10.3390/medicina59030441
Chicago/Turabian StyleAlmutairi, Halah S., Mohammed M. Alanazi, Ibrahim A. Darwish, Ahmed H. Bakheit, Mona M. Alshehri, and Hany W. Darwish. 2023. "Development of Novel Microwell-Based Spectrofluorimetry and High-Performance Liquid Chromatography with Fluorescence Detection Methods and High Throughput for Quantitation of Alectinib in Bulk Powder and Urine Samples" Medicina 59, no. 3: 441. https://doi.org/10.3390/medicina59030441
APA StyleAlmutairi, H. S., Alanazi, M. M., Darwish, I. A., Bakheit, A. H., Alshehri, M. M., & Darwish, H. W. (2023). Development of Novel Microwell-Based Spectrofluorimetry and High-Performance Liquid Chromatography with Fluorescence Detection Methods and High Throughput for Quantitation of Alectinib in Bulk Powder and Urine Samples. Medicina, 59(3), 441. https://doi.org/10.3390/medicina59030441








