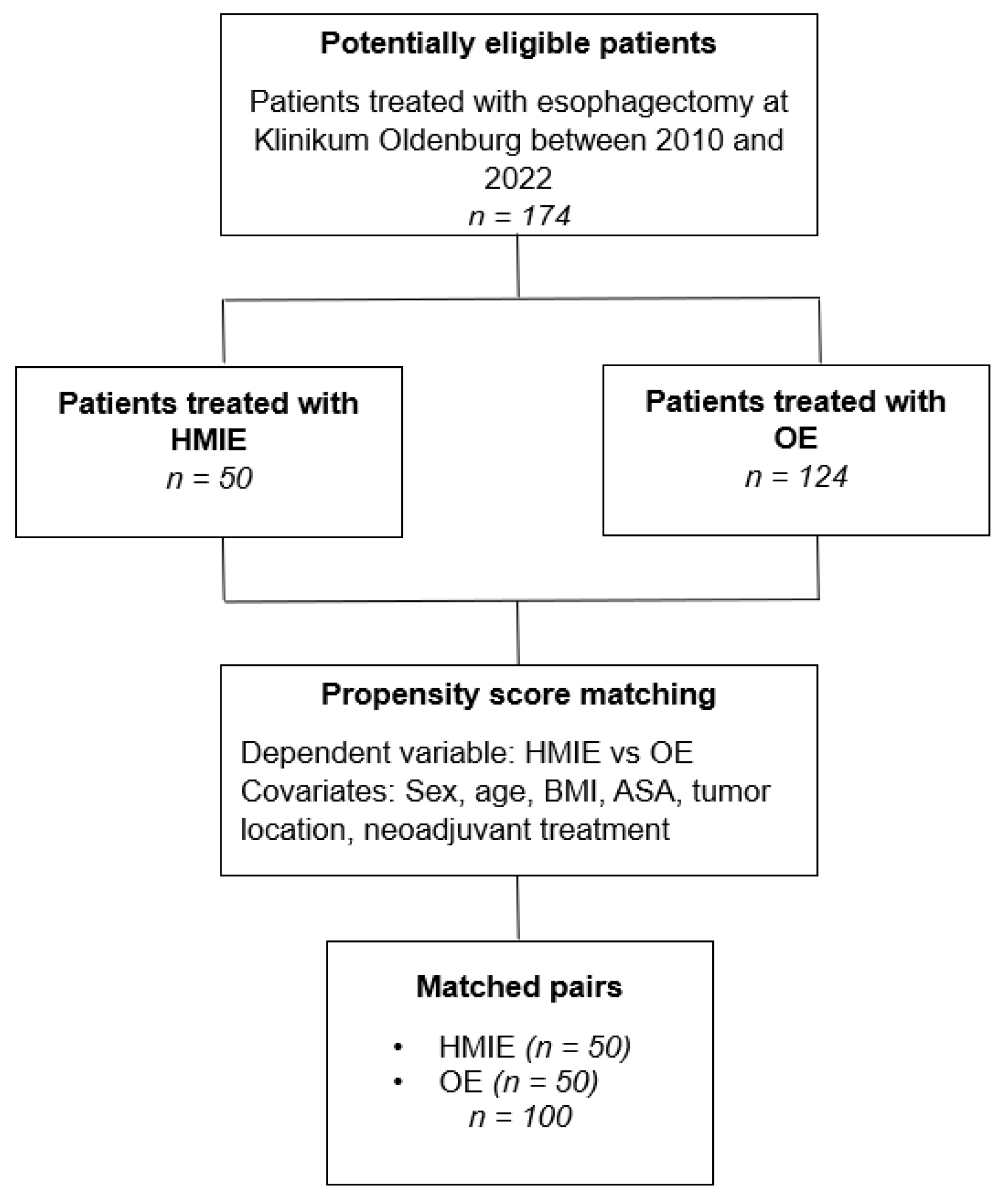
Hybrid Minimally Invasive Esophagectomy vs. Open Esophagectomy: A Retrospective Propensity Score Matched Comparison
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Preoperative Process
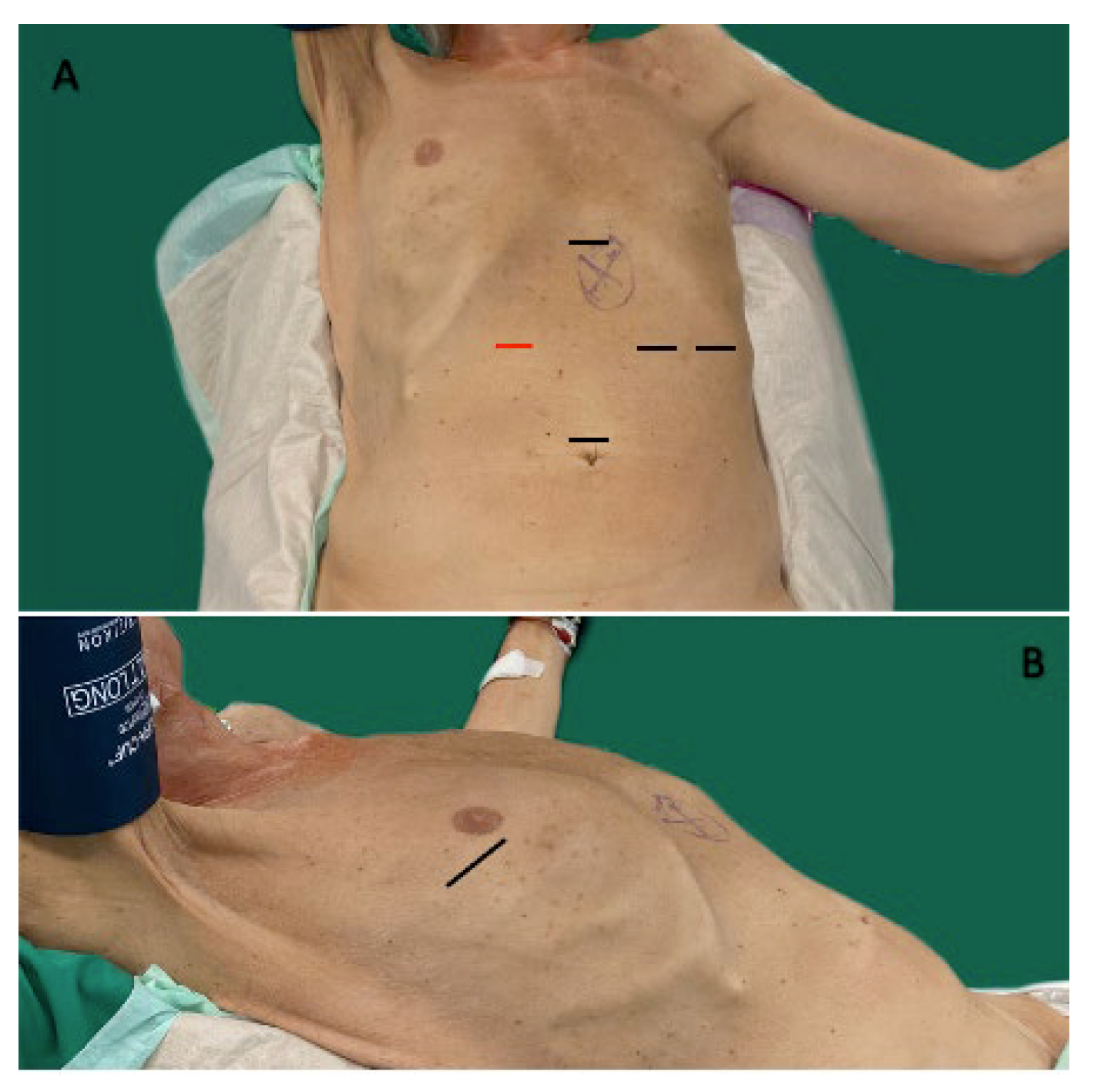
2.3. Surgical Techniques
2.4. Postoperative Management
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Surgical Results
3.3. Perioperative Morbidity and Mortality
3.4. Risk Factor Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Regression Coefficient B | Std Error | Wald | df | p | Exp (B) | ||
|---|---|---|---|---|---|---|---|
| Step 1 a | ICU Stay (days) | 0.039 | 0.028 | 1.947 | 1 | 0.163 | 1.040 |
| Hospital Stay (days) | −0.045 | 0.021 | 4.601 | 1 | 0.032 | 0.956 | |
| Number of RBCs | −1.455 | 0.819 | 3.156 | 1 | 0.076 | 0.233 | |
| Constant | 1.030 | 0.426 | 5.856 | 1 | 0.016 | 2.801 | |
Appendix B
| Regression Coefficient B | Std Error | Wald | df | p | Exp (B) | ||
|---|---|---|---|---|---|---|---|
| Step 2 a | ICU Stay (days) | 0.036 | 0.028 | 1.597 | 1 | 0.206 | 1.037 |
| Hospital Stay (days) | −0.043 | 0.021 | 4.395 | 1 | 0.036 | 0.958 | |
| Number of RBCs | −1.617 | 0.840 | 3.700 | 1 | 0.054 | 0.199 | |
| Gender Female | −0.050 | 0.548 | 0.008 | 1 | 0.927 | 0.951 | |
| BMI >/= 25 | 0.000 | 0.445 | 0.000 | 1 | 0.999 | 1.000 | |
| ASA 3/4 | 0.607 | 0.443 | 1.876 | 1 | 0.171 | 1.835 | |
| Constant | 0.719 | 0.565 | 1.620 | 1 | 0.203 | 2.052 | |
Appendix C
| Regression Coefficient B | Std Error | Wald | df | p | Exp (B) | ||
|---|---|---|---|---|---|---|---|
| Step 3 a | ICU Stay (days) | 0.030 | 0.031 | 0.945 | 1 | 0.331 | 1.031 |
| Hospital Stay (days) | −0.043 | 0.022 | 3.821 | 1 | 0.051 | 0.958 | |
| Number of RBCs | −1.656 | 0.953 | 3.019 | 1 | 0.082 | 0.191 | |
| Gender Female | −0.068 | 0.567 | 0.014 | 1 | 0.904 | 0.934 | |
| BMI >/= 25 | 0.178 | 0.465 | 0.147 | 1 | 0.701 | 1.195 | |
| ASA 3/4 | 0.813 | 0.499 | 2.658 | 1 | 0.103 | 2.256 | |
| Neoadjuvant Treatment | 0.203 | 0.329 | 0.380 | 1 | 0.537 | 1.225 | |
| Smoking | 0.418 | 0.475 | 0.774 | 1 | 0.379 | 1.518 | |
| Pulmonary Comorbidities | 0.437 | 0.688 | 0.403 | 1 | 0.526 | 1.547 | |
| Cardiac Comorbidities | −0.859 | 0.503 | 2.925 | 1 | 0.087 | 0.423 | |
| Diabetes mellitus | −0.144 | 0.837 | 0.030 | 1 | 0.863 | 0.866 | |
| Alcohol abuse | 0.284 | 0.840 | 0.114 | 1 | 0.735 | 1.328 | |
| Constant | 0.687 | 0.700 | 0.964 | 1 | 0.326 | 1.989 | |
Appendix D
| Pearson-Chi-Square Value | df | p | φ | |
|---|---|---|---|---|
| Anastomotic leakage | 19.048 | 1 | <0.001 | 0.436 |
| Pneumonia | 41.360 | 1 | <0.001 | 0.643 |
| ARDS | 8.696 | 1 | 0.003 | 0.295 |
| Pulmonary complication | 44.444 | 1 | <0.001 | 0.667 |
| Pulmonary embolism | 4.167 | 1 | 0.041 | 0.204 |
| Death | 5.983 | 1 | 0.014 | 0.245 |
References
- AWMF and Leitlinienprogramm Onkologie. S3-Leitlinie Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus: Version 3.1., AWMF-Registernummer: 021/023OL; AWMF and Leitlinienprogramm Onkologie: Berlin, Germany, 2021. [Google Scholar]
- Moehler, M.; Al-Batran, S.E.; Andus, T.; Anthuber, M.; Arends, J.; Arnold, D.; Aust, D.; Baier, P.; Baretton, G.; Bernhardt, J.; et al. German S3-guideline “Diagnosis and treatment of esophagogastric cancer. Z. Fur Gastroenterol. 2011, 49, 461–531. (In German) [Google Scholar] [CrossRef] [PubMed]
- Schröder, W.; Gisbertz, S.S.; Voeten, D.M.; Gutschow, C.A.; Fuchs, H.F.; van Berge Henegouwen, M.I. Surgical Therapy of Esophageal Adenocarcinoma-Current Standards and Future Perspectives. Cancers 2021, 13, 5834. [Google Scholar] [CrossRef] [PubMed]
- van Workum, F.; Klarenbeek, B.R.; Baranov, N.; Rovers, M.M.; Rosman, C. Totally minimally invasive esophagectomy versus hybrid minimally invasive esophagectomy: Systematic review and meta-analysis. Dis. Esophagus 2020, 33, doaa021. [Google Scholar] [CrossRef] [PubMed]
- Harriott, C.B.; Angeramo, C.A.; Casas, M.A.; Schlottmann, F. Open versus hybrid versus totally minimally invasive Ivor Lewis esophagectomy: Systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2022, 164, e233–e254. [Google Scholar] [CrossRef]
- Mariette, C.; Markar, S.R.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrère, N.; et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N. Engl. J. Med. 2019, 380, 152–162. [Google Scholar] [CrossRef]
- Nuytens, F.; Dabakuyo-Yonli, T.S.; Meunier, B.; Gagnière, J.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrère, N.; Mabrut, J.Y.; et al. Five-Year Survival Outcomes of Hybrid Minimally Invasive Esophagectomy in Esophageal Cancer: Results of the MIRO Randomized Clinical Trial. JAMA Surg. 2021, 156, 323–332. [Google Scholar] [CrossRef]
- Biere, S.S.; van Berge Henegouwen, M.I.; Maas, K.W.; Bonavina, L.; Rosman, C.; Garcia, J.R.; Gisbertz, S.S.; Klinkenbijl, J.H.; Hollmann, M.W.; De Lange, E.S.; et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicentre, open-label, randomised controlled trial. Lancet 2012, 379, 1887–1892. [Google Scholar] [CrossRef]
- Van der Wilk, B.J.; Hagens, E.R.; Eyck, B.M.; Gisbertz, S.S.; van Hillegersberg, R.; Nafteux, P.; Schröder, W.; Nilsson, M.; Wijnhoven, B.P.; Lagarde, S.M.; et al. Outcomes after totally minimally invasive versus hybrid and open Ivor Lewis oesophagectomy: Results from the International Esodata Study Group. Br. J. Surg. 2022, 109, 283–290. [Google Scholar] [CrossRef]
- AWMF and Leitlinienprogramm Onkologie. S3-Leitlinie Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus.: Version 2.0. 2018. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/oesophaguskarzinom/ (accessed on 27 September 2022).
- Porschen, R.; Buck, A.; Fischbach, W.; Gockel, I.; Görling, U.; Grenacher, L.; Hollerbach, S.; Hölscher, A.; Körber, J.; Messmann, H.; et al. S3-Leitlinie Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus (Langversion 1.0—September 2015, AWMF-Registernummer: 021/023OL). Z. Fur Gastroenterol. 2015, 53, 1288–1347. (In German) [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.; Ge, K.; Yang, J.L. Efficacy of hybrid minimally invasive esophagectomy vs. open esophagectomy for esophageal cancer: A meta-analysis. World J. Gastrointest. Oncol. 2019, 11, 1081–1091. [Google Scholar] [CrossRef]
- Glatz, T.; Marjanovic, G.; Kulemann, B.; Sick, O.; Hopt, U.T.; Hoeppner, J. Hybrid minimally invasive esophagectomy vs. open esophagectomy: A matched case analysis in 120 patients. Langenbeck’s Arch. Surg. 2017, 402, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.; Khan, O.; Willows, E.; Somers, S.; Mercer, S.; Toh, S. Open and laparoscopically assisted oesophagectomy: A prospective comparative study. Eur. J. Cardio-Thorac. Surg. 2013, 43, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Lacy, A.M.; García-Valdecasas, J.C.; Delgado, S.; Castells, A.; Taurá, P.; Piqué, J.M.; Visa, J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: A randomised trial. Lancet 2002, 359, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet. Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef]
- Bjelovic, M.; Babic, T.; Spica, B.; Gunjic, D.; Veselinovic, M.; Trajkovic, G. Could hybrid minimally invasive esophagectomy improve the treatment results of esophageal cancer? Eur. J. Surg. Oncol. 2016, 42, 1196–1201. [Google Scholar] [CrossRef]
- Briez, N.; Piessen, G.; Claret, A.; Triboulet, J.; Mariette, C. Is minimally invasive œsophagectomy for cancer decreasing pulmonary complications? Results from a case-control study. J. Clin. Oncol. 2010, 28 (Suppl. 15), 4071. [Google Scholar] [CrossRef]
- Sparn, M.B.; Widmann, B.; Pietsch, U.; Weitzendorfer, M.; Warschkow, R.; Steffen, T. Risk factors and outcomes of postoperative aspiration pneumonia in abdominal surgery patients: An exact matching and weighting analysis. Surgery 2021, 170, 1432–1441. [Google Scholar] [CrossRef]
- Baba, H.; Tokai, R.; Hirano, K.; Watanabe, T.; Shibuya, K.; Hashimoto, I.; Hojo, S.; Yoshioka, I.; Okumura, T.; Nagata, T.; et al. Risk factors for postoperative pneumonia after general and digestive surgery: A retrospective single-center study. Surg. Today 2020, 50, 460–468. [Google Scholar] [CrossRef]
- Charles, M.P.; Kali, A.; Easow, J.M.; Joseph, N.M.; Ravishankar, M.; Srinivasan, S.; Kumar, S.; Umadevi, S. Ventilator-associated pneumonia. Australas. Med. J. 2014, 7, 334–344. [Google Scholar] [CrossRef]
- Fabbi, M.; Hagens, E.R.C.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Anastomotic leakage after esophagectomy for esophageal cancer: Definitions, diagnostics, and treatment. Dis. Esophagus 2021, 34, doaa039. [Google Scholar] [CrossRef]
- Welte, M.; Saugel, B.; Reuter, D.A. Perioperative blood pressure management: What is the optimal pressure? Anaesthesist 2020, 69, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, M.; Gwam, C.U.; Mohamed, N.; Khlopas, A.; Newman, J.M.; Khan, R.; Nadhim, A.; Shaffiy, S.; Mont, M.A. The Epidemiology and Risk Factors for Postoperative Pneumonia. J. Clin. Med. Res. 2017, 9, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D. The Clavien–Dindo Classification of Surgical Complications. In Treatment of Postoperative Complications after Digestive Surgery; Cuesta, M., Bonjer, H.J., Eds.; Springer: London, UK, 2014; pp. 13–17. [Google Scholar]
| Open (n = 50) | Hybrid (n = 50) | |||||||
|---|---|---|---|---|---|---|---|---|
| n | % | Median | n | % | Median | p | ||
| Sex | Male | 41 | 82.0% | 40 | 80.0% | 1.000 | ||
| Female | 9 | 18.0% | 10 | 20.0% | 1.000 | |||
| Age in years | 64.00 | 64.50 | 0.373 | |||||
| Preoperative BMI | 25.50 | 26.00 | 0.371 | |||||
| ASA-Classification | ASA I | 0 | 0.0% | 0 | 0.0% | |||
| ASA II | 26 | 52.0% | 21 | 42.0% | 0.423 | |||
| ASA III | 21 | 42.0% | 28 | 56.0% | 0.230 | |||
| ASA IV | 3 | 6.0% | 1 | 2.0% | 0.617 | |||
| ASA V | 0 | 0.0% | 0 | 0.0% | ||||
| Neoadjuvant Treatment | None | 24 | 48.0% | 25 | 50.0% | 1.000 | ||
| Chemotherapy | 20 | 40.0% | 18 | 36.0% | 0.837 | |||
| Radio-Chemotherapy | 6 | 12.0% | 6 | 12.0% | 1.000 | |||
| Endoscopic Submucosal Dissection | 0 | 0.0% | 1 | 2.0% | 1.000 | |||
| Postoperative UICC-Classification | 0 | 7 | 8 | 1.000 | ||||
| IA | 14 | 12 | 0.820 | |||||
| IB | 2 | 9 | 0.051 | |||||
| IIA | 4 | 7 | 0.525 | |||||
| IIB | 9 | 0 | 0.003 | |||||
| III | 14 | 12 | 0.820 | |||||
| IV | 0 | 2 | 0.495 | |||||
| Histology of Tumor | No malignancy | 1 | 2.0% | 0 | 0.0% | 1.000 | ||
| Adenocarcinoma | 40 | 81.6% | 43 | 86.0% | 0.595 | |||
| Squamous Cell Carcinoma | 8 | 16.3% | 7 | 14.0% | 1.000 | |||
| Location of Tumor | Lower third | 42 | 84.0% | 42 | 84.0% | 1.000 | ||
| Middle third | 8 | 16.0% | 7 | 14.0% | 1.000 | |||
| Upper third | 0 | 0.0% | 1 | 2.0% | 1.000 | |||
| Smoking | 16 | 32.0% | 23 | 46.0% | 0.218 | |||
| Alcohol abuse | 3 | 6.0% | 5 | 10.0% | 0.715 | |||
| Diabetes mellitus | 5 | 10.0% | 4 | 8.0% | 1.000 | |||
| Cardiac Comorbidities | 34 | 68.0% | 27 | 54.0% | 0.218 | |||
| Pulmonary Comorbidities | 8 | 16.0% | 8 | 16.0% | 1.000 | |||
| Open (n = 50) | Hybrid (n = 50) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Maximum | Minimum | n (%) | Median | Maximum | Minimum | n (%) | p | ||
| Number of Harvested Lymphnodes | 23.50 | 41.00 | 12.00 | 21.00 | 38.00 | 13.00 | 0.666 | |||
| Duration of Surgery | 288.00 | 482.00 | 127.00 | 225.50 | 337.00 | 122.00 | 0.243 | |||
| Tumor Margin | R0 | 49 (98.00) | 50 (100.00) | 1.000 | ||||||
| R1 | 0 | 0 | ||||||||
| R2 | 1 (2.00) | 0 | 1.000 | |||||||
| Required RBC Transfusion | 6 (12.00) | 1 (2.00) | 0.112 | |||||||
| Number of RBCs | 0.00 | 6.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.041 | |||
| Hospital Stay (days) | 23.00 | 123.00 | 7.00 | 16.50 | 142.00 | 9.00 | 0.004 | |||
| IMC Stay (days) | 4.00 | 15.00 | 0.00 | 2.00 | 8.00 | 0.00 | 0.061 | |||
| ICU Stay (days) | 5.50 | 71.00 | 2.00 | 3.00 | 117.00 | 1.00 | 0.003 | |||
| Open (n = 50) | Hybrid (n = 50) | ||||
|---|---|---|---|---|---|
| n | % | n | % | p | |
| Any Complication | 35 | 70.0% | 15 | 30.0% | <0.001 |
| Clavien Dindo I-II | 26 | 52.0% | 42 | 84.0% | 0.001 |
| Clavien Dindo III-V | 24 | 48.0% | 8 | 16.0% | 0.001 |
| Anastomotic Leakage | 11 | 22.0% | 5 | 10.0% | 0.171 |
| ECCG I | 0 | 0% | 0 | 0% | |
| ECCG II | 6 | 12% | 5 | 10% | 1.000 |
| ECCG III | 5 | 10% | 0 | 0% | 0.056 |
| Pulmonary Embolism | 3 | 6.0% | 1 | 2.0% | 0.617 |
| Pulmonary Complication | 23 | 46.0% | 13 | 26.0% | 0.060 |
| Pneumonia | 19 | 38.0% | 13 | 26.0% | 0.284 |
| ARDS | 6 | 12.0% | 2 | 4.0% | 0.269 |
| Perioperative Death | 7 | 14.0% | 2 | 4.0% | 0.160 |
| Any Complication | Clavien Dindo III–V | |||||||
|---|---|---|---|---|---|---|---|---|
| OE/ HMIE (%) | pa | OR a (95% CI) | pb | OE/HMIE (%) | pa | OR a (95% CI) | pb | |
| Surgical Technique (OE/HMIE) | 70/30 | <0.001 | 0.184 (0.078–0.432) | < 0.001 | 48/16 | 0.001 | 0.206 (0.081–0.527) | <0.001 |
| Sex (male/female) | 86/14 | 0.308 | 0.103 | 52/12 | 1.000 | 0.483 | ||
| Age (<65 years/≥65 years) | 54/46 | 0.689 | 0.277 | 36/28 | 0.524 | 0.238 | ||
| BMI (≥25/<25) | 60/40 | 1.000 | 0.420 | 38/26 | 1.000 | 0.479 | ||
| ASA (I–II/ III–IV) | 40/60 | 0.229 | 0.082 | 28/36 | 0.674 | 0.329 | ||
| Histology (adeno/SCC) | 40/16 | 0.595 | 0.215 | 54/10 | 1.000 | 0.411 | ||
| Neoadjuvant treatment (any/none) | 48/52 | 1.000 | 0.422 | 36/28 | 0.524 | 0.306 | ||
| Smoking (yes/no) | 34/66 | 0.412 | 0.155 | 26/38 | 0.829 | 0.260 | ||
| Pulmonary Comorbidities (yes/no) | 20/80 | 0.414 | 0.140 | 12/52 | 0.771 | 0.057 | ||
| Cardiac Comorbidities (yes/no) | 72/28 | 0.040 | 2.571 (1.122–5.895) | 0.012 | 42/22 | 0.661 | 0.331 | |
| Diabetes mellitus (yes/no) | 14/86 | 0.160 | 0.041 | 10/54 | 0.140 | 0.402 | ||
| Alcohol abuse (yes/no) | 4/96 | 0.269 | 0.072 | 4/60 | 1.000 | 0.238 | ||
| Pulmonary Complication | In-Hospital Death | |||||||
|---|---|---|---|---|---|---|---|---|
| OE/HMIE (%) | pa | OR a (95% CI) | pb | OE/HMIE (%) | pa | OR a (95% CI) | pb | |
| Surgical Technique (OE/HMIE) | 46/26 | 0.060 | 0.019 | 14/4 | 0.160 | 0.041 | ||
| Sex (male/ female) | 60/12 | 0.793 | 0.330 | 16/2 | 1.000 | 0.266 | ||
| Age (<65 years/≥65 years) | 40/32 | 0.537 | 0.250 | 4/14 | 0.089 | 0.036 | ||
| BMI (≥25/<25) | 44/28 | 0.833 | 0.375 | 12/6 | 0.733 | 0.314 | ||
| ASA (I–II/III–IV) | 30/42 | 0.532 | 0.214 | 2/16 | 0.034 | 1.212 (0.521–2.822) | 0.012 | |
| Histology (adeno/SCC) | 60/12 | 1.000 | 0.474 | 18/0 | 0.351 | 0.079 | ||
| Neoadjuvant treatment (any/none) | 36/36 | 1.000 | 0.441 | 8/10 | 0.738 | 0.342 | ||
| Smoking (yes/no) | 22/50 | 0.209 | 0.099 | 8/10 | 0.733 | 0.364 | ||
| Pulmonary Comorbidities (yes/no) | 18/54 | 0.089 | 0.033 | 4/14 | 0.633 | 0.299 | ||
| Cardiac Comorbidities (yes/no) | 50/22 | 0.209 | 0.099 | 16/2 | 0.086 | 0.037 | ||
| Diabetes mellitus (yes/no) | 6/66 | 1.000 | 0.432 | 4/14 | 0.186 | 0.075 | ||
| Alcohol abuse (yes/no) | 4/68 | 0.707 | 0.252 | 0/18 | 1.00 | 0.179 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincke, A.; Miftode, S.; Alfarawan, F.; Bockhorn, M.; El-Sourani, N. Hybrid Minimally Invasive Esophagectomy vs. Open Esophagectomy: A Retrospective Propensity Score Matched Comparison. Medicina 2023, 59, 434. https://doi.org/10.3390/medicina59030434
Vincke A, Miftode S, Alfarawan F, Bockhorn M, El-Sourani N. Hybrid Minimally Invasive Esophagectomy vs. Open Esophagectomy: A Retrospective Propensity Score Matched Comparison. Medicina. 2023; 59(3):434. https://doi.org/10.3390/medicina59030434
Chicago/Turabian StyleVincke, Anna, Sorin Miftode, Fadl Alfarawan, Maximilian Bockhorn, and Nader El-Sourani. 2023. "Hybrid Minimally Invasive Esophagectomy vs. Open Esophagectomy: A Retrospective Propensity Score Matched Comparison" Medicina 59, no. 3: 434. https://doi.org/10.3390/medicina59030434
APA StyleVincke, A., Miftode, S., Alfarawan, F., Bockhorn, M., & El-Sourani, N. (2023). Hybrid Minimally Invasive Esophagectomy vs. Open Esophagectomy: A Retrospective Propensity Score Matched Comparison. Medicina, 59(3), 434. https://doi.org/10.3390/medicina59030434






