Abstract
Gestational diabetes mellitus (GDM) is one of the most common pregnancy complications and one of the main causes of adverse pregnancy outcomes. An early diagnosis of GDM is of fundamental importance in clinical practice. However, the major professional organizations recommend universal screening for GDM, using a 75 g oral glucose tolerance test at 24–28 weeks of gestation. A selective screening at an early stage of pregnancy is recommended only if there are maternal risk factors for diabetes. As a result, the GDM diagnosis is often delayed and established after the appearance of complications. The manifestation of GDM is directly related to insulin resistance, which is closely associated with endothelial dysfunction. The placenta, the placental peptides and hormones play a pivotal role in the manifestation and progression of insulin resistance during pregnancy. Recently, the placental growth factor (PlGF) and plasma-associated protein-A (PAPP-A), have been shown to significantly affect both insulin sensitivity and endothelial function. The principal function of PAPP-A appears to be the cleavage of circulating insulin-like growth factor binding protein-4 while PlGF has been shown to play a central role in the development and maturation of the placental vascular system and circulation. On one hand, these factors are widely used as early predictors (11–13 weeks of gestation) of complications during pregnancy, such as preeclampsia and fetal aneuploidies, in most countries. On the other hand, there is increasing evidence for their predictive role in the development of carbohydrate disorders, but some studies are rather controversial. Therefore, this review aims to summarize the available literature about the potential of serum levels of PlGF and PAPP-A as early predictors in the diagnosis of GDM.
1. Introduction
Gestational diabetes mellitus (GDM) is defined as a disorder of glucose metabolism recognized for the first time during pregnancy, even though the abnormalities may have existed before pregnancy and may persist after the delivery [1]. It has been estimated that approximately 7% of all pregnancies are complicated by GDM, resulting in more than 200,000 worldwide cases annually [2,3]. The prevalence may vary from 1% to 14% of all pregnancies depending on the population studied and the diagnostic tests used [2]. GDM is the most common cause of hyperglycemia during pregnancy, accounting for more than 85% of all cases [3,4]. The remaining 15% are due to pre-existing type 1 or type 2 diabetes mellitus. In addition, GDM is highly associated with many other serious maternal and fetal complications [4,5]. Therefore, more research and clinical efforts should be focused on preventing GDM risk factors and especially on its early diagnosis. The universal screening for GDM includes a 75 g oral glucose tolerance test at 24–28 weeks of gestation, but often then is too late and both GDM and its complications may have already occurred [3,4,5,6,7,8]. On the other hand, selective screening in the early stages of pregnancy is recommended mainly in the presence of risk factors in mothers (maternal overweight or obesity, age, ethnicity and/or family history of diabetes, previous macrosomia, GDM in previous pregnancies) [1,6,9,10,11]. Hence, an important question arises: Whether biochemical markers that are widely tested during the first trimester of pregnancy in terms of screening for aneuploidies, such as placental growth factor (PlGF) and plasma-associated protein-A (PAPP-A), could also be included in the recommendations for GDM screening?
In this narrative review we discuss the potential role of serum biomarkers—PlGF and PAPP-A—as early predictors of GDM diagnosis.
2. Pathogenesis of GDM
Pregnancy is a condition of physiological insulin resistance (IR) [12]. The factors contributing to this IR include increased maternal adiposity, decrease in insulin sensitivity, placental hormone production, elevated levels of cortisol and various inflammatory markers such as tumor necrosis factor-α and interleukin 6 [12,13]. IR is a state in which a given concentration of insulin produces a less-than-expected biological effect in target tissues such as adipose tissue, muscle, and liver [12,13,14,15]. As a result, there is reduced glucose uptake, mainly in the muscle cells and adipocytes [14]. The compensatory mechanism is the activation of glycogenolysis and increased hepatic glucose production [15]. In addition, the ability of insulin to suppress whole-body lipolysis is also reduced during pregnancy, contributing to a greater postprandial increase in free fatty acids [16,17,18]. In the initial stages of IR, the reduced tissue sensitivity and response have been compensated by hyperinsulinemia. Gradually, however, the pancreatic β-cells become depleted, their secretory function declines, and hyperglycemia appears [12,15]. The decrease in insulin sensitivity is most pronounced in the second half of pregnancy and its major function is to limit the absorption of glucose by the mother, which meets the needs of the developing fetus. During a normal pregnancy, IR is compensated by the increase of insulin production by the mother’s pancreas [14]. In some pregnant women, especially those with obesity and pre-pregnancy IR, the IR status worsens as the pregnancy progresses, and the pancreatic beta cells cannot continue to maintain the normoglycemia secreting more insulin. Consequently, abnormal blood glucose levels develop, marking the onset of GDM [19].
With the formation of the placenta, several hormones, proteins (cytokines, growth factors, glycoproteins) and other signaling molecules begin to exert their effect on IR [20]. (Figure 1).
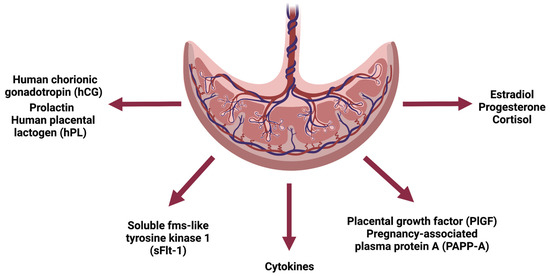
Figure 1.
Schematic representation of the endocrine function of the placenta. Created with BioRender.com (accessed on 17 December 2022).
The advance in pregnancy and the growth of the placenta consequently leads to an increased production of these hormones and factors. Since most placental products have insulin-antagonistic effects, IR also worsens [13].
As mentioned above, these changes are usually most noticeable after the second trimester of pregnancy. Therefore, the recommended period for performing GDM screening is 24–28 weeks of gestation [1,7,21,22].
However, during the first trimester (11–14 weeks of gestation) all pregnant women undergo a screening for fetal aneuploidy. This can help to determine whether the fetus is at risk for a chromosomal abnormality and also might be used to assess the risk of preeclampsia. First-trimester screening includes the measurement of several biochemical markers like human chorionic gonadotropin (hCG), PIGF, soluble fms-like tyrosine kinase-1 (sflt-1) and PAPP-A [23]. These markers are already considered as established predictors of chromosomal abnormalities and some maternal complications (e.g., PlGF and PAPP-A are widely used in the screening for pre-eclampsia) [24,25,26]. In addition, first trimester PAPP-A levels have been associated with early prediction of pregnancy-induced hypertension [27]. Furthermore, some recent studies have suggested that PAPP-A and PlGF may also be relevant markers for carbohydrate disorders manifested during pregnancy [24,28,29,30,31].
3. PAPP-A
PAPP-A is a zinc-containing metalloproteinase, belonging to the metzincin superfamily, first described in 1974 as a protein present in the plasma of pregnant women [32]. The structure of PAPP-A is similar to that of human placental lactogen and hCG. PAPP-A concentration increases with the progress of pregnancy until delivery [33]. It is produced by the syncytiotrophoblast and acts as a protease for the IGF-binding protein-4 [34]. In addition to the placenta, this protein is also expressed in the fibroblasts, osteoblasts, endothelial cells, or smooth-muscle cells [20]. The primary mechanism of action of PAPP-A is to cleave the molecule of circulating IGFBP-2,4,5 [20,29]. Thus, an improvement in the activity of IGF is achieved. The reduction of PAPP-A concentration is associated with higher levels of IGFBP and low levels of free IGF, respectively [29].
The reason for the reduced synthesis of PAPP-A from the placenta is the disturbance in the trophoblast invasion. Low protease levels during the first trimester in pregnant women without chromosomal abnormalities are associated with adverse perinatal outcomes, including intrauterine growth restriction, miscarriage, and low birth weight [20,35].
The mechanism of the effects of PAPP-A in the pathogenesis of GDM is not fully understood. It has been assumed that women with low PAPP-A levels also have lower IGF levels [35]. IGF, in turn, plays a role in fetal growth regulation. It takes part in the autocrine and paracrine control of trophoblast invasion [36]. IGF is a stimulator of muscle protein synthesis and the utilization of free fatty acids [37]. The action of PAPP-A is considered to be indirect, as it is the reduced level of IGF that leads to hyperinsulinemia, impaired glucose metabolism, and is therefore inversely correlated with the severity of insulin resistance [24,28,29].
Studies have shown that one of the binding IGF proteins in adipocytes, namely IGFBP-5 and the protease PAPP-A, may be involved in the pathogenesis of GDM [29,37] This is due to a change in the regulation of functional levels of IGF, fat stores, and their metabolism.
During a normal pregnancy, the induction of IGFBP-5 in adipose tissue increases the levels of sequestered IGF-1 and IGF-2, and PAPP-A degrades IGFBP-5, resulting in the release of insulin-like growth factors. This leads to angiogenesis and hyperplastic expansion of adipocytes. In women with GDM, insufficient IGFBP-5 levels and possibly decreased PAPP-A levels lead to reduced IGF bioavailability. Inadequate angiogenesis occurs, resulting in adipocytic hypertrophy and decreased capillary density. Low-grade inflammation and lipotoxicity are manifested, respectively, leading to further insulin resistance and impaired carbohydrate tolerance [38] (Figure 2).
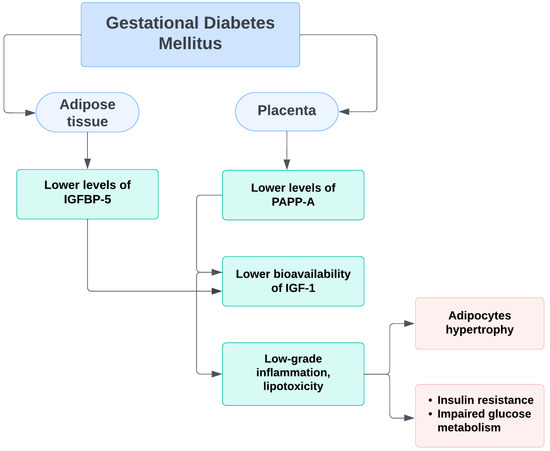
Figure 2.
Schematic representation of the probable mechanism of PAPP-A impact on insulin resistance and glucose homeostasis during pregnancy.
In recent years, serious attention has been directed to placental products. Some of them have been considered as prognostic markers for the occurrence of complications during pregnancy. Several studies have shown that low serum levels of PAPP-A in the first trimester of pregnancy could be associated with the onset of GDM and adverse pregnancy outcomes. [28,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] (Table 1).

Table 1.
Studies showing an inverse relationship between serum PAPP-A levels and GDM risk.
However, the cited studies do not use the same criteria for diagnosing GDM. In addition, there is also a difference in age, ethnicity and body mass index of women included in the analysis. Despite this, most of the results are identical. Therefore, it could be assumed, that lower levels of PAPP-A in the first trimester of pregnancy may be associated with an increased risk of GDM. In accordance with this, several studies have also shown that women with pre-existing diabetes mellitus (DM) have significantly lower PAPP-A levels than those without DM [54,55].
Nevertheless, few other investigations have not found such a relationship between PAPP-A and GDM [55,56,57,58,59]. This discrepancy is probably due to the lack of identical criteria and characteristics of the analyzed patients. For example, the study conducted by Husslein et al. has failed to demonstrate alterations in PAPP-A levels, but the authors investigated only women who developed GDM and needed insulin treatment at 11–14 weeks of gestation [58]. Hence, PAPP-A is not yet considered an early indicator of carbohydrate disorders during pregnancy and further studies are warranted to prove its predictive value.
4. PlGF
Placental growth factor (PlGF), first isolated in 1991, is a homodimeric glycoprotein belonging to the vascular endothelial growth factor (VEGF) family [60,61]. It is expressed mainly in the syncytiotrophoblast and cytotrophoblast of the placenta, but low levels have also been registered in the endothelial cells and bone marrow erythroblasts [62,63]. Since the placenta is the major source of PlGF, its circulating levels are markedly elevated during pregnancy, but the expression of PlGF alters at different stages of placental development [61]. PlGF has been shown to induce the proliferation, migration, and activation of endothelial cells, thus playing a central role in development and maturation of the placental vascular system and circulation [63].
In an uncomplicated pregnancy, PlGF concentrations in the first trimester are low, increasing from 11–12 weeks of gestation with a peak at the 30th week, after which there is a gradual decrease in its levels [64]. PlGF is considered as one of the major predictors of the occurrence of a complication, such as preeclampsia and fetal growth restriction [62,65].
Noteworthily, elevated levels of PlGF have been also observed in pregnant women with GDM. The possible explanation might be due to the fact that hyperglycemia affects angiogenesis and maternal hyperglycemia stimulates placental neovascularization [66]. Moreover, PlGF has been shown to play a role in development and the function of the placental vascular network, and there has been evidence that the placentas of women with GDM and pre-existing type 1 and 2 diabetes mellitus are characterized by a higher total area of the terminal villi of the placenta and an increased number of small vessels [67,68,69].
The changes in PlGF levels have been shown to be positively associated primarily with fasting blood glucose. This may also explain its predictive role in women who do not have risk factors for diabetes or pre-pregnancy IR [70,71,72,73]. Table 2 summarizes some of the studies showing a positive association between increased serum PlGF levels and GDM.

Table 2.
Studies establishing a significant relationship between elevated serum PlGF levels and GDM.
Although the cited data suggest a significant relationship between the serum PlGF concentrations and GDM, there are studies that have not demonstrated differences in PlGF levels between women who developed GDM and controls [74,75]. Tsiakkas et al. observed that maternal serum PlGF levels were even reduced in women with pre-existing diabetes [75]. Therefore, PlGF, like PAPP-A, is not yet considered as an early screening marker for the risk of GDM development.
5. Conclusions
GDM is a health problem associated with the risk of many complications for both mother and fetus, childbirth, and beyond. Hence, the early diagnosis and timely treatment of this condition is of pivotal importance. Nonetheless, the diagnosis of GDM is very often delayed as a result of the universal screening for carbohydrate disorders during pregnancy, carried out in the interval 24–28 weeks of gestation. The establishment of maternal-fetal medicine as an independent specialty has brought indisputable positive changes in the early diagnosis of many anomalies and diseases. Markers of placentation, such as PlGF and PAPP-A, are routinely tested in almost all pregnant women at the end of the first trimester of pregnancy as biomarkers of pre-eclampsia and aneuploidies. Therefore, it would be useful to evaluate their predictive potential also for GDM. The current review summarized the available data for the use of these markers as predictors of carbohydrate disorders in pregnant women. Despite a few conflicting results, most analyses have shown that low levels of PAPP-A and elevated levels of PlGF are significantly associated with a higher risk of GDM. However, further large-scale studies are needed to conclude whether these markers could be used in early screening for GDM.
Author Contributions
Conceptualization, V.Y. and R.S.; methodology, V.Y. and R.S.; investigation, V.Y., T.S. and R.S; writing—original draft preparation, V.Y.; writing—review and editing, V.Y., T.S. and R.S; visualization, R.S.; supervision, V.Y., R.S. and Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research is supported by the Bulgarian Ministry of Education and Science under the National Program “Young Scientists and Postdoctoral Students-2”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care 2022, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef] [PubMed]
- Modzelewski, R.; Stefanowicz-Rutkowska, M.M.; Matuszewski, W.; Bandurska-Stankiewicz, E.M. Gestational Diabetes Mellitus-Recent Literature Review. J. Clin. Med. 2022, 11, 5736. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; di Renzo, G.C.; Roura, L.C.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S3), S173–S211. [Google Scholar] [CrossRef] [PubMed]
- Farahvar, S.; Walfisch, A.; Sheiner, E. Gestational diabetes risk factors and long-term consequences for both mother and offspring: A literature review. Expert Rev. Endocrinol. Metab. 2019, 14, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.X.; Hamblin, P.S. Selective versus universal screening for gestational diabetes mellitus: An evaluation of predictive risk factors. Med. J. Aust. 2001, 174, 118–121. [Google Scholar] [CrossRef]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Farrar, D.; Fairley, L.; Wright, J.; Tuffnell, D.; Whitelaw, D.; Lawlor, D.A. Evaluation of the impact of universal testing for gestational diabetes mellitus on maternal and neonatal health outcomes: A retrospective analysis. BMC Pregnancy Childbirth 2014, 14, 317. [Google Scholar] [CrossRef]
- Cosson, E.; Benbara, A.; Pharisien, I.; Nguyen, M.T.; Revaux, A.; Lormeau, B.; Sandre-Banon, D.; Assad, N.; Pillegand, C.; Valensi, P.; et al. Diagnostic and prognostic performances over 9 years of a selective screening strategy for gestational diabetes mellitus in a cohort of 18,775 subjects. Diabetes Care 2013, 36, 598–603. [Google Scholar] [CrossRef]
- Miailhe, G.; Kayem, G.; Girard, G.; Legardeur, H.; Mandelbrot, L. Selective rather than universal screening for gestational diabetes mellitus? Eur. J. Obstet. Gynecol. Reprod. Boil. 2015, 191, 95–100. [Google Scholar] [CrossRef]
- Benhalima, K.; Mathieu, C.; Van Assche, A.; Damm, P.; Devlieger, R.; Mahmood, T.; Dunne, F. Survey by the European Board and College of Obstetrics and Gynaecology on Screening for Gestational Diabetes in Europe. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 201, 197–202. [Google Scholar] [CrossRef]
- Agha-Raffar, R.; Oliver, N.; Jhonston, D.; Robinson, S. Gestational diabetes mellitus: Does an effective prevention strategy exist? Nat. Rev. Endocrinol. 2016, 12, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J. Diabetes Res. 2019, 2019, 5320156. [Google Scholar] [CrossRef]
- Sonagra, A.D.; Biradar, S.M.; Dattatreya, K.; Jayaprakash Murthy, D.S. Normal Pregnancy—A State of Insulin Resistance. J. Clin. Diagn. Res. 2014, 8, CC01–CC03. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar]
- Zavalza-Gómez, A.B.; Anaya-Prado, R.; Rincón-Sánchez, A.R.; Mora-Martínez, J.M. Adipokines and insulin resistance during pregnancy. Diabetes Res. Clin. Pract. 2008, 80, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F. Carbohydrate and lipid metabolism in pregnancy: Normal compared with gestational diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 1256S–1261S. [Google Scholar] [CrossRef] [PubMed]
- Trivett, C.; Lees, Z.J.; Freeman, D.J. Adipose tissue function in healthy pregnancy, gestational diabetes mellitus and pre-eclampsia. Eur. J. Clin. Nutr. 2021, 75, 1745–1756. [Google Scholar] [CrossRef]
- Moyce, B.L.; Dolinsky, V.W. Maternal β-Cell Adaptations in Pregnancy and Placental Signalling: Implications for Gestational Diabetes. Int. J. Mol. Sci. 2018, 19, 3467. [Google Scholar] [CrossRef]
- Costa, M.A. The endocrine function of human placenta: An overview. Reprod. Biomed. Online 2016, 32, 14–43. [Google Scholar] [CrossRef]
- Webber, J.; Charlton, M.; Johns, N. Diabetes in pregnancy: Management of diabetes and its complications from preconception to the postnatal period (NG3). Br. J. Diabetes 2015, 15, 107–111. [Google Scholar] [CrossRef]
- Rani, P.R.; Begum, J. Screening and Diagnosis of Gestational Diabetes Mellitus, Where Do We Stand. J. Clin. Diagn. Res. 2016, 10, QE01–QE04. [Google Scholar] [CrossRef]
- Kagan, K.O.; Sonek, J.; Wagner, P.; Hoopmann, M. Principles of first trimester screening in the age of non-invasive prenatal diagnosis: Screening for chromosomal abnormalities. Arch. Gynecol. Obstet. 2017, 296, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Saruhan, Z.; Ozekinci, M.; Simsek, M.; Mendilcioglu, I. Association of first trimester low PAPP-A levels with adverse pregnancy outcomes. Clin. Exp. Obstet. Gynecol. 2012, 39, 225–228. [Google Scholar] [PubMed]
- Pandya, P.; Wright, D.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Maternal serum placental growth factor in prospective screening for aneuploidies at 8-13 weeks’ gestation. Fetal Diagn Ther. 2012, 31, 87–93. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, N.; Wright, D.; Syngelaki, A.; Akolekar, R.; Wright, A.; Poon, L.C.; Nicolaides, K.H. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am. J. Obstet. Gynecol. 2016, 214, 103.e1–103.e12. [Google Scholar] [CrossRef]
- Meloni, P.; D’Angeli, I.; Piazze, J.; Cerekya, A.; Simari, T.; Pala, A.; Anceschi, M.M.; Guglietta, M.; Izzo, P.; Izzo, L. First Trimester PAPP-A Levels Associated with Early Prediction of Pregnancy Induced Hypertension. Hypertens. Pregnancy 2009, 28, 361–368. [Google Scholar] [CrossRef]
- Petry, C.J.; Ong, K.K.; Hughes, I.A.; Acerini, C.L.; Frystyk, J.; Dunger, D.B. Early Pregnancy-Associated Plasma Protein A Concentrations Are Associated with Third Trimester Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2017, 102, 2000–2008. [Google Scholar] [CrossRef]
- Rojas-Rodriguez, R.; Ziegler, R.; DeSouza, T.; Majid, S.; Madore, A.S.; Amir, N.; Pace, V.A.; Nachreiner, D.; Alfego, D.; Mathew, J.; et al. PAPPA-mediated adipose tissue remodeling mitigates insulin resistance and protects against gestational diabetes in mice and humans. Sci. Transl. Med. 2020, 12, eaay4145. [Google Scholar] [CrossRef]
- Talasaz, Z.H.; Sadeghi, R.; Askari, F.; Dadgar, S.; Vatanchi, A. First trimesters Pregnancy-Associated Plasma Protein-A levels value to Predict Gestational diabetes Mellitus: A systematic review and meta-analysis of the literature. Taiwan J. Obstet. Gynecol. 2018, 57, 181–189. [Google Scholar] [CrossRef]
- Donovan, B.M.; Nidey, N.L.; Jasper, E.A.; Robinson, J.G.; Bao, W.; Saftlas, A.F.; Ryckman, K.K. First trimester prenatal screening biomarkers and gestational diabetes mellitus, A systematic review and meta-analysis. PLoS ONE 2018, 13, e0201319. [Google Scholar] [CrossRef]
- Lin, T.M.; Halbert, S.P.; Spellacy, W.N. Measurement of pregnancy-associated plasma proteins during human gestation. J. Clin. Investig. 1974, 54, 576–582. [Google Scholar] [CrossRef]
- Conover, C.A. Key Questions and Answers about Pregnancy-Associated Plasma Protein-A. Trends Endocrinol. Metab. 2012, 23, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.B.; Oxvig, C.; Overgaard, M.T.; Sottrup-Jensen, L.; Gleich, G.J.; Hays, L.G.; Yates, J.R., 3rd; Conover, C.A. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc. Natl. Acad. Sci. USA 1999, 96, 3149–3153. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Panchanadikar, T.M.; Wagh, G. Variation of PAPP-A level in the first trimester of pregnancy and its clinical outcome. J. Obstet. Gynaecol. India 2014, 64, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Sferruzzi-Perri, A.N.; Sandovici, I.; Constancia, M.; Fowden, A.L. Placental Phenotype and the Insulin-like Growth Factors: Resource Allocation to Fetal Growth. J. Physiol. 2017, 595, 5057–5093. [Google Scholar] [CrossRef]
- Clemmons, D.R. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol. Metab. Clin. N. Am. 2012, 41, 425–443. [Google Scholar] [CrossRef]
- Rojas-Rodriguez, R.; Lifshitz, L.M.; Bellve, K.D.; Min, S.Y.; Pires, J.; Leung, K.; Boeras, C.; Sert, A.; Draper, J.T.; Corvera, S.; et al. Human adipose tissue expansion in pregnancy is impaired in gestational diabetes mellitus. Diabetologia 2015, 58, 2106–2114. [Google Scholar] [CrossRef]
- Ong, C.Y.; Liao, A.W.; Spencer, K.; Munim, S.; Nicolaides, K.H. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG Int. J. Obstet. Gynaecol. 2000, 107, 1265–1270. [Google Scholar] [CrossRef]
- Beneventi, F.; Simonetta, M.; Lovati, E.; Albonico, G.; Tinelli, C.; Locatelli, E.; Spinillo, A. First trimester pregnancy-associated plasma protein-A in pregnancies complicated by subsequent gestational diabetes. Prenat. Diagn. 2011, 31, 523–528. [Google Scholar] [CrossRef]
- Lovati, E.; Beneventi, F.; Simonetta, M.; Laneri, M.; Quarleri, L.; Scudeller, L.; Albonico, G.; Locatelli, E.; Cavagnoli, C.; Tinelli, C.; et al. Gestational diabetes mellitus: Including serum pregnancy-associated plasma protein-A testing in the clinical management of primiparous women? A case–control study. Diabetes Res. Clin. Pr. 2013, 100, 340–347. [Google Scholar] [CrossRef]
- Spencer, K.; Cowans, N.J. The association between gestational diabetes mellitus and first trimester aneuploidy screening markers. Ann. Clin. Biochem. 2013, 50, 603–610. [Google Scholar] [CrossRef]
- Kulaksizoglu, S.; Kulaksizoglu, M.; Kebapcilar, A.G.; Torun, A.N.; Ozcimen, E.; Turkoglu, S. Can first-trimester screening program detect women at high risk for gestational diabetes mellitus? Gynecol Endocrinol. 2013, 29, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Beneventi, F.; Simonetta, M.; Locatelli, E.; Cavagnoli, C.; Badulli, C.; Lovati, E.; Garbin, G.; Genini, E.; Albertini, R.; Tinelli, C.; et al. Temporal variation in soluble human leukocyte antigen-G (sHLA-G) and pregnancy-associated plasma protein A (PAPP-A) in pregnancies complicated by gestational diabetes mellitus and in controls. Am. J. Reprod. Immunol. 2014, 72, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Bleicher, K.; Han, X.; McShane, M.; Chan, Y.F.; Bartlett, A.; White, C.; Lau, S.M. Maternal Diabetes, Large for Gestational Age Births and First Trimester Pregnancy Associated Plasma Protein-A. J. Clin. Endocrinol. Metab. 2015, 100, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Syngelaki, A.; Kotecha, R.; Pastides, A.; Wright, A.; Nicolaides, K.H. First-trimester biochemical markers of placentation in screening for gestational diabetes mellitus. Metabolism 2015, 64, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, T.; Pinto, P.; Martins, S.; Guimarães, J.T.; Montenegro, N.; Ramalho, C. 754: Serum PAPP-A as a predictor of gestational diabetes. Am. J. Obstet. Gynecol. 2015, 212, S366e7. [Google Scholar] [CrossRef]
- Xiao, D.; Chenhong, W.; Yanbin, X.; Lu, Z. Gestational diabetes mellitus and first trimester pregnancy-associated plasma protein A: A case-control study in a Chinese population. J. Diabetes Investig. 2018, 9, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, S.; Ahmadi, M.; Saqhafi, H.; Alipoor, M. Association of Pregnancy-Associated Plasma Protein A (PAPP-A) and Gestational Diabetes. Iran. J. Obstet. Gynecol. Infertil. 2017, 20, 61–69. [Google Scholar]
- Ramezani, S.; Doulabi, M.A.; Saqhafi, H.; Alipoor, M. Prediction of Gestational Diabetes by Measuring the Levels of Pregnancy Associated Plasma Protein-A (PAPP-A) During Gestation Weeks 11-14. J. Reprod. Infertil. 2020, 21, 130–137. [Google Scholar]
- Ren, Z.; Zhe, D.; Li, Z.; Sun, X.-P.; Yang, K.; Lin, L. Study on the correlation and predictive value of serum pregnancy-associated plasma protein A, triglyceride and serum 25-hydroxyvitamin D levels with gestational diabetes mellitus. World J. Clin. Cases 2020, 8, 864–873. [Google Scholar] [CrossRef]
- Caliskan, R.; Atis, A.; Aydin, Y.; Acar, D.; Kiyak, H.; Topbas, F. PAPP-A concentrations change in patients with gestational diabetes. J. Obstet Gynaecol. 2020, 40, 190–194. [Google Scholar] [CrossRef]
- Yanachkova, V.E.; Staynova, R.; Bochev, I.; Kamenov, Z. Potential role of biochemical placentation markers—Pregnancy associated plasma protein-A and human chorionic gonadotropin for early gestational diabetes screening—A pilot study. Ginekol. Pol. 2022, 93, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Kuc, S.; Wortelboer, E.; Koster, M.; de Valk, H.; Schielen, P.; Visser, G. Prediction of macrosomia at birth in type-1 and 2 diabetic pregnancies with biomarkers of early placentation. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 748–754. [Google Scholar] [CrossRef]
- Savvidou, M.D.; Syngelaki, A.; Muhaisen, M.; Emelyanenko, E.; Nicolaides, K.H. First trimester maternal serum free beta-human chorionic gonadotropin and pregnancy-associated plasma protein A in pregnancies complicated by diabetes mellitus. Br. J. Obstet. Gynaecol. 2012, 119, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, Q.K.; Lo, T.K.; Wong, S.F.; Lee, C.P. Association between pregnancy-associated plasma protein-A levels in the first trimester and gestational diabetes mellitus in Chinese women. Hong Kong Med. J. 2016, 22, 30–38. [Google Scholar] [CrossRef]
- Tul, N.; Pusenjak, S.; Osredkar, J.; Spencer, K.; Novak-Antolic, Z. Predicting complications of pregnancy with first-trimester maternal serum free-betahCG, PAPP-A and inhibin-A. Prenat. Diagn. 2003, 23, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Husslein, H.; Lausegger, F.; Leipold, H.; Worda, C. Association between pregnancy-associated plasma protein-A and gestational diabetes requiring insulin treatment at 11–14 weeks of gestation. J. Matern. Fetal Neonatal Med. 2012, 25, 2230–2233. [Google Scholar] [CrossRef]
- Visconti, F.; Quaresima, P.; Chiefari, E.; Caroleo, P.; Arcidiacono, B.; Puccio, L.; Mirabelli, M.; Foti, D.P.; Di Carlo, C.; Vero, R.; et al. First Trimester Combined Test (FTCT) as a Predictor of Gestational Diabetes Mellitus. Int. J. Environ. Res. Public Health 2019, 16, 3654. [Google Scholar] [CrossRef]
- Maglione, D.; Guerriero, V.; Viglietto, G.; Delli-Bovi, P.; Persico, M.G. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc. Natl. Acad. Sci. USA 1991, 88, 9267–9271. [Google Scholar] [CrossRef]
- De Falco, S. The discovery of placenta growth factor and its biological activity. Exp. Mol. Med. 2012, 44, 1–9. [Google Scholar] [CrossRef]
- Depoix, C.; Tee, M.K.; Taylor, R.N. Molecular regulation of human placental growth factor (PlGF) gene expression in placental villi and trophoblast cells is mediated via the protein kinase a pathway. Reprod. Sci. 2011, 18, 219–228. [Google Scholar] [CrossRef]
- Newell, L.F.; Holtan, S.G. Placental growth factor: What hematologists need to know. Blood Rev. 2017, 31, 57–62. [Google Scholar] [CrossRef]
- Chau, K.; Hennessy, A.; Makris, A. Placental growth factor and pre-eclampsia. J. Hum. Hypertens. 2017, 31, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Sibiude, J.; Guibourdenche, J.; Dionne, M.D.; Le Ray, C.; Anselem, O.; Serreau, R.; Goffinet, F.; Tsatsaris, V. Placental growth factor for the prediction of adverse outcomes in patients with suspected preeclampsia or intrauterine growth restriction. PLoS ONE 2012, 7, e50208. [Google Scholar] [CrossRef] [PubMed]
- Cvitic, S.; Desoye, G.; Hiden, U. Glucose, insulin, and oxygen interplay in placental hypervascularisation in diabetes mellitus. BioMed. Res. Int. 2014, 2014, 145846. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Dawson, D.; Roberts, D.; Bentley-Lewis, R. A systematic review of placental pathology in maternal diabetes mellitus. Placenta 2015, 36, 101–114. [Google Scholar] [CrossRef]
- Ahmed, A.; Dunk, C.; Ahmad, S.; Khaliq, A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen—A review. Placenta 2000, 21, S16–S24. [Google Scholar] [CrossRef]
- Nuzzo, A.M.; Giuffrida, D.; Moretti, L.; Re, P.; Grassi, G.; Menato, G.; Rolfo, A. Placental and maternal sFlt1/PlGF expression in gestational diabetes mellitus. Sci. Rep. 2021, 11, 2312. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.Y.T.; Lao, T.T.; Spencer, K.; Nicolaides, K.H. Maternal serum level of placental growth factor in diabetic pregnancies. J. Reprod. Med. 2004, 49, 477–480. [Google Scholar] [PubMed]
- Eleftheriades, M.; Papastefanou, I.; Lambrinoudaki, I.; Kappou, D.; Lavranos, D.; Akalestos, A.; Souka, A.P.; Pervanidou, P.; Hassiakos, D.; Chrousos, G.P. Elevated placental growth factor concentrations at 11–14 weeks of gestation to predict gestational diabetes mellitus. Metabolism 2014, 63, 1419–1425. [Google Scholar] [CrossRef]
- Gorkem, U.; Togrul, C.; Arslan, E. Relationship between elevated serum level of placental growth factor and status of gestational diabetes mellitus. J. Matern. Fetal. Neonatal. Med. 2020, 33, 4159–4163. [Google Scholar] [CrossRef] [PubMed]
- Yanachkova, V.; Staynova, R.; Naseva, E.; Kamenov, Z. The Role of Placental Growth Factor in the Prediction of Carbohydrate and Thyroid Disorders during Pregnancy. Medicina 2022, 58, 232. [Google Scholar] [CrossRef]
- Mosimann, B.; Amylidi, S.; Risch, L.; Wiedemann, U.; Surbek, D.; Baumann, M.; Stettler, C.; Raio, L. First-Trimester Placental Growth Factor in Screening for Gestational Diabetes. Fetal Diagn. Ther. 2016, 39, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Tsiakkas, A.; Duvdevani, N.; Wright, A.; Wright, D.; Nicolaides, K.H. Serum placental growth factor in the three trimesters of pregnancy: Effects of maternal characteristics and medical history. Ultrasound Obstet. Gynecol. 2015, 45, 591–598. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).