The Impact of Nutritional Status on Sexual Function in Male Kidney Transplant Recipients
Abstract
1. Introduction
2. Subjects and Methods
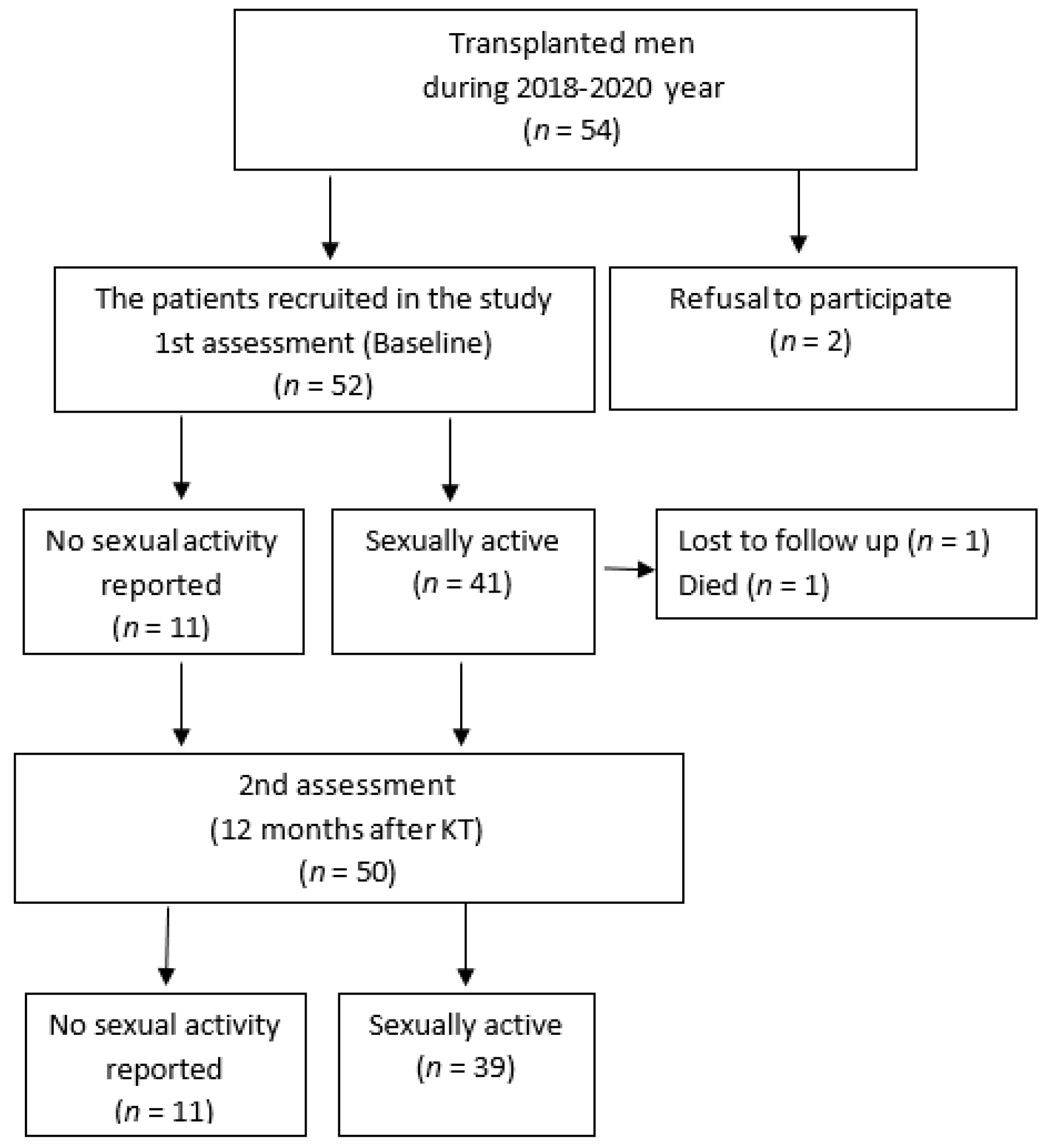
2.1. Study Design and Patients
2.2. Ethics
2.3. Laboratory Data
2.4. Assessment of International Index of Erectile Function
2.5. Evaluation of Nutritional Status
2.5.1. Anthropometric Data
2.5.2. Handgrip Strength Assessment
2.5.3. Bioelectrical Impedance Analysis
2.5.4. Nutrition-Related Questionnaires
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Sexual Activity and Nutritional Status in Kidney Transplant Recipients before Kidney Transplantation
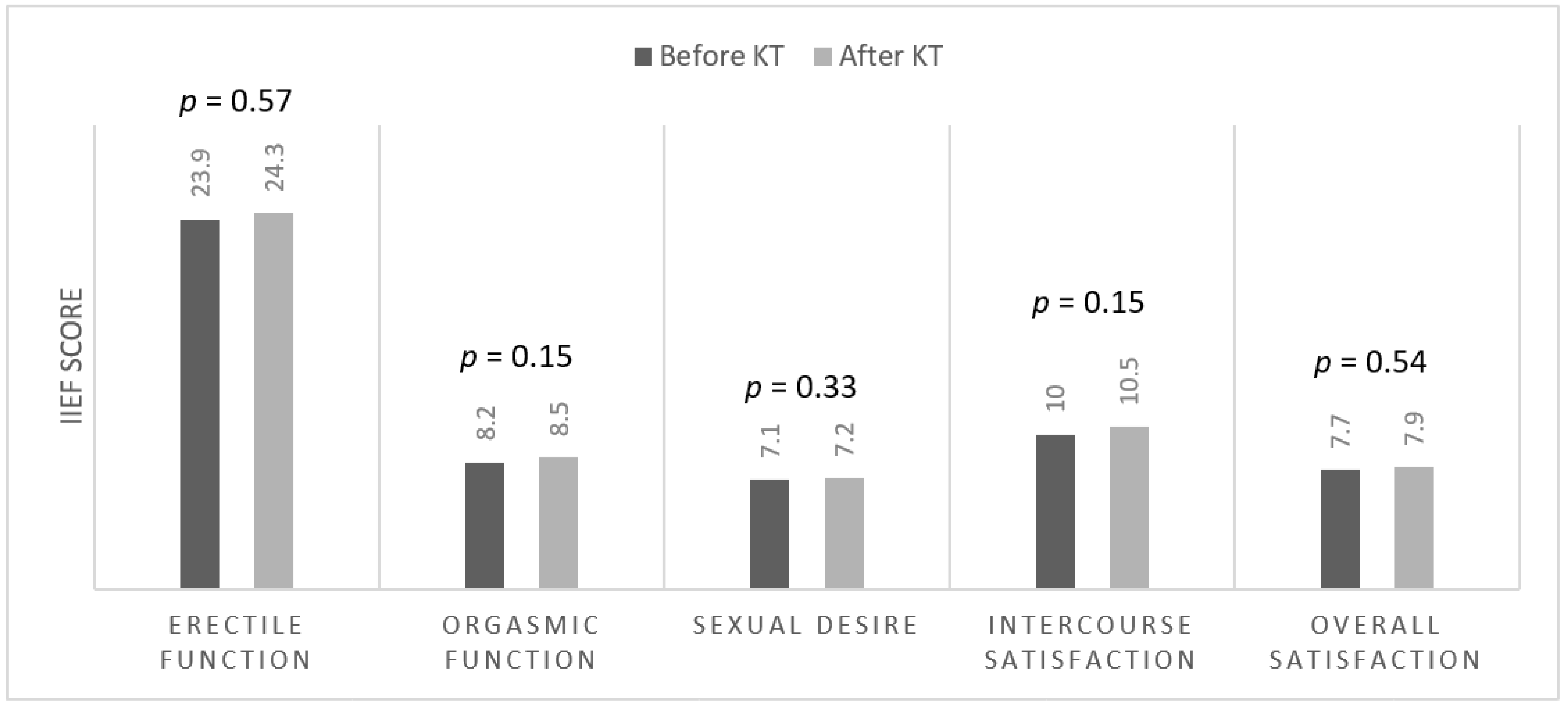
3.3. Nutritional Parameters and Erectile Function before and after Kidney Transplantation
4. Discussion
5. Conclusions/Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papadopoulou, E.; Varouktsi, A.; Lazaridis, A.; Boutari, C.; Doumas, M. Erectile dysfunction in chronic kidney disease: From pathophysiology to management. World J. Nephrol. 2015, 4, 379. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.R.; Ponciano, V.C.; Costa, T.R.; De Oliveira, A.M.; Gomes, C.P.; De Oliveira, E.C. Prevalence and factors associated with erectile dysfunction in patients with chronic kidney disease on conservative treatment. Int. J. Impot. Res. 2017, 29, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Vecchio, M.; Johnson, D.W.; Saglimbene, V.; Graziano, G.; Pellegrini, F.; Lucisano, G.; Craig, J.C.; Ruospo, M.; Gentile, G.; et al. Prevalence and Correlates of Self-Reported Sexual Dysfunction in CKD: A Meta-analysis of Observational Studies. Am. J. Kidney Dis. 2010, 56, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; D’Alessandro, C.; Fumagalli, G.; Vigo, V.; Meola, M.; Cianchi, C.; Egidi, M.F. Nutrition and Physical Activity in CKD patients. Kidney Blood Press. Res. 2014, 39, 107–113. [Google Scholar] [CrossRef]
- Zarifi, S.H.; Shadnoush, M.; Pahlavani, N.; Malekahmadi, M.; Firouzi, S.; Sabbagh, M.G.; Rezaiyan, M.K.; Islam, S.M.S.; Yahyapoor, F.; Arabi, S.M.; et al. Nutritional status in kidney transplant patients before and 6-month after transplantation: Result of PNSI study. Clin. Nutr. ESPEN 2020, 41, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.A.; Rasyid, N.; Birowo, P.; Atmoko, W. Effects of renal transplantation on erectile dysfunction: A systematic review and meta-analysis. Int. J. Impot. Res. 2021, 34, 456–466. [Google Scholar] [CrossRef]
- Pizzol, D.; Xiao, T.; Yang, L.; Demurtas, J.; McDermott, D.; Garolla, A.; Nardelotto, A.; Grabovac, I.; Soysal, P.; Kazancioglu, R.T.; et al. Prevalence of erectile dysfunction in patients with chronic kidney disease: A systematic review and meta-analysis. Int. J. Impot. Res. 2020, 33, 508–515. [Google Scholar] [CrossRef]
- Rosen, R.C.; Cappelleri, J.C.; Gendrano, N. The International Index of Erectile Function (IIEF): A state-of-the-science review. Int. J. Impot. Res. 2002, 14, 226–244. [Google Scholar] [CrossRef]
- Cappelleri, J.C.; Rosen, R.C.; Smith, M.D.; Mishra, A.; Osterloh, I.H. Diagnostic evaluation of the erectile function domain of the international index of erectile function. Urology 1999, 54, 346–351. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Walter-Kroker, A.; Kroker, A.; Mattiucci-Guehlke, M.; Glaab, T. A practical guide to bioelectrical impedance analysis using the example of chronic obstructive pulmonary disease. Nutr. J. 2011, 10, 35. [Google Scholar] [CrossRef]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional Risk Screening and Assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Compher, C.; Ellen, D.M. American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. Clinical Guidelines. J. Parenter. Enter. Nutr. 2011, 35, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Furuya, R.; Takita, T.; Maruyama, Y.; Yamaguchi, Y.; Ohkawa, S.; Kumagai, H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008, 87, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Rosas, S.E.; Joffe, M.; Franklin, E.; Strom, B.L.; Kotzker, W.; Brensinger, C.; Grossman, E.; Glasser, D.B.; Feldman, H.I. Association of decreased quality of life and erectile dysfunction in hemodialysis patients. Kidney Int. 2003, 64, 232–238. [Google Scholar] [CrossRef]
- Jansz, T.T.; Bonenkamp, A.A.; Boereboom, F.T.J.; van Reekum, F.E.; Verhaar, M.C.; van Jaarsveld, B.C. Health-related quality of life compared between kidney transplantation and nocturnal hemodialysis. PLoS ONE 2018, 13, e0204405. [Google Scholar] [CrossRef] [PubMed]
- Mirone, V.; Longo, N.; Fusco, F.; Verze, P.; Creta, M.; Parazzini, F.; Imbimbo, C. Renal Transplantation Does Not Improve Erectile Function in Hemodialysed Patients. Eur. Urol. 2009, 56, 1047–1054. [Google Scholar] [CrossRef]
- Al Khallaf, H.H. Analysis of sexual functions in male nondiabetic hemodialysis patients and renal transplant recipients. Transpl. Int. 2010, 23, 176–181. [Google Scholar] [CrossRef]
- Suzuki, E.; Nishimatsu, H.; Oba, S.; Takahashi, M.; Homma, Y. Chronic kidney disease and erectile dysfunction. World J. Nephrol. 2014, 3, 220–229. [Google Scholar] [CrossRef]
- Antonucci, M.; Palermo, G.; Recupero, S.M.; Bientinesi, R.; Presicce, F.; Foschi, N.; Bassi, P.; Gulino, G. Male sexual dysfunction in patients with chronic end-stage renal insufficiency and in renal transplant recipients. Arch. Ital. Urol. Androl. 2016, 87, 299–305. [Google Scholar] [CrossRef]
- Carrero, J.J.; Qureshi, A.R.; Parini, P.; Arver, S.; Lindholm, B.; Bárány, P.; AccentNy, P.B.A.; DiaeresisRger, O.H.; Stenvinkel, P. Low Serum Testosterone Increases Mortality Risk among Male Dialysis Patients. J. Am. Soc. Nephrol. 2009, 20, 613–620. [Google Scholar] [CrossRef]
- Edey, M.M. Male Sexual Dysfunction and Chronic Kidney Disease. Front. Med. 2017, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Skeldon, S.C.; Detsky, A.S.; Goldenberg, S.L.; Law, M.R. Erectile Dysfunction and Undiagnosed Diabetes, Hypertension, and Hypercholesterolemia. Ann. Fam. Med. 2015, 13, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Fiuk, J.V.; Tadros, N.N. Erectile dysfunction in renal failure and transplant patients. Transl. Androl. Urol. 2019, 8, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.; Loureiro, L.; Fernandes, M. Sexual function of kidney transplant recipients. Rev. Enferm. Ref. 2019, 2019, 47–56. [Google Scholar] [CrossRef]
- Han, T.S.; Tajar, A.; O’Neill, T.W.; Jiang, M.; Bartfai, G.; Boonen, S.; Casanueva, F.; Finn, J.D.; Forti, G.; Giwercman, A.; et al. Impaired quality of life and sexual function in overweight and obese men: The European Male Ageing Study. Eur. J. Endocrinol. 2011, 164, 1003–1011. [Google Scholar] [CrossRef]
- Kapoor, D.; Aldred, H.; Clark, S.; Channer, K.S.; Jones, T.H. Clinical and Biochemical Assessment of Hypogonadism in Men with Type 2 DiabetesCorrelations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007, 30, 911–917. [Google Scholar] [CrossRef]
- Couillard, C.; Gagnon, J.; Bergeron, J.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H.; Després, J.-P.; Bouchard, C. Contribution of Body Fatness and Adipose Tissue Distribution to the Age Variation in Plasma Steroid Hormone Concentrations in Men: The HERITAGE Family Study*. J. Clin. Endocrinol. Metab. 2000, 85, 1026–1031. [Google Scholar] [CrossRef]
- Lapauw, B.; Kaufman, J.-M. Management of Endocrine Disease: Rationale and current evidence for testosterone therapy in the management of obesity and its complications. Eur. J. Endocrinol. 2020, 183, R167–R183. [Google Scholar] [CrossRef]
- Selberg, O.; Selberg, D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur. J. Appl. Physiol. 2002, 86, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Habedank, D.; Kung, T.; Karhausen, T.; von Haehling, S.; Doehner, W.; Schefold, J.C.; Hasper, D.; Reinke, S.; Anker, S.D.; Reinke, P. Exercise capacity and body composition in living-donor renal transplant recipients over time. Nephrol. Dial. Transplant. 2009, 24, 3854–3860. [Google Scholar] [CrossRef] [PubMed]
- van den Ham, E.C.H.; Kooman, J.P.; Schols, A.M.W.J.; Nieman, F.H.M.; Does, J.D.; Franssen, F.M.E.; Akkermans, M.; Janssen, P.; van Hooff, J. Similarities in Skeletal Muscle Strength and Exercise Capacity Between Renal Transplant and Hemodialysis Patients. Am. J. Transplant. 2005, 5, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Raggi, M.C.; Siebert, S.B.; Friess, H.; Schremmer-Danninger, E.; Thorban, S.; Dinkel, A. Sexual and relationship functioning before and after renal transplantation: A descriptive study with patients and partners. Scand. J. Urol. Nephrol. 2012, 46, 431–436. [Google Scholar] [CrossRef]
- Shamsa, A.; Motavalli, S.M.; Aghdam, B. Erectile Function in End-Stage Renal Disease Before and After Renal Transplantation. Transplant. Proc. 2005, 37, 3087–3089. [Google Scholar] [CrossRef]
- Akbari, F.; Alavi, M.; Esteghamati, A.; Mehrsai, A.; Djaladat, H.; Zohrevand, R.; Pourmand, G. Effect of renal transplantation on sperm quality and sex hormone levels. BJU Int. 2003, 92, 281–283. [Google Scholar] [CrossRef]
- Kang, J.; Tian, J.; Lu, Y.; Song, Y.; Liu, X. Erectile function after kidney transplantation: A meta-analysis. Transl. Androl. Urol. 2020, 9, 1967–1979. [Google Scholar] [CrossRef]
- Branco, F.; Cavadas, V.; Rocha, A.; Vidinha, J.; Osório, L.; Martins, L.; Braga, I.; Cabral, J.; Dias, L.; Henriques, C.; et al. Living Versus Cadaveric-donor Renal Transplant Recipients: A Comparison on Sexual Function. Transplant. Proc. 2013, 45, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
| Variable | n = 52 |
|---|---|
| Age, year | 40.8 (11) |
| Residual kidney function, yes | 31 (60) |
| SGA | A 30 (58) B 22 (42) |
| GNRI score | 114 (10) |
| MIS score | 4 (2) |
| Dialysis modality | HD 42 (81) PD 10 (19) |
| Dialysis vintage, months | 19 (20) |
| Having a partner, yes | 42 (81) |
| Comorbidities Diabetes Hypertension Cardiovascular disease Dyslipidemia Neurological disorders | 4 (8) 50 (96) 9 (17) 33 (63) 3 (6) |
| Variable | No Sexual Activity (n = 11) | Sexually Active (n = 41) | p Value |
|---|---|---|---|
| Dialysis vintage | 16 (17) | 19 (21) | 0.76 |
| Age, year | 44 (13) | 40 (10) | 0.20 |
| Weight, kg | 91 (25) | 84 (15) | 0.35 |
| BMI, kg/m2 | 28 (6) | 25 (4) | 0.05 |
| Muscle mass, kg | 39 (9) | 40 (6) | 0.67 |
| Muscle mass index kg/m2 | 12 (2) | 12 (1) | 0.90 |
| Lean mass, kg | 66 (14) | 67 (9) | 0.84 |
| Handgrip strength, kg | 44 (8) | 48 (11) | 0.26 |
| Phase angle, ° | 6 (1) | 7 (1) | 0.04 |
| Lean tissue index, kg/m2 | 20 (3) | 20 (2) | 0.76 |
| Fat mass, kg | 22 (15) | 11 (12) | 0.07 |
| Fat mass index, kg/m2 | 7 (5) | 3 (4) | 0.04 |
| Body fat (%) | 22 (15) | 13 (12) | 0.05 |
| Fat free mass, kg | 70 (16) | 71 (9) | 0.85 |
| Fat free mass index, kg/m2 | 22 (4) | 22 (2) | 0.73 |
| Body cell mass, kg | 45 (10) | 46 (6) | 0.75 |
| Body cell mass index, kg/m | 14 (2) | 14 (2) | 0.89 |
| Waist circumference, cm | 105 (21) | 94 (12) | 0.13 |
| MIS score | 4 (2) | 5 (2) | 0.83 |
| GNRI score | 118 (12) | 114 (10) | 0.24 |
| Prealbumin, mg/dL | 43 (8) | 42 (8) | 0.81 |
| Albumin, g/L | 45 (3) | 45 (4) | 0.81 |
| Dysfunction Rate. | |||||
|---|---|---|---|---|---|
| Severe, n (%) | Moderate, n (%) | Mild to Moderate, n (%) | Mild, n (%) | No Dysfunction, n (%) | |
| Before KT (n = 41) | 3 (7%) | 2 (5%) | 6 (15%) | 7 (17%) | 23 (56%) |
| After KT (n = 39) | 3 (8%) | 2 (5%) | 5 (13%) | 3 (8%) | 26 (66%) |
| p-value | 0.94 | 0.95 | 0.81 | 0.20 | 0.33 |
| Before KT (n = 41) | 12 Months after KT (n = 39) | |||||
|---|---|---|---|---|---|---|
| ED (n = 18) | No ED (n = 23) | p Value | ED (n = 13) | No ED (n = 26) | p Value | |
| Age, year | 40 (12) | 39 (9) | 0.79 | 42 (13) | 41 (9) | 0.76 |
| Dialysis vintage, months | 23 (21) | 18 (20) | 0.62 | - | - | - |
| Delayed graft function, yes (%) | - | - | - | 7 (54%) | 16 (61%) | 0.64 |
| BMI, kg/m2 | 25 (4) | 26 (4) | 0.28 | 25 (8) | 25 (4) | 0.83 |
| Waist circumference, cm | 91 (16) | 91(16) | 0.46 | 97 (11) | 98 (12) | 0.67 |
| Handgrip strength, kg | 50 (10) | 46 (12) | 0.19 | 44 (11) | 53 (15) | 0.13 |
| Muscle mass, kg | 38 (7) | 42 (4) | 0.05 | 36 (4) | 38 (5) | 0.17 |
| Muscle mass index, kg/m2 | 12 (2) | 12 (1) | 0.25 | 11 (1) | 12 (1) | 0.59 |
| Fat mass, kg | 10 (9) | 12 (15) | 0.41 | 13 (9) | 15 (12) | 0.43 |
| Body fat % | 14 (9) | 14 (8) | 0.78 | 18 (8) | 19 (9) | 0.81 |
| Fat free mass, kg | 67 (11) | 74 (7) | 0.04 | 65 (7) | 68 (8) | 0.19 |
| Fat free mass index, kg/m2 | 21 (3) | 22 (2) | 0.24 | 20 (2) | 21 (2) | 0.66 |
| Fat mass index, kg/m2 | 3 (3) | 4 (4) | 0.58 | 5 (4) | 4 (3) | 0.98 |
| Body cell mass, kg | 44 (7) | 48 (4) | 0.05 | 42 (4) | 44 (5) | 0.17 |
| Body cell mass index, kg/m2 | 14 (2) | 14 (2) | 0.27 | 13 (1) | 13 (1) | 0.62 |
| PhaseA, ° | 7 (1) | 7 (1) | 0.77 | 6 (1) | 6 (1) | 0.26 |
| Lean mass, kg | 64 (10) | 70 (7) | 0.03 | 61 (6) | 65 (8) | 0.19 |
| Lean tissue index, kg/m2 | 20 (2) | 21 (2) | 0.24 | 18 (2) | 19 (2) | 0.43 |
| Albumin, g/l | 46 (5) | 45 (4) | 0.76 | 44 (3) | 45 (3) | 0.07 |
| Prealbumin, mg/dL | 43 (8) | 43 (8) | 0.83 | 33 (8) | 37 (8) | 0.25 |
| GNRI score | 112 (10) | 115 (9) | 0.38 | 112 (6) | 115 (9) | 0.21 |
| MIS score | 5 (2) | 5 (2) | 0.83 | 1(1) | 1(2) | 0.61 |
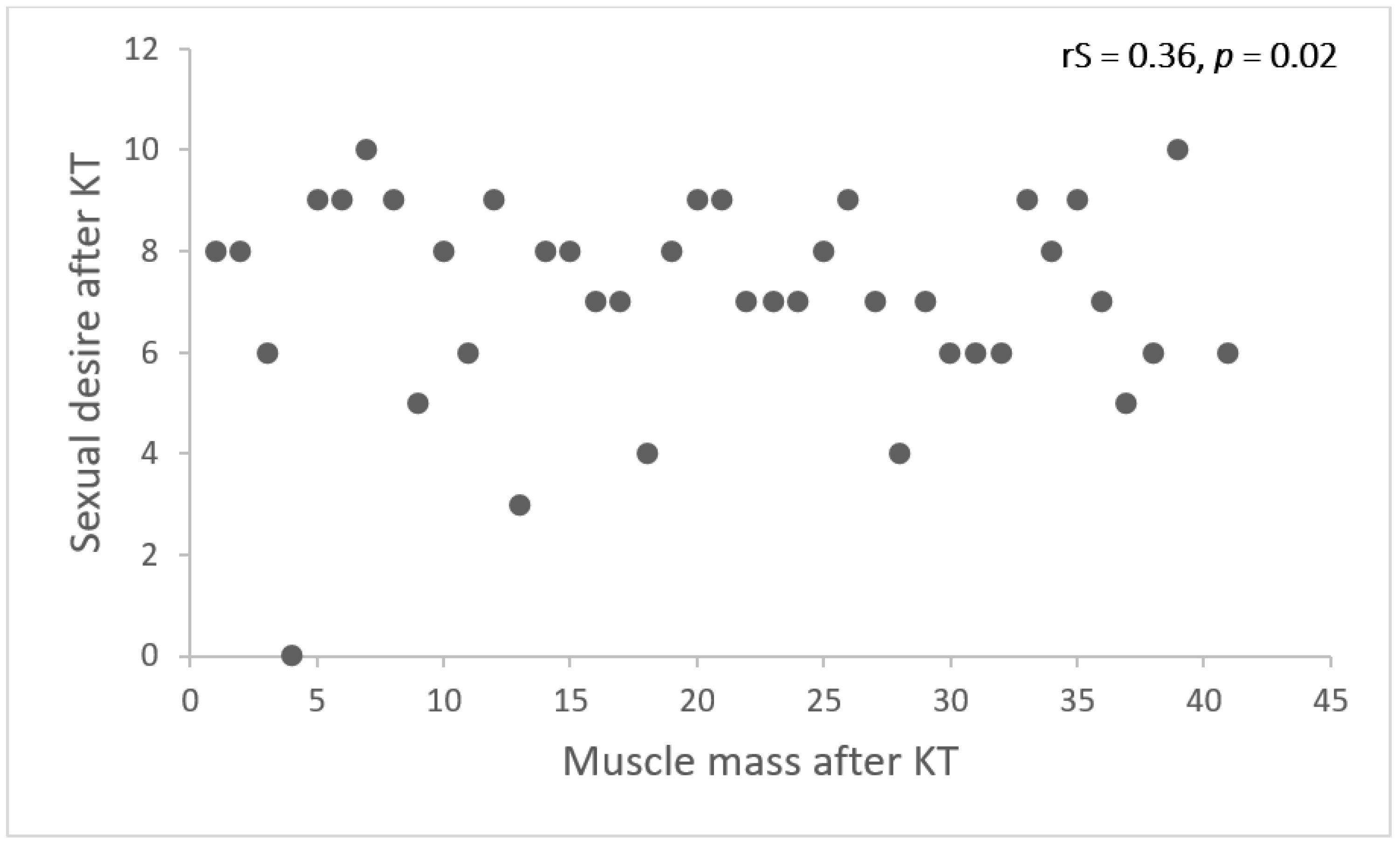
| Sexual Desire | |||
|---|---|---|---|
| Beta | SE | p-Value | |
| Muscle mass, kg | 0.102 | 0.05 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukackiene, D.; Adomaitis, R.; Miglinas, M. The Impact of Nutritional Status on Sexual Function in Male Kidney Transplant Recipients. Medicina 2023, 59, 376. https://doi.org/10.3390/medicina59020376
Sukackiene D, Adomaitis R, Miglinas M. The Impact of Nutritional Status on Sexual Function in Male Kidney Transplant Recipients. Medicina. 2023; 59(2):376. https://doi.org/10.3390/medicina59020376
Chicago/Turabian StyleSukackiene, Diana, Robertas Adomaitis, and Marius Miglinas. 2023. "The Impact of Nutritional Status on Sexual Function in Male Kidney Transplant Recipients" Medicina 59, no. 2: 376. https://doi.org/10.3390/medicina59020376
APA StyleSukackiene, D., Adomaitis, R., & Miglinas, M. (2023). The Impact of Nutritional Status on Sexual Function in Male Kidney Transplant Recipients. Medicina, 59(2), 376. https://doi.org/10.3390/medicina59020376






