HPV and RAD51 as Prognostic Factors for Survival in Inoperable Oral and Oropharyngeal Cancer in Patients Unfit for Chemotherapy Treated with Hyperfractionated Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Radiotherapy
2.3. Molecular Genetics (HPV)
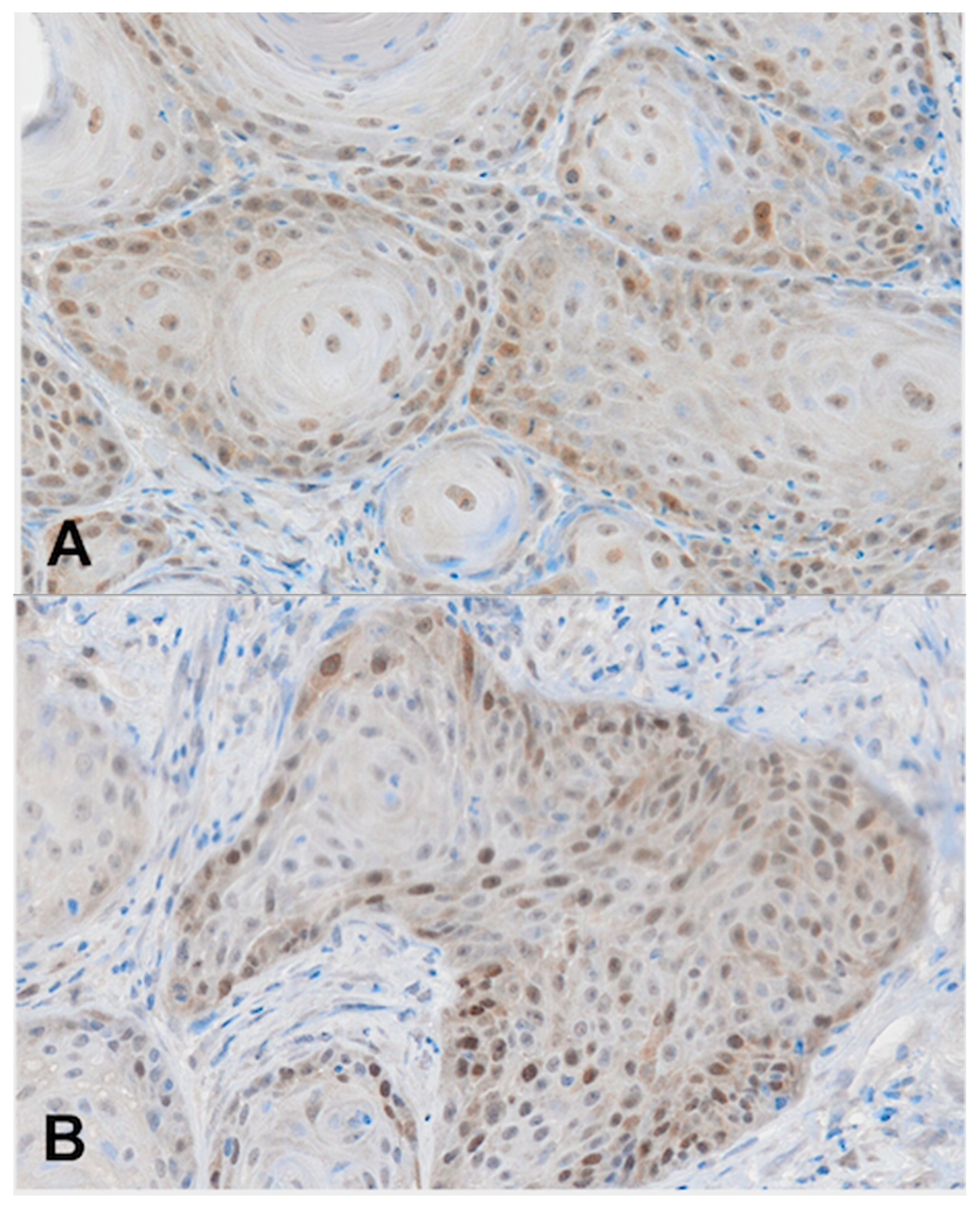
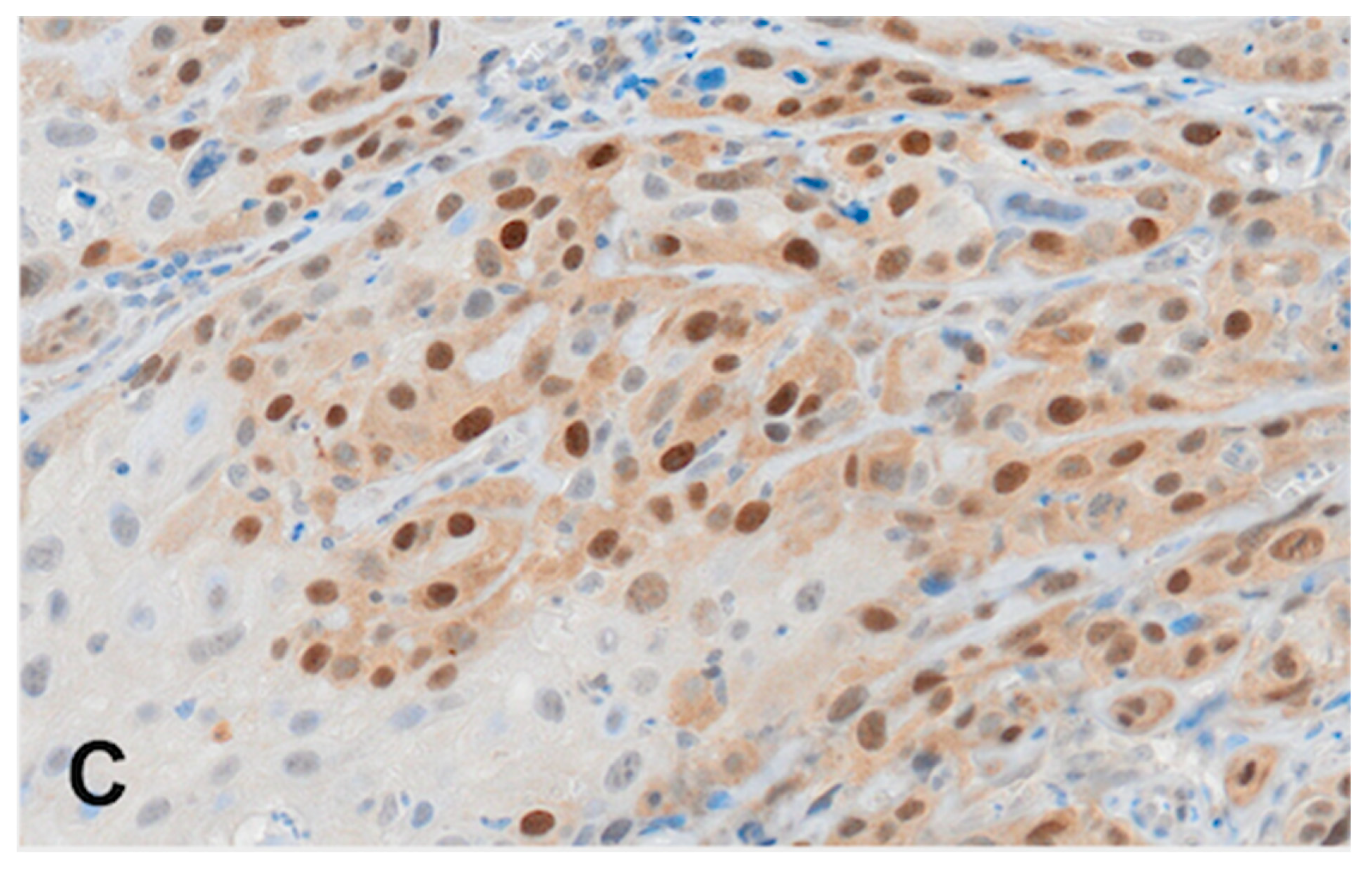
2.4. Immunohistochemistry (RAD51)
2.5. Statistical Analysis
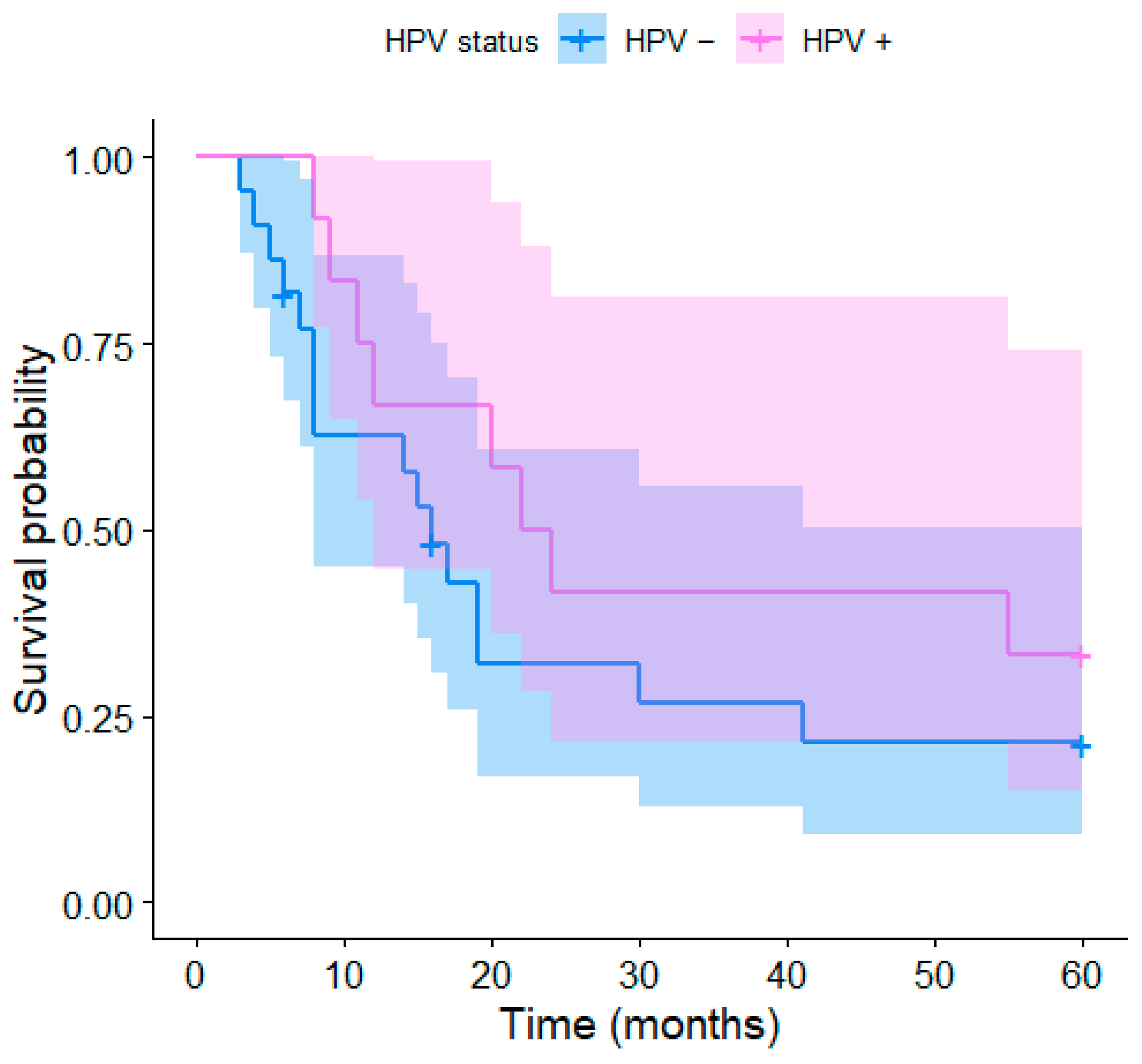
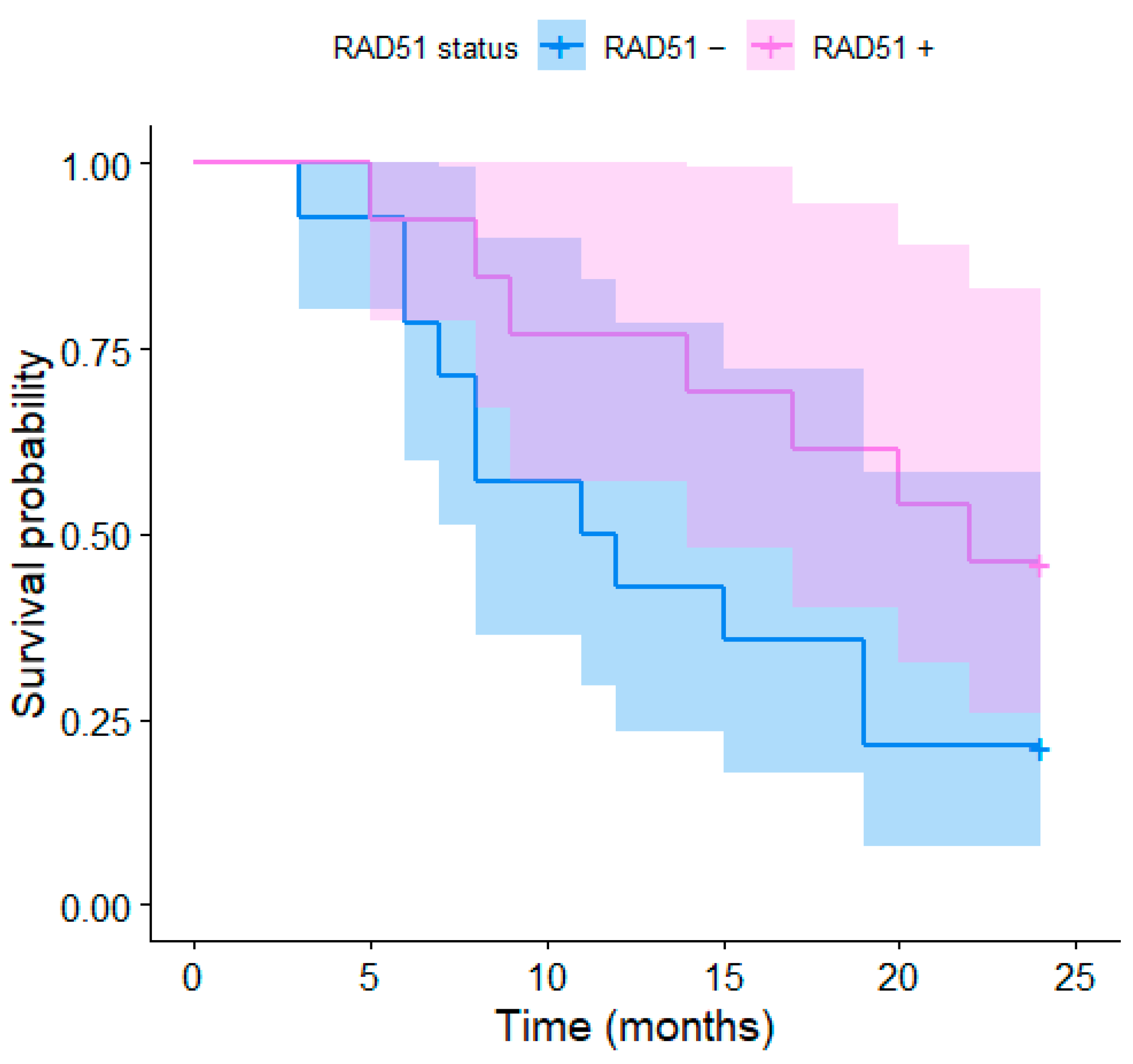
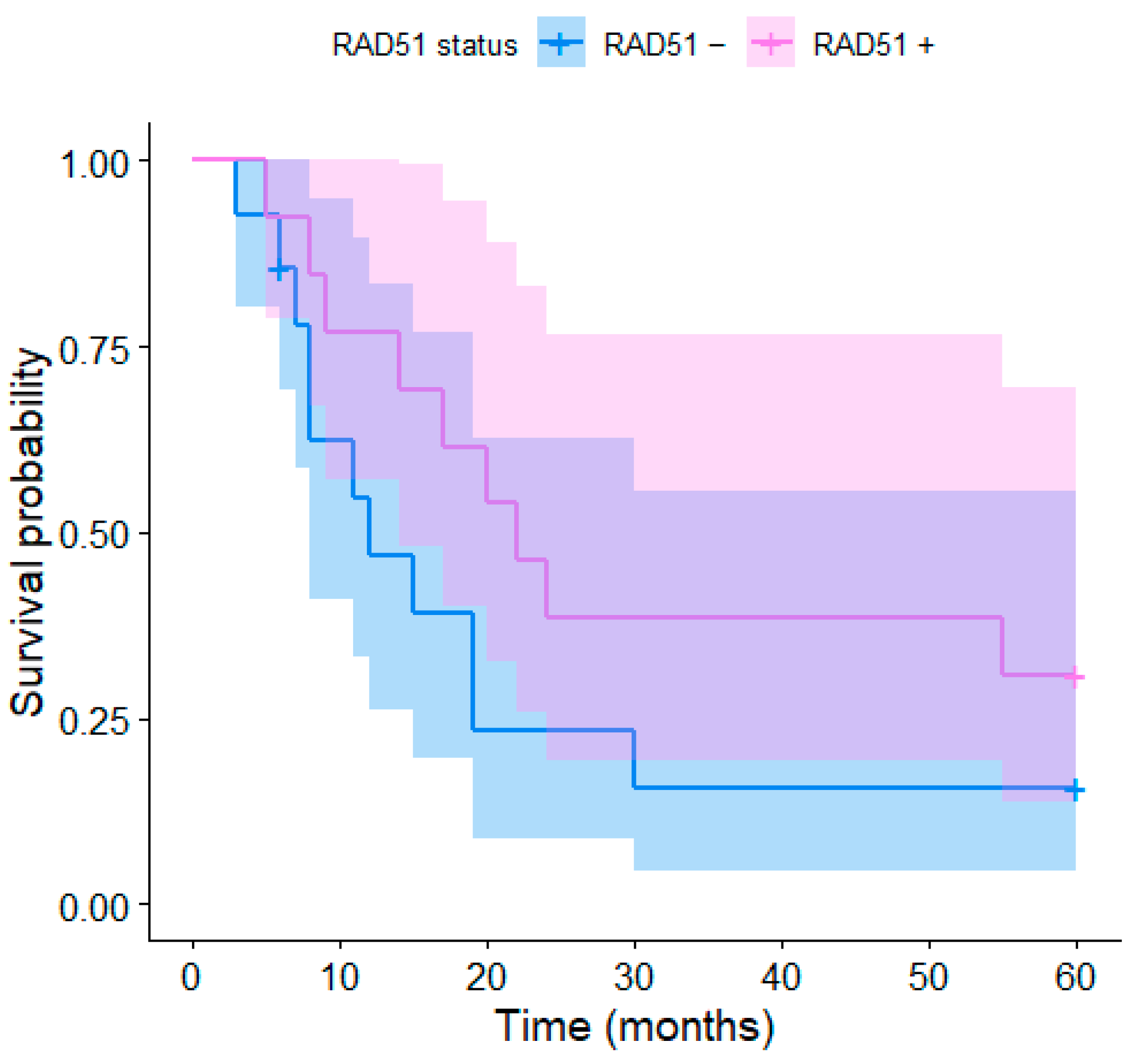
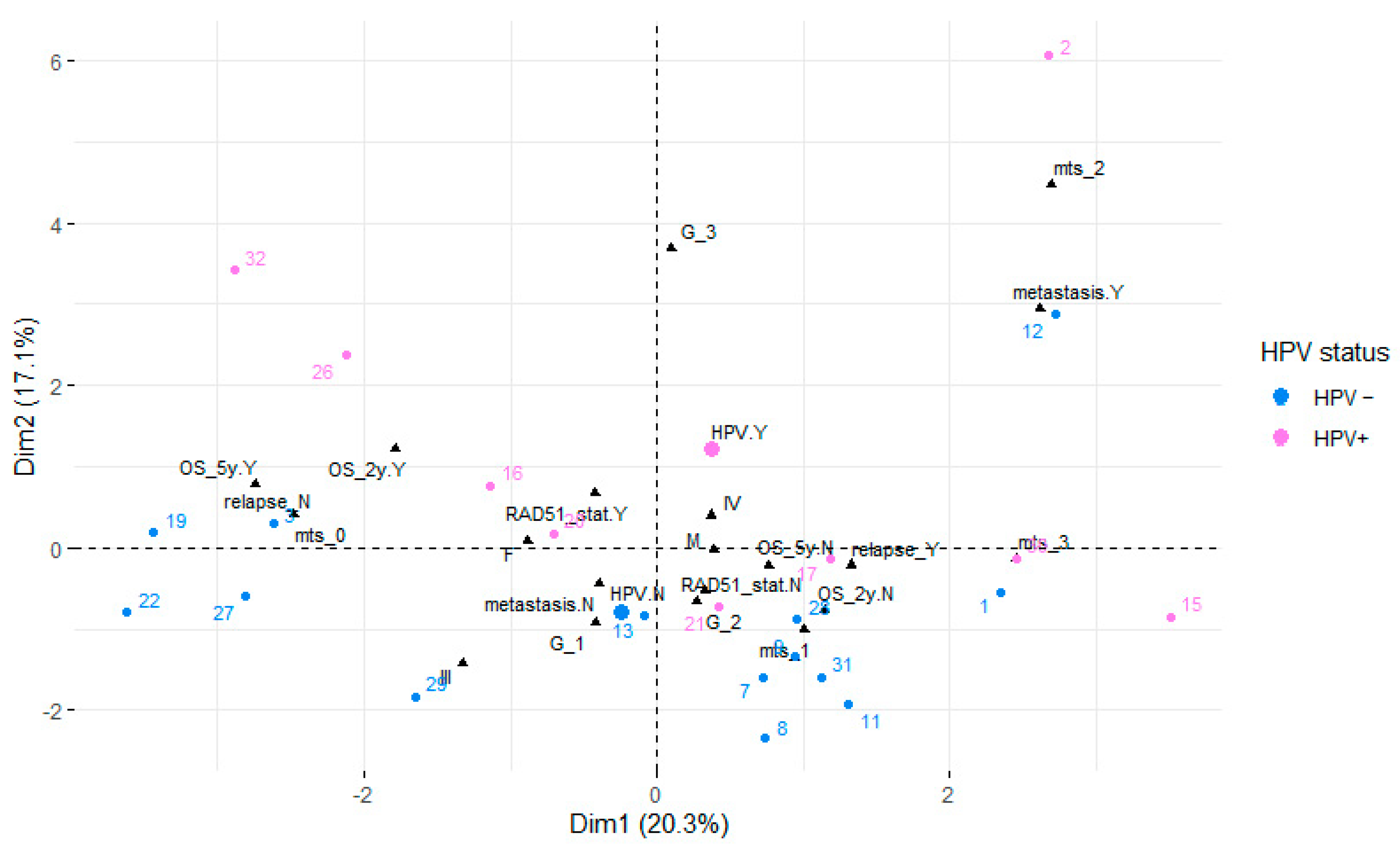
3. Results
4. Discussion
4.1. HPV Subtypes and Tumor Development
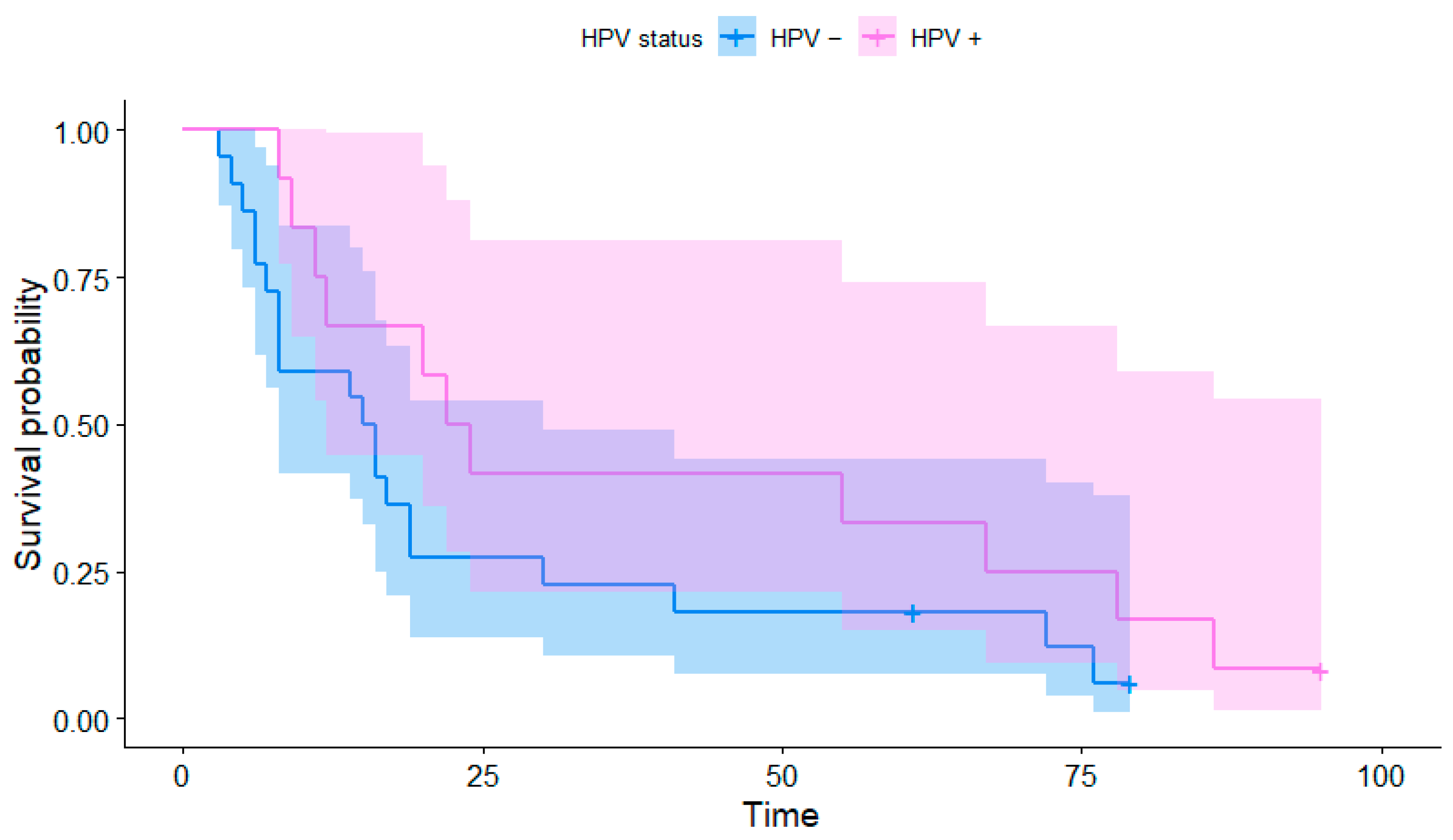
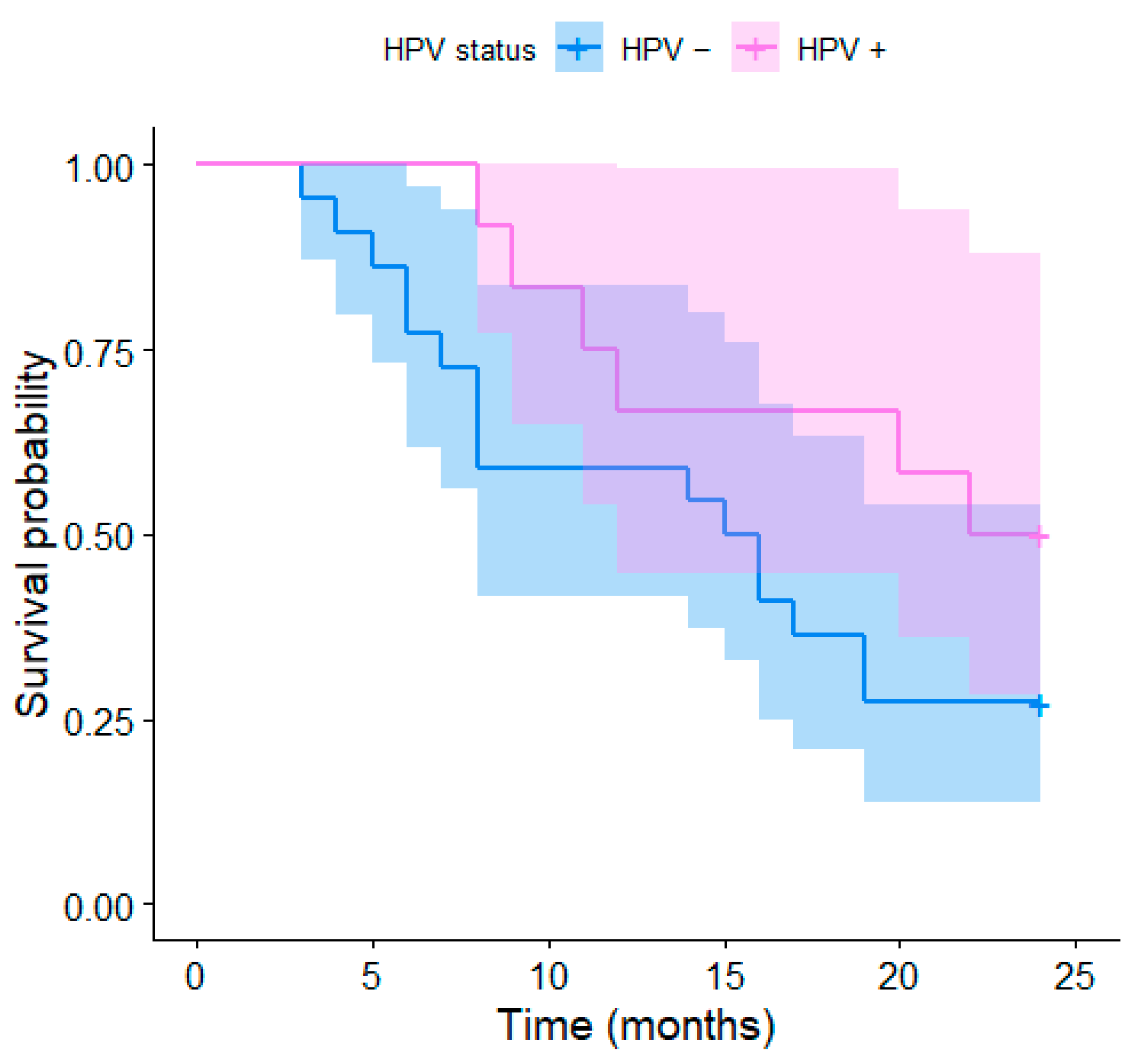
4.2. HPV Status and Survival
4.3. RAD51 Overexpression and Survival
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warnakulasuriya, S. Global Epidemiology of Oral and Oropharyngeal Cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Vrînceanu, D.; Dumitru, M.; Ştefan, A.A.; Mogoantă, C.A.; Sajin, M. Giant pleomorphic sarcoma of the tongue base—A cured clinical case report and literature review. Rom. J. Morphol. Embryol. 2020, 61, 1323–1327. [Google Scholar] [CrossRef]
- Chow, L.T.; Broker, T.R.; Steinberg, B.M. The Natural History of Human Papillomavirus Infections of the Mucosal Epithelia. APMIS 2010, 118, 422–449. [Google Scholar] [CrossRef] [PubMed]
- SVOD. Available online: https://www.svod.cz (accessed on 17 January 2023).
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 12 February 2023).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
- Kassambara, A.; Kosinski, M.; Biecek, P.; Fabian, S. Survminer: Drawing Survival Curves Using “ggplot2”. Available online: https://cran.r-project.org/web/packages/survminer/index.html (accessed on 12 February 2023).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: AnRPackage for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 12 February 2023).
- Di Gravio, E.J.; Lang, P.; Kim, H.A.J.; Chinnery, T.; Mundi, N.; MacNeil, S.D.; Mendez, A.; Yoo, J.; Fung, K.; Mymryk, J.S.; et al. Modern Treatment Outcomes for Early T-Stage Oropharyngeal Cancer Treated with Intensity-Modulated Radiation Therapy at a Tertiary Care Institution. Radiat. Oncol. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Fakhry, C.; Zhang, Q.; Nguyen-Tan, P.F.; Rosenthal, D.; El-Naggar, A.; Garden, A.S.; Soulieres, D.; Trotti, A.; Avizonis, V.; Ridge, J.A.; et al. Human Papillomavirus and Overall Survival after Progression of Oropharyngeal Squamous Cell Carcinoma. J. Clin. Oncol. 2014, 32, 3365–3373. [Google Scholar] [CrossRef]
- Pannone, G.; Santoro, A.; Papagerakis, S.; Lo Muzio, L.; De Rosa, G.; Bufo, P. The Role of Human Papillomavirus in the Pathogenesis of Head & Neck Squamous Cell Carcinoma: An Overview. Infect. Agents Cancer 2011, 6, 4. [Google Scholar]
- de Villiers, E.-M.; Fauquet, C.; Broker, T.R.; Bernard, H.-U.; zur Hausen, H. Classification of Papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- van Monsjou, H.S.; van Velthuysen, M.L.F.; van den Brekel, M.W.M.; Jordanova, E.S.; Melief, C.J.M.; Balm, A.J.M. Human Papillomavirus Status in Young Patients with Head and Neck Squamous Cell Carcinoma. Int. J. Cancer 2011, 130, 1806–1812. [Google Scholar] [CrossRef]
- Garden, A.S.; Kies, M.S.; Morrison, W.H.; Weber, R.S.; Frank, S.J.; Glisson, B.S.; Gunn, G.B.; Beadle, B.M.; Ang, K.K.; Rosenthal, D.I.; et al. Outcomes and Patterns of Care of Patients with Locally Advanced Oropharyngeal Carcinoma Treated in the Early 21st Century. Radiat. Oncol. 2013, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; Young, R.J.; Fisher, R.; Fox, S.B.; Le, Q.-T.; Peters, L.J.; Solomon, B.; Choi, J.; O’Sullivan, B.; Kenny, L.M.; et al. Prognostic Significance of P16INK4A and Human Papillomavirus in Patients with Oropharyngeal Cancer Treated on TROG 02.02 Phase III Trial. J. Clin. Oncol. 2010, 28, 4142–4148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, N.; Yao, W.; Li, S.; Ren, Z. RAD51 Is a Potential Marker for Prognosis and Regulates Cell Proliferation in Pancreatic Cancer. Cancer Cell Int. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gachechiladze, M.; Škarda, J.; Skanderová, D.; Überall, I.; Kolek, V.; Smičkova, P.; Vojta, P.; Vbrková, J.; Hajdúch, M.; Shani, I.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes (TILs) and Their Association with PD-L1 Expression and DNA Repair Protein RAD51 in Patients with Resected Non-Small Cell Lung Carcinoma. Lung Cancer 2020, 147, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Che, W.; Wang, W.; Su, G.; Zhen, T.; Jiang, Z. CDKN3 Promotes Tumor Progression and Confers Cisplatin Resistance via RAD51 in Esophageal Cancer. Cancer Manag. Res. 2019, 11, 3253–3264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, P.; Dong, Y.; Zhu, X.; Zhang, X. Relationship between Rad51 G135C and G172T Variants and the Susceptibility to Cancer: A Meta-Analysis Involving 54 Case-Control Studies. PLoS ONE 2014, 9, e87259. [Google Scholar] [CrossRef] [PubMed]
- Connell, P.P.; Jayathilaka, K.; Haraf, D.J.; Weichselbaum, R.R.; Vokes, E.E.; Lingen, M.W. Pilot Study Examining Tumor Expression of RAD51 and Clinical Outcomes in Human Head Cancers. Int. J. Oncol. 2006, 28, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Blažek, T.; Zděblová Čermáková, Z.; Knybel, L.; Hurník, P.; Štembírek, J.; Resová, K.; Paračková, T.; Formánek, M.; Cvek, J.; Soumarová, R. Dose Escalation in Advanced Floor of the Mouth Cancer: A Pilot Study Using a Combination of IMRT and Stereotactic Boost. Radiat. Oncol. 2021, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]

| Median Age | St III n | ST IV n | Ki 60 n | Ki 70 n | Ki 80 n | Ki 90 n | GTV Med | Gtvtu Med | GTVLN Med | |
|---|---|---|---|---|---|---|---|---|---|---|
| HPV+ | 60 | 0 | 11 | 0 | 3 | 5 | 3 | 70 mL | 38 mL | 8 mL |
| HPV− | 61 | 5 | 17 | 1 | 5 | 9 | 7 | 32 mL | 30 mL | 1 mL |
| RAD51+ | 64 | 1 | 12 | 0 | 6 | 6 | 1 | 60 mL | 40 mL | 5 mL |
| RAD51− | 60.5 | 3 | 11 | 1 | 2 | 5 | 6 | 30.5 mL | 30 mL | 0 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zděblová Čermáková, Z.; Hurník, P.; Konvalinka, D.; Štembírek, J.; Paračková, T.; Resová, K.; Cvek, J.; Blažek, T.; Knybel, L.; Formánek, M.; et al. HPV and RAD51 as Prognostic Factors for Survival in Inoperable Oral and Oropharyngeal Cancer in Patients Unfit for Chemotherapy Treated with Hyperfractionated Radiotherapy. Medicina 2023, 59, 361. https://doi.org/10.3390/medicina59020361
Zděblová Čermáková Z, Hurník P, Konvalinka D, Štembírek J, Paračková T, Resová K, Cvek J, Blažek T, Knybel L, Formánek M, et al. HPV and RAD51 as Prognostic Factors for Survival in Inoperable Oral and Oropharyngeal Cancer in Patients Unfit for Chemotherapy Treated with Hyperfractionated Radiotherapy. Medicina. 2023; 59(2):361. https://doi.org/10.3390/medicina59020361
Chicago/Turabian StyleZděblová Čermáková, Zuzana, Pavel Hurník, David Konvalinka, Jan Štembírek, Tereza Paračková, Kamila Resová, Jakub Cvek, Tomáš Blažek, Lukáš Knybel, Martin Formánek, and et al. 2023. "HPV and RAD51 as Prognostic Factors for Survival in Inoperable Oral and Oropharyngeal Cancer in Patients Unfit for Chemotherapy Treated with Hyperfractionated Radiotherapy" Medicina 59, no. 2: 361. https://doi.org/10.3390/medicina59020361
APA StyleZděblová Čermáková, Z., Hurník, P., Konvalinka, D., Štembírek, J., Paračková, T., Resová, K., Cvek, J., Blažek, T., Knybel, L., Formánek, M., Gachechiladze, M., Joerger, M., Soltermann, A., Škarda, J., Motyka, O., & Janoutová, J. (2023). HPV and RAD51 as Prognostic Factors for Survival in Inoperable Oral and Oropharyngeal Cancer in Patients Unfit for Chemotherapy Treated with Hyperfractionated Radiotherapy. Medicina, 59(2), 361. https://doi.org/10.3390/medicina59020361







