Exploring the Radioprotective Indium (III) Oxide Screens for Mammography Scans Using a Three-Layer Heterogeneous Breast Phantom and MCNPX: A Comparative Study Using Clinical Findings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Definition of the Utilized Breast Model through MCNPX Code
2.2. Validation of MCNPX Code
3. Results and Discussion
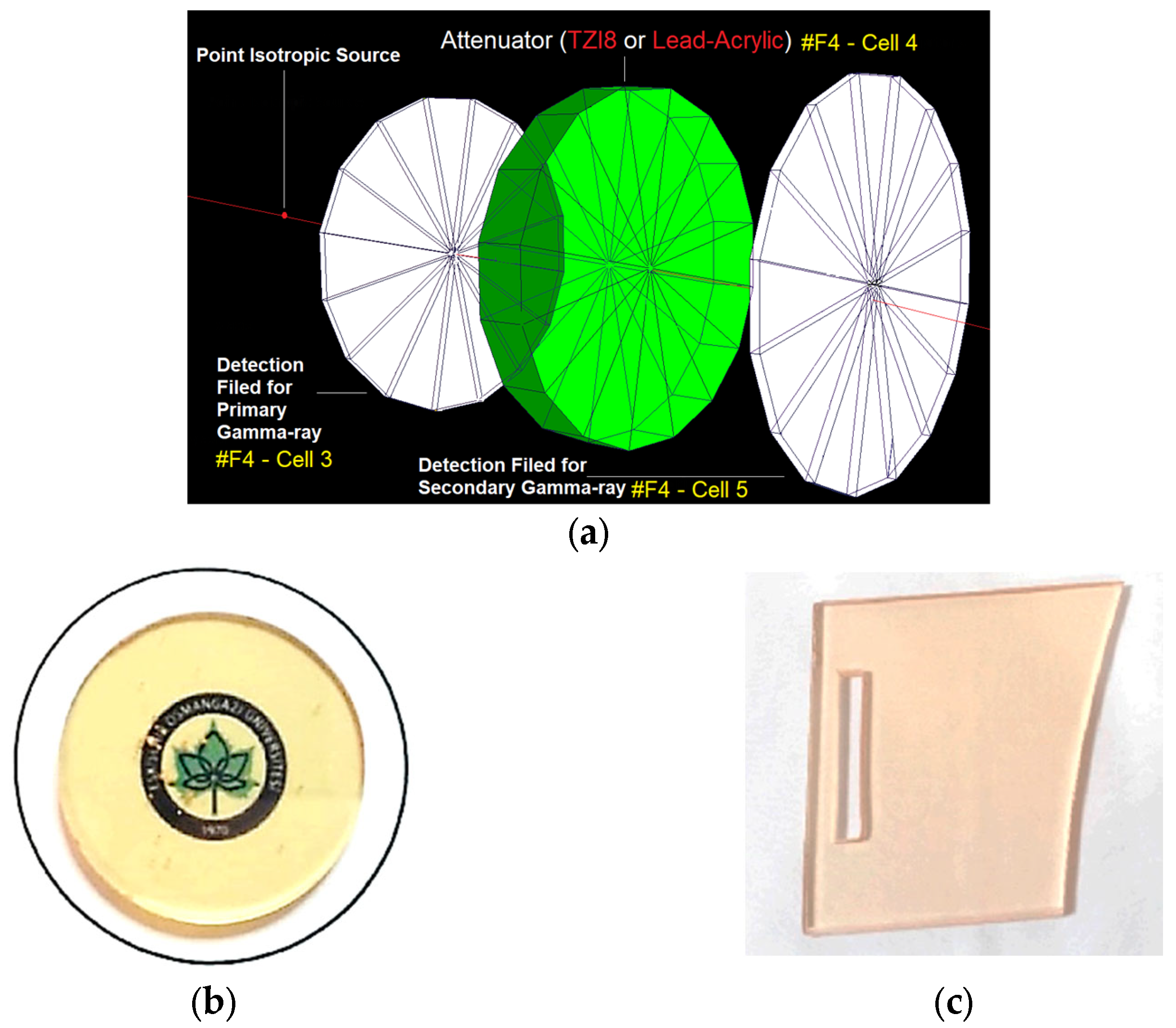
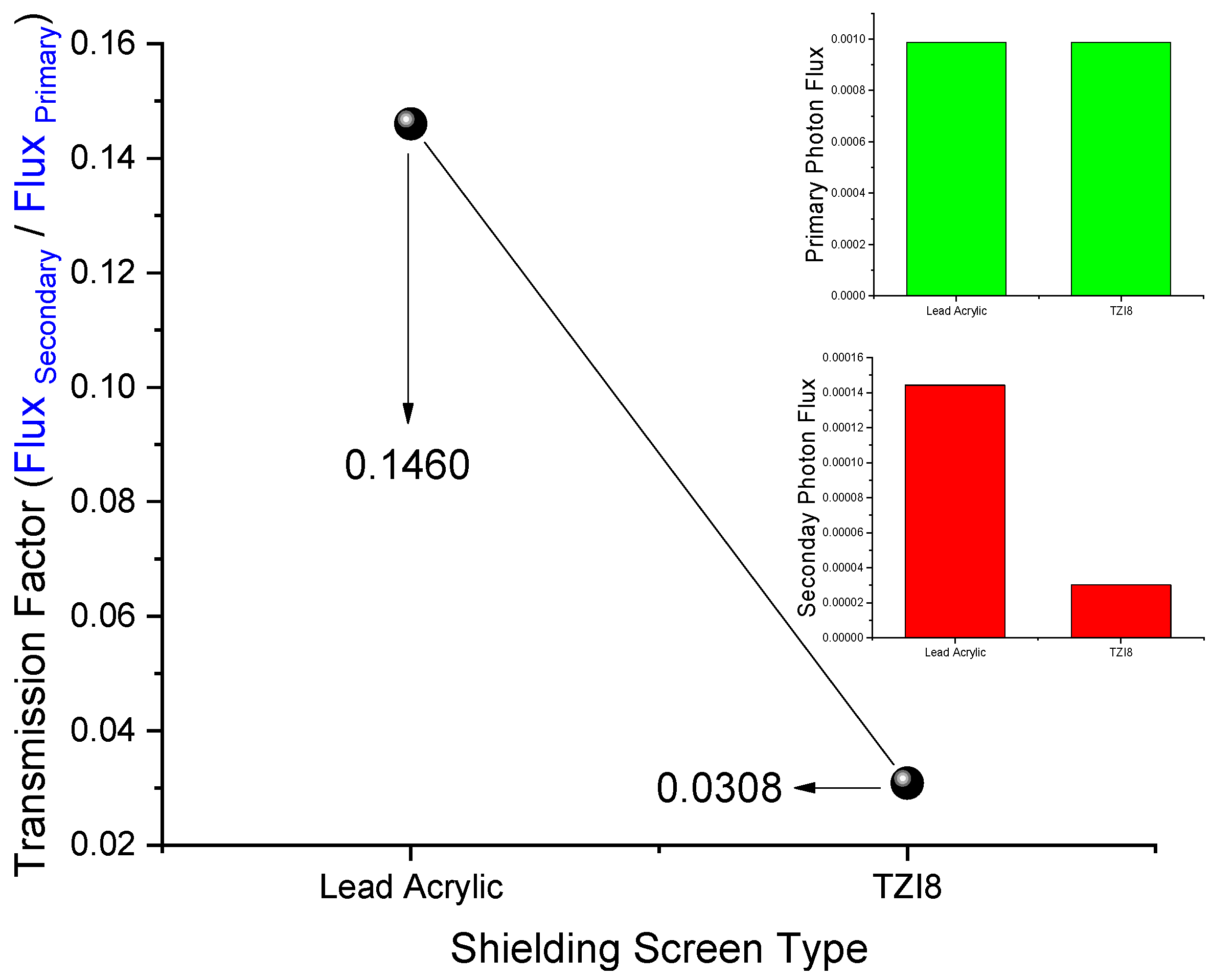
3.1. Initial Assessment on Transmission-Factor (TFs) Values of Lead Acrylic and TZI8
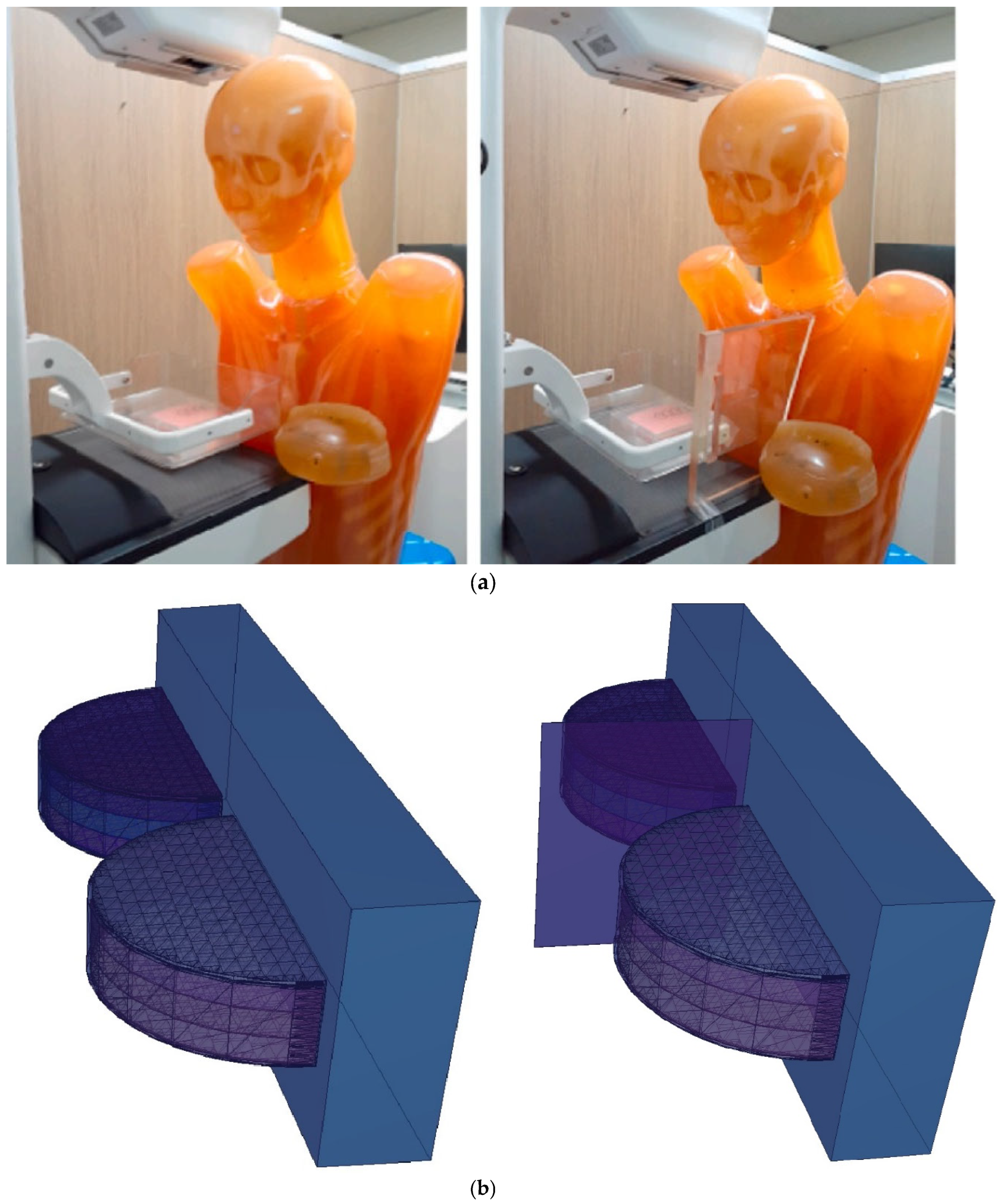
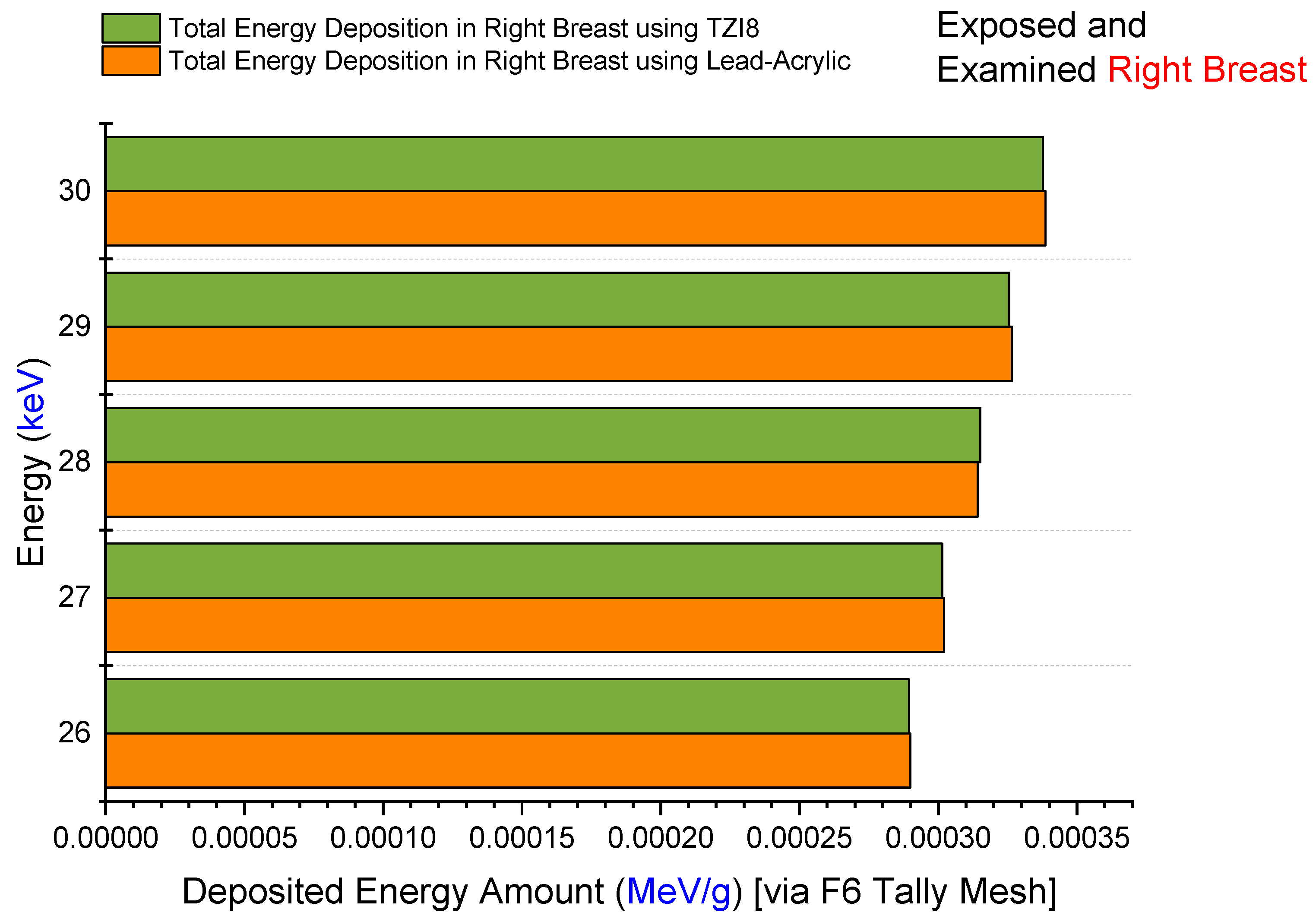
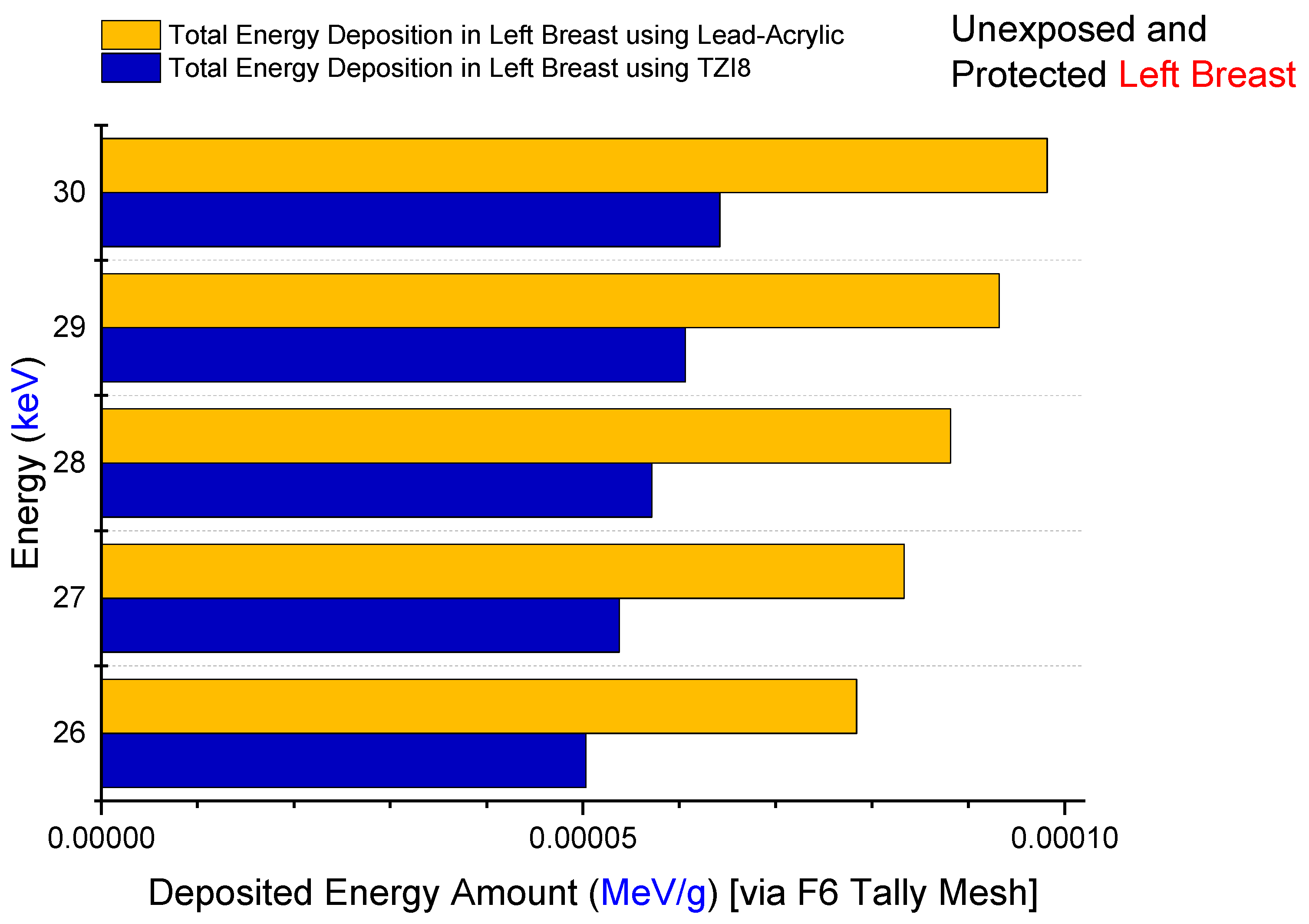
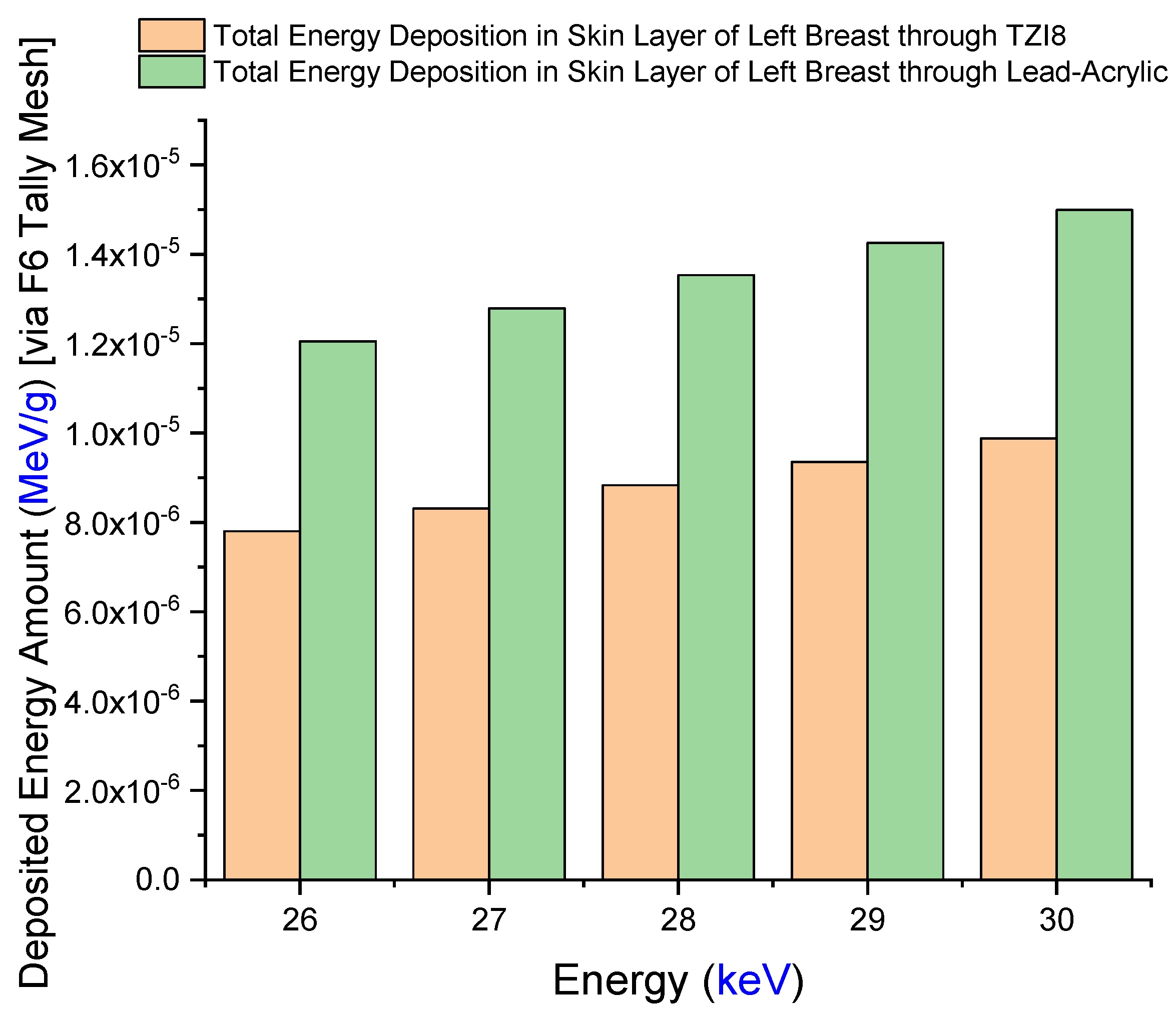
3.2. Assesment of Lead-Acrylic and TZI8 Shielding Screens on Breast Protection
4. Conclusions
- The protection given by the TZI8-glass screen is 60–65% greater than that of the lead-acrylic screen.
- Given the drawbacks of hazardous Pb in the structure of lead-acrylic material, it can be implied that glasses rich in indium (III) oxide might be used for mammography tests, as they would provide a greater radioprotection compared to the lead acrylic, as well as significantly reducing the dose to the unexposed breast.
- Such a decrease will lower the risk of breast cancer (as a result of radiation exposure), and may be advantageous in reducing medium- and long-term concerns, by further decreasing the dose amount for the existing mammography practice.
- Given the flexibility of synthesis, the superior mechanical and thermal features of glass materials, and the transparency of TZI8 in the visible-light spectrum, it is anticipated that such a material would contribute to medical applications as a suitable breast shield.
- Using glass-shielding materials during mammography tests may be more convenient for radiographers in terms of radiographic imaging and patient positioning (owing to their transparency and, accordingly, real-time patient monitoring).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schönherr, R. Clinical Relevance of Ion Channels for Diagnosis and Therapy of Cancer. J. Membr. Biol. 2005, 205, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.; Stockton, D.; Møller, H.; Quinn, M.; Babb, P.; De Angelis, R.; Micheli, A. Cancer prevalence in the UK: Results from the EUROPREVAL study. Ann. Oncol. 2003, 14, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Chen, W. Epidemiology of lung cancer in China. Thorac. Cancer 2019, 10, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chen, H.-D.; Yu, Y.-W.; Li, N.; Chen, W.-Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef]
- Durham, D.D.; Abraham, L.A.; Roberts, M.C.; Khan, C.P.; Smith, R.A.; Kerlikowske, K.; Miglioretti, D.L. Breast cancer incidence among women with a family history of breast cancer by relative’s age at diagnosis. Cancer 2022, 128, 4232–4240. [Google Scholar] [CrossRef]
- Lee, A.; Mavaddat, N.; Wilcox, A.N.; Msc, A.P.C.; Carver, T.; Hartley, S.; de Villiers, C.B.; Izquierdo, A.; Simard, J.; Schmidt, M.K.; et al. BOADICEA: A comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Anesthesia Analg. 2019, 21, 1708–1718. [Google Scholar] [CrossRef]
- Rejani, Y.; Selvi, S.T. Early detection of breast cancer using SVM classifier technique. arXiv 2009, arXiv:0912.2314. [Google Scholar]
- Dibden, A.; Offman, J.; Duffy, S.W.; Gabe, R. Worldwide Review and Meta-Analysis of Cohort Studies Measuring the Effect of Mammography Screening Programmes on Incidence-Based Breast Cancer Mortality. Cancers 2020, 12, 976. [Google Scholar] [CrossRef]
- Nothacker, M.; Duda, V.; Hahn, M.; Warm, M.; Degenhardt, F.; Madjar, H.; Weinbrenner, S.; Albert, U.-S. Early detection of breast cancer: Benefits and risks of supplemental breast ultrasound in asymptomatic women with mammographically dense breast tissue. A systematic review. BMC Cancer 2009, 9, 335. [Google Scholar] [CrossRef]
- Morris, E.A. Breast cancer imaging with MRI. Radiol. Clin. North Am. 2002, 40, 443–466. [Google Scholar] [CrossRef]
- Olsen, O.; Gøtzsche, P.C. Screening for breast cancer with mammography. Cochrane Database Syst. Rev. 2001. [Google Scholar]
- Bushong, S.C. Radiologic Science for Technologists E-Book: Physics, Biology, and Protection; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Stines, J.; Tristant, H. The normal breast and its variations in mammography. Eur. J. Radiol. 2005, 54, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Golubicic, I.; Borojevic, N.; Pavlovic, T. Risk factors for breast cancer: Is ionizing radiation among them? J. BUON 2008, 13, 487–494. [Google Scholar] [PubMed]
- Brown, M.; Covington, M.F. Comparative Benefit-to–Radiation Risk Ratio of Molecular Breast Imaging, Two-Dimensional Full-Field Digital Mammography with and without Tomosynthesis, and Synthetic Mammography with Tomosynthesis. Radiol. Imaging Cancer 2019, 1, e190005. [Google Scholar] [CrossRef]
- Eklund, G.W. The art of mammographic positioning. Radiol. Diagn. Breast Dis. 2000, 75–88. [Google Scholar] [CrossRef]
- Koo, B.-Y.; Lee, K.-S. Reduction of scattered radiation dose by X-ray shielding during mammography. Radiat. Phys. Chem. 2020, 177, 109111. [Google Scholar] [CrossRef]
- Drukteinis, J.S.; Mooney, B.P.; Flowers, C.I.; Gatenby, R.A. Beyond Mammography: New Frontiers in Breast Cancer Screening. Am. J. Med. 2013, 126, 472–479. [Google Scholar] [CrossRef]
- Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55. [Google Scholar]
- Kurtulus, R.; Kurtulus, C.; Kavas, T. Nuclear radiation shielding characteristics and physical, optical, mechanical, and thermal properties of lithium-borotellurite glass doped with Rb2O. Prog. Nucl. Energy 2021, 141, 103961. [Google Scholar] [CrossRef]
- Kurtulus, R.; Kavas, T.; Akkurt, I.; Gunoglu, K. A significant study for radiation shielding applications: Synthesis of waste CRT-derived glass systems containing CoO. Appl. Phys. A 2021, 127, 737. [Google Scholar] [CrossRef]
- Kilic, G.; Kavaz, E.; Ilik, E.; ALMisned, G.; Tekin, H.O. CdO-rich quaternary tellurite glasses for nuclear safety purposes: Synthesis and experimental gamma-ray and neutron radiation assessment of high-density and transparent samples. Opt. Mater. 2022, 129, 112512. [Google Scholar] [CrossRef]
- Olarinoye, I.O.; El-Agawany, F.I.; Gamal, A.; Yousef, E.S.; Rammah, Y.S. Investigation of mechanical properties, photons, neutrons, and charged particles shielding characteristics of Bi2O3/B2O3/SiO2 glasses. Appl. Phys. A 2021, 127, 223. [Google Scholar] [CrossRef]
- Kilic, G.; Ilik, E.; Issa, S.A.; Issa, B.; Issever, U.; Zakaly, H.M.; Tekin, H. Fabrication, structural, optical, physical and radiation shielding characterization of indium (III) oxide reinforced 85TeO2-(15–x)ZnO-xIn2O3 glass system. Ceram. Int. 2021, 47, 27305–27315. [Google Scholar] [CrossRef]
- RSICC Computer Code Collection, MCNPX user’s manual version 2.4.Monte Carlo N-Particle Transport Code System for Multiple and High Energy Applications, 2002.
- Sechopoulos, I.; Ali, E.S.M.; Badal, A.; Badano, A.; Boone, J.M.; Kyprianou, I.S.; Mainegra-Hing, E.; McMillan, K.L.; McNitt-Gray, M.F.; Rogers, D.W.O.; et al. Monte Carlo reference data sets for imaging research: Executive summary of the report of AAPM Research Committee Task Group 195. Med. Phys. 2015, 42, 5679–5691. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-Y.; Lai, K.-J.; Tu, C.-Y.; Wu, J. Three-layer heterogeneous mammographic phantoms for Monte Carlo simulation of normalized glandular dose coefficients in mammography. Sci. Rep. 2020, 10, 2234. [Google Scholar] [CrossRef] [PubMed]
- Almisned, G.; Baykal, D.S.; Susoy, G.; Kilic, G.; Zakaly, H.M.H.; Ene, A.; Tekin, H.O. Determination of gamma-ray transmission factors of WO3–TeO2–B2O3 glasses using MCNPX Monte Carlo code for shielding and protection purposes. Appl. Rheol. 2022, 32, 166–177. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Sayyed, M. BaO–Li2O–B2O3 glass systems: Potential utilization in gamma radiation protection. Prog. Nucl. Energy 2020, 129, 103511. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Almurayshid, M.; Almasoud, F.; Sayyed, M.I.; Mahmoud, K.A. Examinations the optical, mechanical, and shielding properties of Ag2O doped B2O3–Bi2O3–SrF2–Na2O glasses for gamma ray shield applications. Sci. Rep. 2022, 12, 3548. [Google Scholar] [CrossRef]
- Tekin, H.O.; Almisned, G.; Rammah, Y.S.; Susoy, G.; Ali, F.T.; Baykal, D.S.; Elshami, W.; Zakaly, H.M.H.; Issa, S.A.M. The significant role of WO3 on high-dense BaO–P2O3 glasses: Transmission factors and a comparative investigation using commercial and other types of shields. Appl. Phys. A 2022, 128, 470. [Google Scholar] [CrossRef]
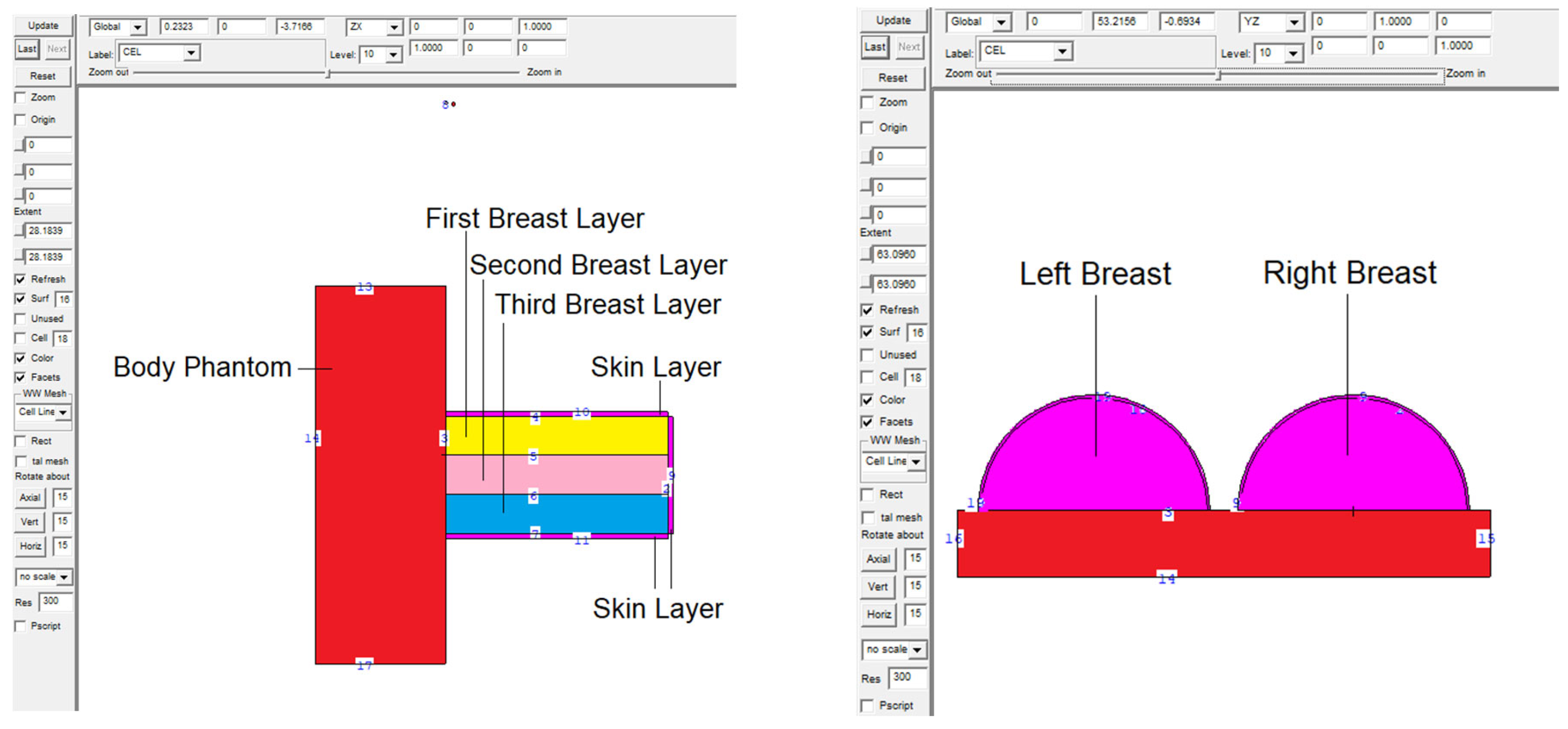
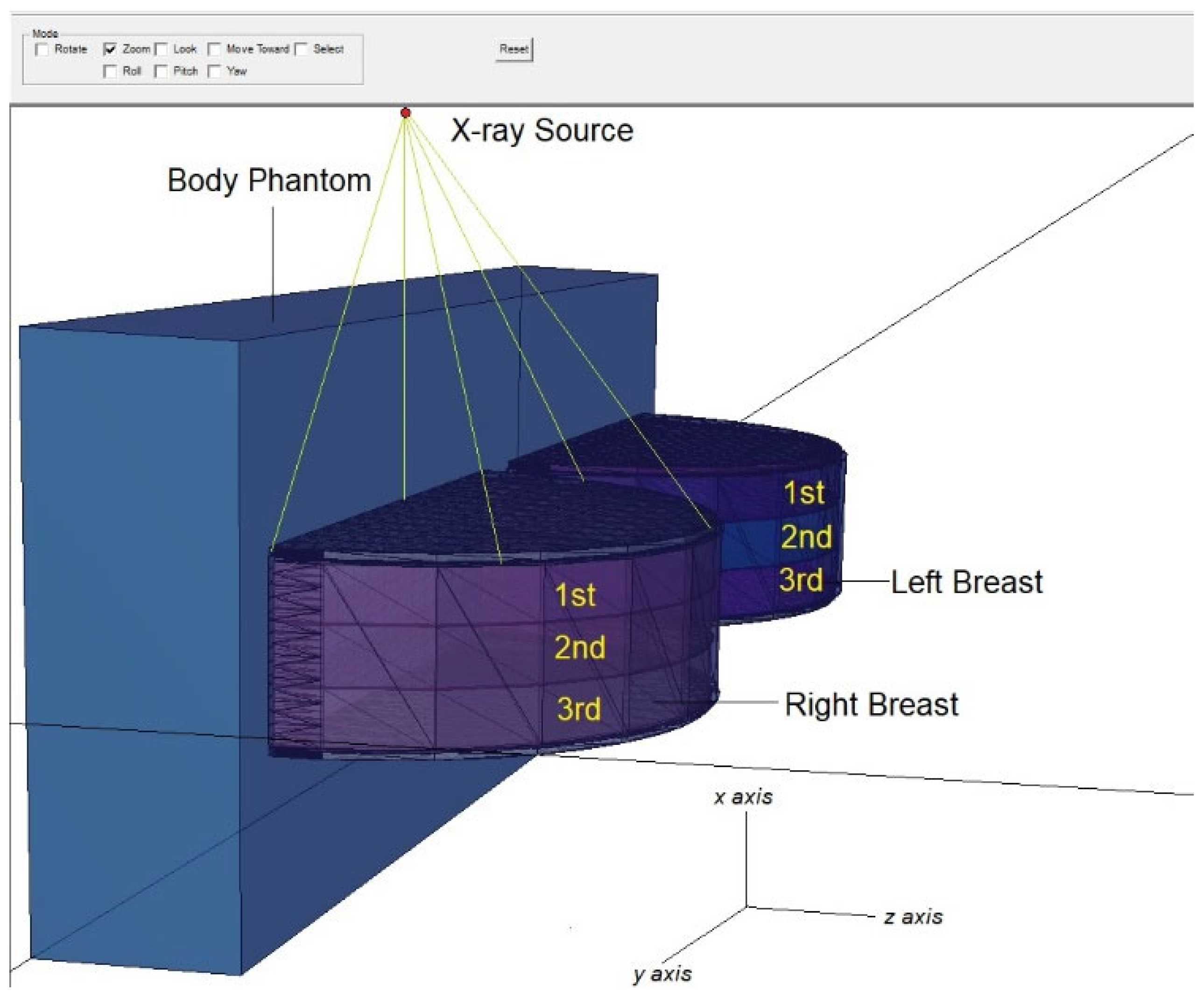
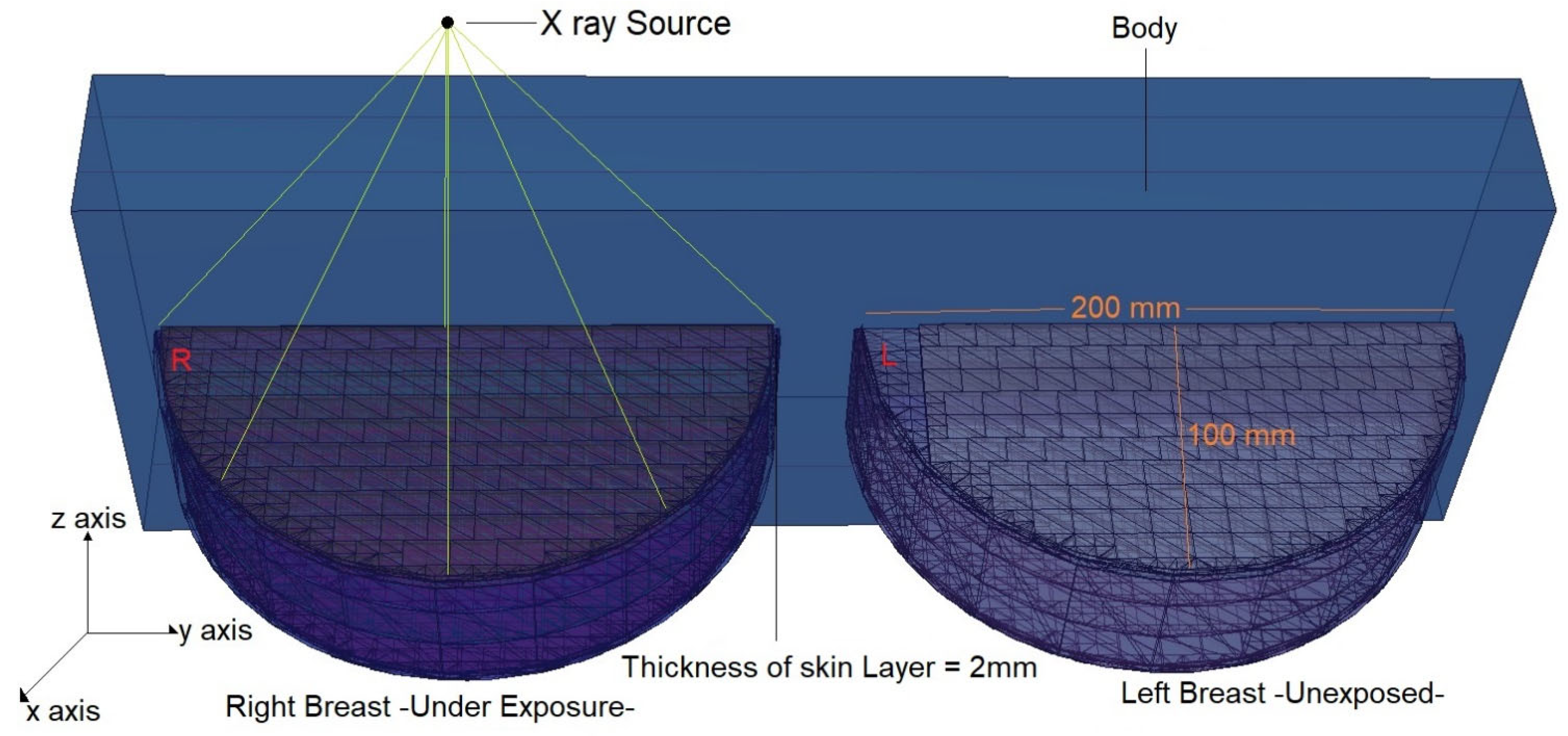
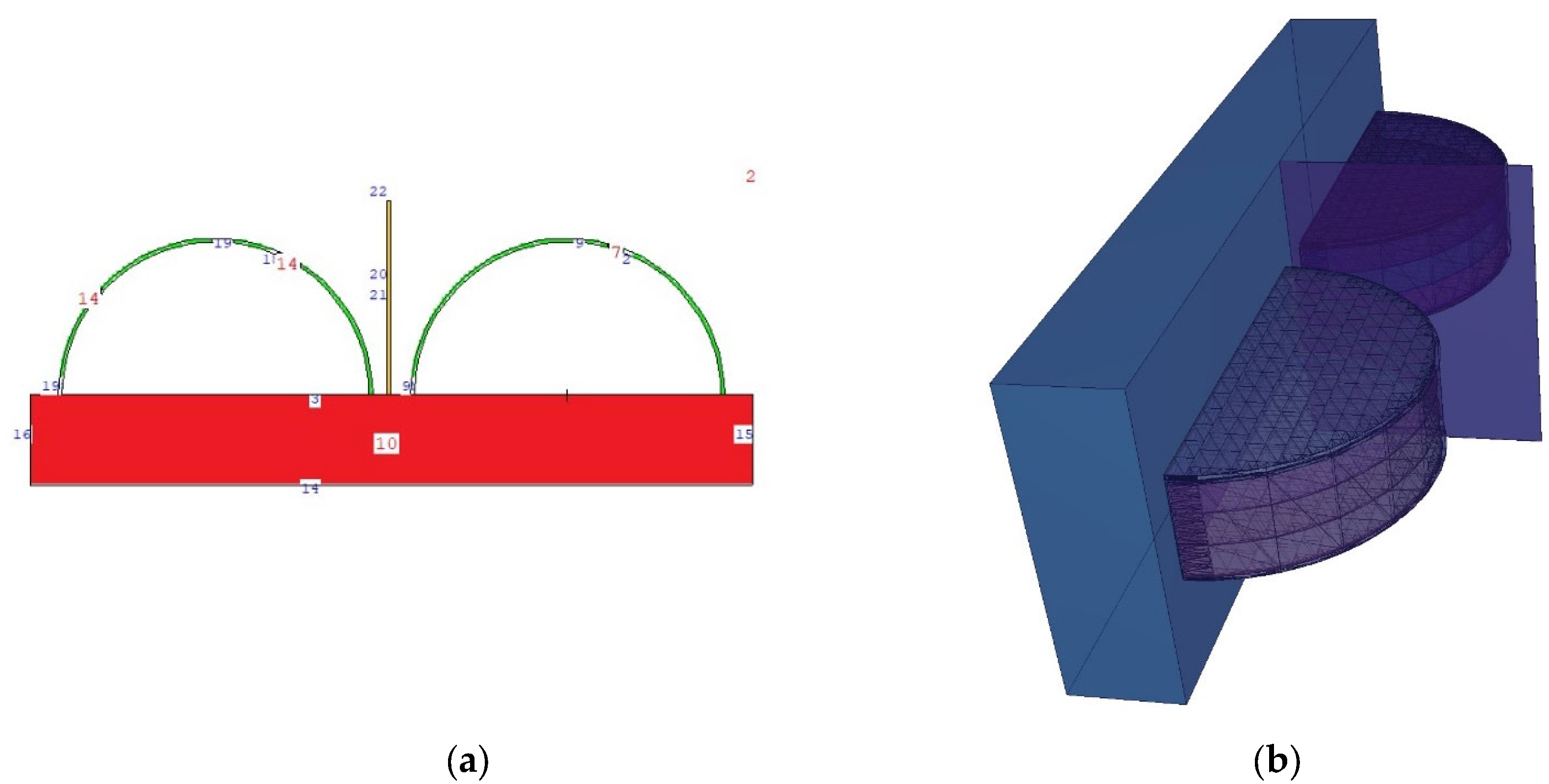
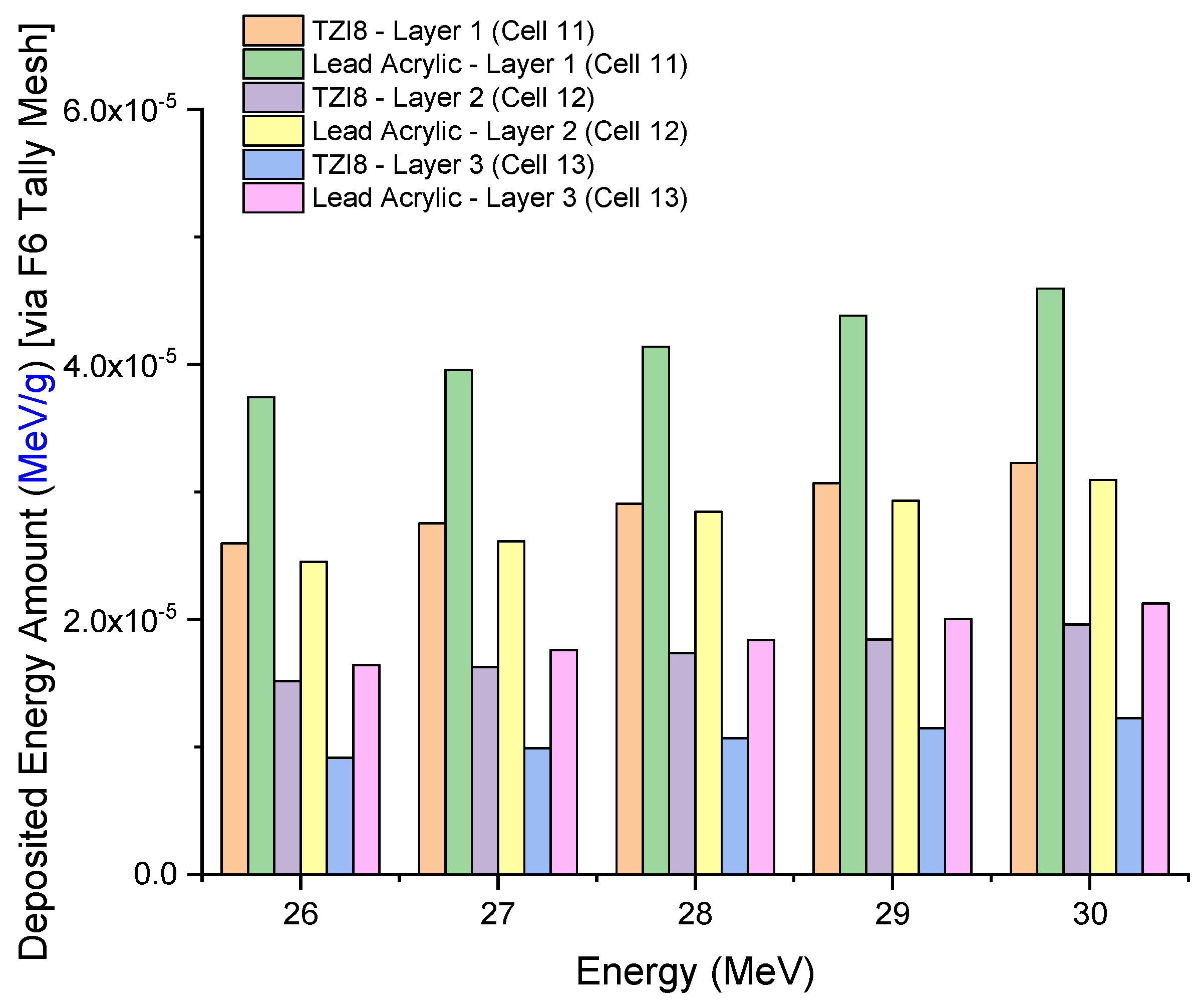
| Weight Percentage (%) | |||||
|---|---|---|---|---|---|
| Tissue | Density (g/cm3) | H | C | N | O |
| GF Tissue (25%) | 0.955 | 11 | 51 | 2.1 | 35.7 |
| GF Tissue (50%) | 0.982 | 10.7 | 40.1 | 2.5 | 46.4 |
| GF Tissue (75%) | 1.010 | 10.5 | 29.3 | 2.9 | 57 |
| Skin | 1.090 | 9.8 | 17.8 | 5 | 66.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ALMisned, G.; Elshami, W.; Kilic, G.; Ilik, E.; Rabaa, E.; Zakaly, H.M.H.; Ene, A.; Tekin, H.O. Exploring the Radioprotective Indium (III) Oxide Screens for Mammography Scans Using a Three-Layer Heterogeneous Breast Phantom and MCNPX: A Comparative Study Using Clinical Findings. Medicina 2023, 59, 327. https://doi.org/10.3390/medicina59020327
ALMisned G, Elshami W, Kilic G, Ilik E, Rabaa E, Zakaly HMH, Ene A, Tekin HO. Exploring the Radioprotective Indium (III) Oxide Screens for Mammography Scans Using a Three-Layer Heterogeneous Breast Phantom and MCNPX: A Comparative Study Using Clinical Findings. Medicina. 2023; 59(2):327. https://doi.org/10.3390/medicina59020327
Chicago/Turabian StyleALMisned, Ghada, Wiam Elshami, Gokhan Kilic, Erkan Ilik, Elaf Rabaa, Hesham M. H. Zakaly, Antoaneta Ene, and Huseyin O. Tekin. 2023. "Exploring the Radioprotective Indium (III) Oxide Screens for Mammography Scans Using a Three-Layer Heterogeneous Breast Phantom and MCNPX: A Comparative Study Using Clinical Findings" Medicina 59, no. 2: 327. https://doi.org/10.3390/medicina59020327
APA StyleALMisned, G., Elshami, W., Kilic, G., Ilik, E., Rabaa, E., Zakaly, H. M. H., Ene, A., & Tekin, H. O. (2023). Exploring the Radioprotective Indium (III) Oxide Screens for Mammography Scans Using a Three-Layer Heterogeneous Breast Phantom and MCNPX: A Comparative Study Using Clinical Findings. Medicina, 59(2), 327. https://doi.org/10.3390/medicina59020327









