Bony Canal Method of Dexamethasone Injections in Aggressive Form of Central Giant Cell Granuloma—Case Series
Abstract
:1. Introduction
2. Case Series
2.1. Case #1
2.2. Case #2
2.3. Case #3
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, W.J.; Doyle, L.A. Updates from the 2020 World Health Organization Classification of Soft Tissue and Bone Tumours. Histopathology 2021, 78, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Vered, M.; Wright, J.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Odontogenic and Maxillofacial Bone Tumours. Head Neck Pathol. 2022, 16, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Wiatrak, B.J.; Gluckman, J.L.; Fabian, R.L.; Wesseler, T.A. Giant cell reparative granuloma of the ethmoid sinus. Otolaryngol. Head Neck Surg. 1987, 97, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Ciappetta, P.; Salvati, M.; Bernardi, C.; Raco, A.; Di Lorenzo, N. Giant cell reparative granuloma of the skull base mimicking an intra cranial tumor. Case report and review of the literature. Surg Neurol. 1990, 33, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M., Jr.; Gorlin, R.J. Noonas-like/multiple giant cell lesion syndrome. Am. J. Med. Genet. 1991, 40, 159–166. [Google Scholar] [CrossRef]
- Curtis, N.J.; Walker, D.M. A case of aggressive multiple metachronous central giant cell granulomas of the jaws: Differential diagnosis and management options. Int. J. Oral Maxillofac. Surg. 2005, 34, 806–808. [Google Scholar] [CrossRef]
- de Lange, J.; van den Akker, H.P.; van den Berg, H. Central giant cell granuloma of the jaw: A review of the literature with emphasis on therapy options. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 603–615. [Google Scholar] [CrossRef]
- Gawrych, E.; Juszkiewicz, P.; Krzywińska-Zdeb, E. Ziarniniak naprawczy olbrzymiokomórkowy żuchwy u 6-letniego chłopca. Magaz. Stomatol. 2011, 6, 22–25. [Google Scholar]
- Venkateshwarlu, M.; Geetha, P.; Radhika, B. Central Giant Cell Granuloma. IJDA 2010, 2, 134–137. [Google Scholar]
- Kaczmarzyk, T.; Stypułkowska, J.; Tomaszewska, R.; Czopek, J. Nowotwory Zębopochodne i Guzy Nowotworopodobne Kości Szczękowych; Quintessence Publishing: Berlin, Germany, 2009. [Google Scholar]
- Carlos, R.; Sedano, H.O. Intralesional cortico steroids as an alternative treatment for cen tral giant cell granuloma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 2002, 93, 161–166. [Google Scholar] [CrossRef]
- Kurtz, M.; Mesa, M.; Alberto, P. Treatment of a central giant cell lesion of the mandible with intralesional glucocorticosteroids. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 2001, 9, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Łazarczyk, B.; Sianowska, D.; Cylwik, J. Przypadek ziarniniaka olbrzymiokomórkowego żuchwy u 8-letniego dziecka. Czas. Stomat. 1996, 9, 630–632. [Google Scholar]
- Markt, J.C. An endosseous, implant-retained obturator for the rehabilitation of a recurrent central giant cell granuloma: A clinical report. J. Prosthet. Dent. 2001, 85, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Pogorzelska-Stronczak, B.; Szporek, B.; Skowronek, J.; Sabat, D.; Gabriel, A. Centralne olbrzymiokomórkowe guzy szczęk u dzieci. Czas. Stomat. 1997, 5, 343–350. [Google Scholar]
- Adornato, M.C.; Paticoff, K.A. Intralesional corticosteroid injection for treatment of central giant-cell granuloma. J. Am. Dent. Assoc. 2001, 132, 186–190. [Google Scholar] [CrossRef]
- Tallan, E.M.; Olsen, K.D.; McCaffrey, T.V.; Unni, K.K.; Lund, B.A. Advanced giant cell granuloma: A twenty-year study. Otolaryngol. Head Neck Surg. 1994, 110, 413–418. [Google Scholar] [CrossRef]
- Waldron, C.A. Bone pathology. In Oral and Maxillofacial Pathology; Neville, B.W., Damm, D.D., Allen, C.M., Bouquot, J.E., Eds.; WB Saunders: Philadelphia, PA, USA, 1995; pp. 453–458. [Google Scholar]
- Allen, D.T.; Sheats, R.D. A central giant cell granuloma in a patient seeking orthodontic treatment. J. Am. Dent. Assoc. 2001, 132, 1255–1260. [Google Scholar] [CrossRef]
- Theologie-Lygidakis, N.; Telona, P.; Michail-Strantzia, C.; Iatrou, I. Treatment of central giant—cell granulomas of the jaws in children: Conservative or radical surgical approach? J. Craniomaxillofac. Surg. 2010, 39, 639–644. [Google Scholar] [CrossRef]
- Nowak, R.; Pawlak, W.; Kaczkowski, H. Centralny ziarniniak olbrzymiokomórkowy żuchwy u 16-letniego pacjenta—opis przypadku. Dent. Med. Probl. 2004, 41, 803–806. [Google Scholar]
- Zielińska-Kaźmierska, B.; Grodecka, J.; Neskoromna, A.; Kobos, J. Centralny ziarniniak olbrzymiokomórkowy żuchwy o różnym przebiegu klinicznym—przegląd piśmiennictwa i własne obserwacje. Współcz. Onkol. 2006, 5, 250–254. [Google Scholar]
- Szyfter, W.; Mielcarek-Kuchta, D.; Nowaczyk, M.T.; Kaczmarek, J. Central giant cell mandibular granuloma in a 14-year-old boy—case report. Czas. Stomatol. 2009, 62, 486–492. [Google Scholar]
- Niedzielska, I. Nadziaślak—guz zapalny czy nowotworowy [Epuli—The inflammatory or neoplastic tumor]. Pol. Merkur. Lekarski. 2008, 24, 149–150. [Google Scholar]
- Ciorba, A.; Altissimi, G.; Giansanti, M. Giant cell granuloma of the maxilla: Case report. Acta Otorhinolaryngol. Ital. 2004, 24, 26–29. [Google Scholar] [PubMed]
- Mimic, A.; Stajcic, Z. Prognostic significance of cortical perforation in the recurrence of central giant cell granulomas of the jaws. J. Craniomaxillofac. Surg. 1996, 24, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Ardekian, L.; Manor, R.; Peled, M.; Laufer, D. Bilateral central giant cell granulomas in a patient with neurofibromatosis: Report of a case and review of the literature. J. Oral Maxillofac. Surg. 1999, 57, 869–872. [Google Scholar] [CrossRef]
- Chuong, R.; Kaban, L.B.; Kozakewich, H.; Perez-Atayde, A. Central giant cell lesions of the jaws: A clinicopathologic study. J. Oral Maxillofac. Surg. 1986, 44, 708–713. [Google Scholar] [CrossRef]
- Body, J.J.; Jortay, A.M.; de Jager, R.; Ardichvili, D. Treatment with steroids of a giant cell granuloma of the maxilla. J. Surg. Oncol. 1981, 16, 7–13. [Google Scholar] [CrossRef]
- Terry, B.C.; Jacoway, J.R. Management of central giant cell lesions: An alternative to surgical therapy. Oral Maxillofac. Surg. Clin. N. Am. 1994, 6, 579–600. [Google Scholar] [CrossRef]
- Abdo, E.N.; Ahes, C.; Rodrigues, A.S.; Mesąuita, R.A.; Gomez, R.S. Treatment of a central giant cell granuloma with intralesional corticosteroid. Br. J. Oral Maxillofac. Surg. 2005, 43, 74–76. [Google Scholar] [CrossRef]
- Comert, E.; Turanli, M.; Ulu, S. Oral and intrale sional steroid therapy in giant cell granuloma. Acta Otolaryngol. 2006, 126, 664–666. [Google Scholar] [CrossRef]
- Khafif, A.; Krempl, G.; Medina, J.E. Treatment of giant cell granuloma of the maxilla with intralesional injection of steroids. Head Neck 2000, 22, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Kaban, L.B.; Dodson, T.B. Calcitonin therapy for central giant cell granuloma. J. Oral Maxillofac. Surg. 2003, 61, 653–654. [Google Scholar] [CrossRef]
- Suárez-Roa Mde, L.; Reveiz, L.; Ruíz-Godoy Rivera, L.M.; Asbun-Bojalil, J.; Dávila-Serapio, J.E.; Menjívar-Rubio, A.H.; Meneses-García, A. Interventions for central giant cell granuloma (CGCG) of the jaws. Cochrane Database Syst. Rev. 2009, 7, CD007404. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Stanbouly, D.; Litman, E.; Lee, K.C.; Philipone, E. Central giant cell granuloma of the head & neck: A case report and systematic review. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e161–e168. [Google Scholar] [CrossRef]
- Graham, R.M.; Foster, M.E.; Richardson, D. An Unusual Presentation of a Central Giant Cell Granuloma and Initial Treatment with Intralesional Steroids—A Case Report and Review of the Literature. J. Oral Health Comm. Dent. 2008, 2, 65–69. [Google Scholar] [CrossRef]
- Becelli, R.; Cerulli, G.; Gasparini, G. Surgical and implantation reconstruction in a patient with giant-cell reparative granuloma. J. Craniofac. Surg. 1998, 9, 45–47. [Google Scholar] [CrossRef]



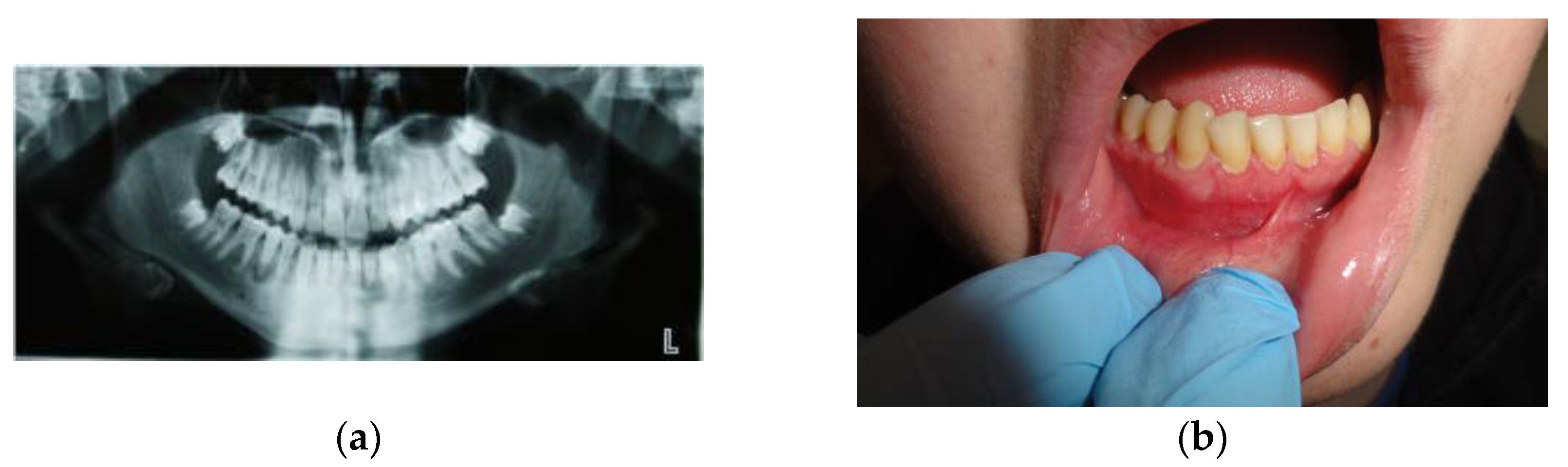
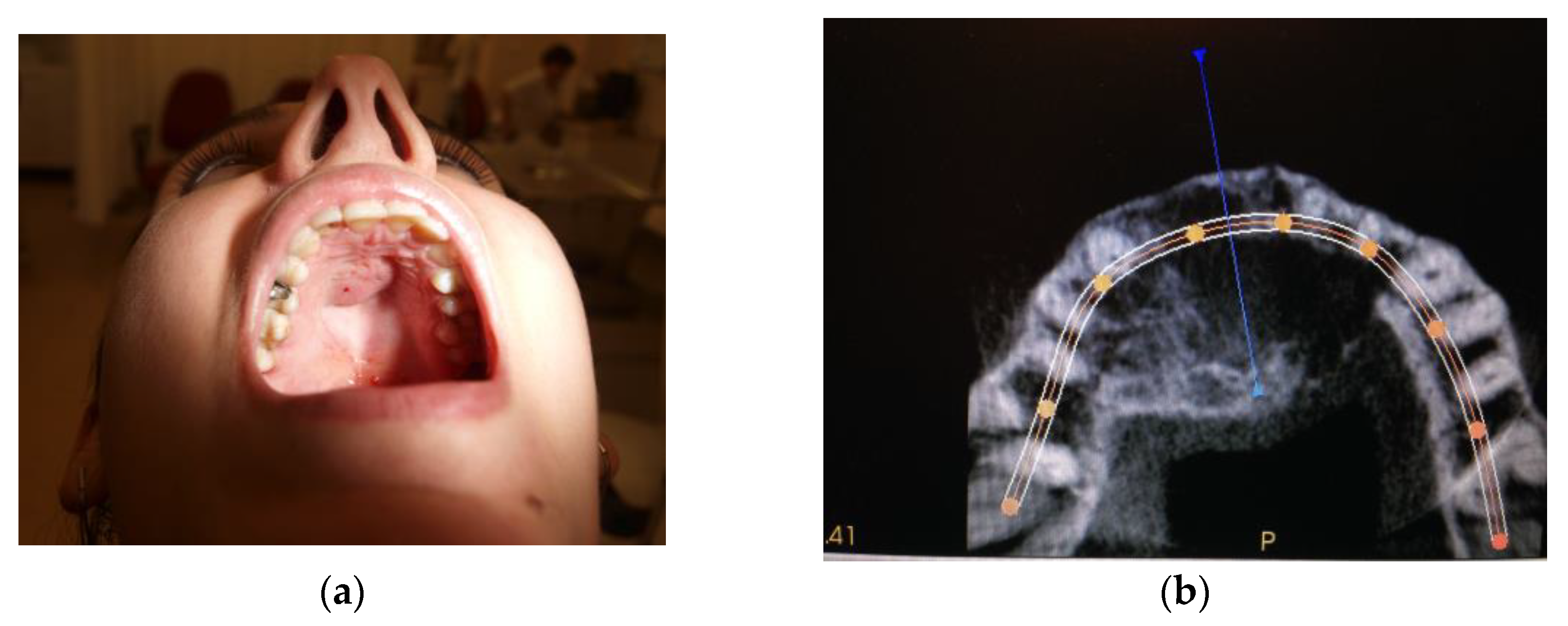

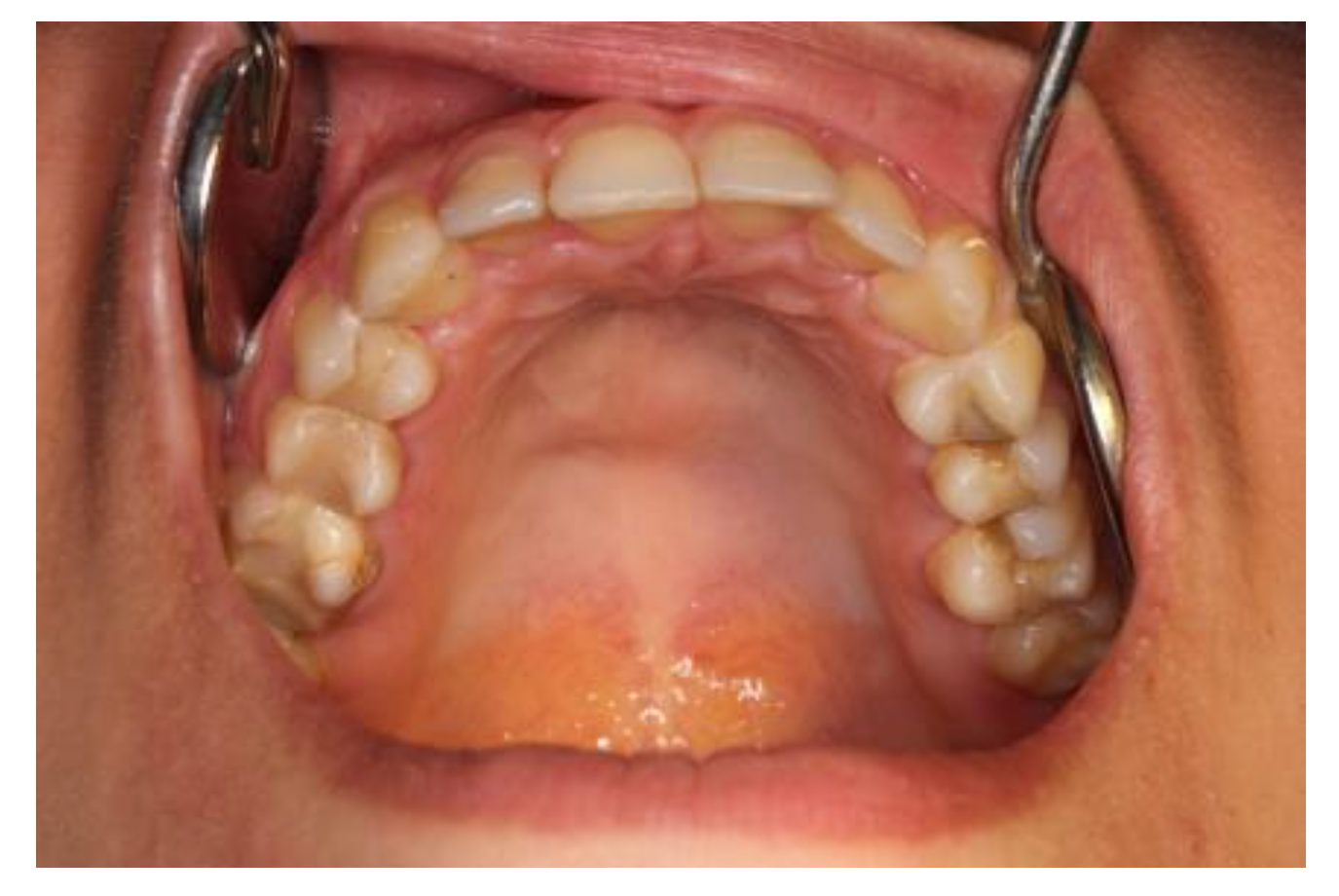
| Case # | Age | Sex | Injections’ Frequency and Doses of the Drug | Tumor Location | Final Histopathological Verification | Treatment Effects | Follow-Up |
|---|---|---|---|---|---|---|---|
| Case #1 | 14 y.o. | Male | Dexamethasone for 3 months 12 mg/3 mL every week and then 8 mg/12 mL every 2 weeks for the next 3 months | Mandible | Yes | Success | 9 years |
| Case #2 | 29 y.o. | Female | Dexamethasone for 4 months 12 mg/3 mL every week and then 8 mg/12 mL every 2 weeks for the next 3 months | Maxilla | Yes | Success | 8 years |
| Case #3 | 18 y.o. | Female | Dexamethasone for 3 months—84 mg/21 mL at 2-day intervals | Mandible | Yes | Surgical treatment was necessary | 5 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedzielska, I.; Bielecki, M.; Bąk, M.; Dziuk, B.; Niedzielski, D. Bony Canal Method of Dexamethasone Injections in Aggressive Form of Central Giant Cell Granuloma—Case Series. Medicina 2023, 59, 250. https://doi.org/10.3390/medicina59020250
Niedzielska I, Bielecki M, Bąk M, Dziuk B, Niedzielski D. Bony Canal Method of Dexamethasone Injections in Aggressive Form of Central Giant Cell Granuloma—Case Series. Medicina. 2023; 59(2):250. https://doi.org/10.3390/medicina59020250
Chicago/Turabian StyleNiedzielska, Iwona, Mateusz Bielecki, Michał Bąk, Barbara Dziuk, and Damian Niedzielski. 2023. "Bony Canal Method of Dexamethasone Injections in Aggressive Form of Central Giant Cell Granuloma—Case Series" Medicina 59, no. 2: 250. https://doi.org/10.3390/medicina59020250
APA StyleNiedzielska, I., Bielecki, M., Bąk, M., Dziuk, B., & Niedzielski, D. (2023). Bony Canal Method of Dexamethasone Injections in Aggressive Form of Central Giant Cell Granuloma—Case Series. Medicina, 59(2), 250. https://doi.org/10.3390/medicina59020250






