Impact of Innovative Treatment Using Biological Drugs for the Modulation of Diffuse Cutaneous Systemic Sclerosis: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Methodological Quality Assessment
2.4. Data Extraction
3. Results
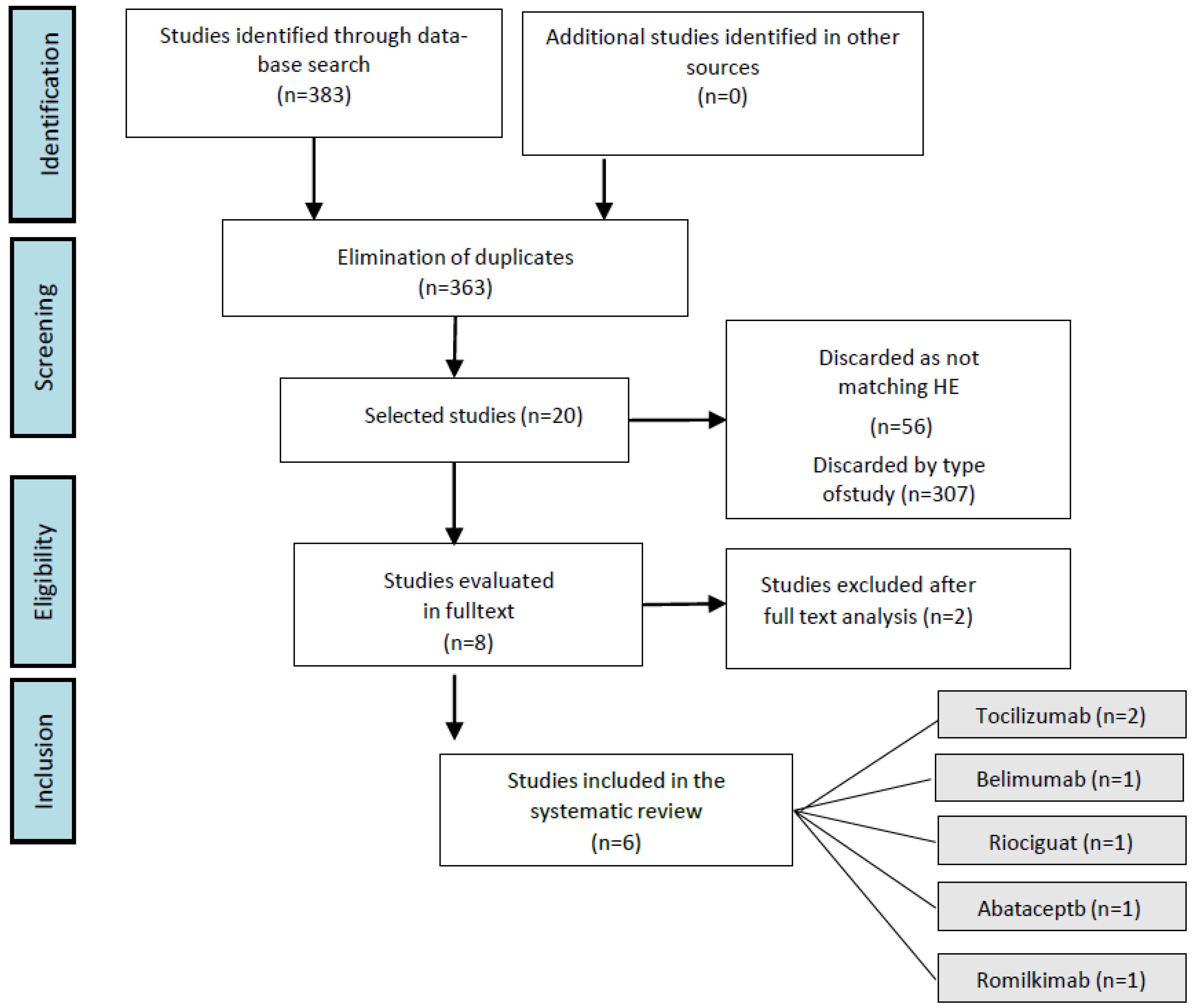
3.1. Selection of Studies
3.2. Methodological Quality Assessment
3.3. Characteristics of the Participants and Interventions
3.4. Outcome Evaluation
3.5. Measure for Skin Disease
3.6. Pulmonary Function Test
3.7. Health Status
3.8. Safety
4. Discussion
4.1. Skin Disease
4.2. Pulmonary Involvement
4.3. Health Status
4.4. Safety
4.5. Clinical Trials of Biological Drugs for the Modulation of Diffuse Cutaneous Systemic Sclerosis
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Sequence Followed for Selection of Articles
| Study | Item | Total | % | Quality Score | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||||
| Allanore et al. (2020) [30] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | 100 | E |
| Gordon et al. (2018) [31] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 11 | 68.7 | G |
| Khanna Dinesh et al. (2018) [32] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 14 | 87.5 | VG |
| Khanna et al. (2020) [33] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 15 | 93.7 | E |
| Khanna et al. (2020) [34] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 15 | 93.7 | E |
| Shima et al. (2019) [35] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 15 | 93.7 | E |
| Study | Item | Total | % | Quality Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||||
| Allanore et al. (2020) [30] | 11 | 100 | E | |||||||||||
| Gordon et al. (2018) [31] | 11 | 100 | E | |||||||||||
| Khanna Dinesh et al. (2018) [32] | 10 | 90.9 | VG | |||||||||||
| Khanna et al. (2020) [33] | 10 | 90.9 | VG | |||||||||||
| Khanna et al. (2020) [34] | 11 | 100 | E | |||||||||||
| Shima et al. (2019) [35] | 11 | 100 | E | |||||||||||
References
- Fett, N. Scleroderma: Nomenclature, etiology, pathogenesis, prognosis, and treatments: Facts and controversies. Clin. Dermatol. 2013, 31, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Suliman, S.; Al Harash, A.; Roberts, W.N.; Perez, R.L.; Roman, J. Scleroderma-related interstitial lung disease. Respir. Med. Case Rep. 2017, 22, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Pizzorni, C.; Riccieri, V.; Decuman, S.; Brusselle, G.; de Pauw, M.; Deschepper, E.; Piette, Y.; Ruaro, B.; Sulli, A.; et al. Stabilization of Microcirculation in Patients with Early Systemic Sclerosis with Diffuse Skin Involvement following Rituximab Treatment: An Open-label Study. J. Rheumatol. 2016, 43, 995–996. [Google Scholar] [CrossRef] [PubMed]
- Sulli, A.; Ruaro, B.; Cutolo, M. Evaluation of blood perfusion by laser speckle contrast analysis in different areas of hands and face in patients with systemic sclerosis. Ann. Rheum. Dis. 2014, 73, 2059–2061. [Google Scholar] [CrossRef] [PubMed]
- Carreira, P.E.; Martín-López, M.; Pablos Álvarez, J.L. Esclerodermia. Med.-Programa Form. 2017, 12, 1448–1457. [Google Scholar] [CrossRef]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the Modified Rodnan Skin Score for Use in Clinical Trials of Systemic Sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef]
- Jaovisidha, K.; Csuka, M.; Almagro, U.A.; Soergel, K.H. Severe gastrointestinal involvement in systemic sclerosis: Report of five cases and review of the literature. Semin. Arthritis Rheum. 2005, 34, 689–702. [Google Scholar] [CrossRef]
- DeMarco, P.J.; Weisman, M.H.; Seibold, J.R.; Furst, D.E.; Wong, W.K.; Hurwitz, E.L.; Mayes, M.; White, B.; Wigley, F.; Barr, W.; et al. Predictors and outcomes of scleroderma renal crisis: The High-Dose Versus Low-Dose D-Penicillamine in early diffuse systemic sclerosis trial. Arthritis Rheum. 2002, 46, 2983–2989. [Google Scholar] [CrossRef]
- Steen, V.D.; Medsger, T.A., Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000, 43, 2437–2444. [Google Scholar] [CrossRef]
- Kuwana, M.; Saito, A.; Sakamoto, W.; Raabe, C.; Saito, K. Incidence Rate and Prevalence of Systemic Sclerosis and Systemic Scle-rosis-Associated Interstitial Lung Disease in Japan: Analysis Using Japanese Claims Databases. Adv. Ther. 2022, 39, 2222–2235. [Google Scholar] [CrossRef]
- De Almeida Chaves, S.; Porel, T.; Mounié, M.; Alric, L.; Astudillo, L.; Huart, A.; Lairez, O.; Michaud, M.; Prévot, G.; Ribes, D.; et al. Sine scleroderma, limited cutaneous, and diffused cutaneous systemic sclerosis survival and predictors of mortality. Arthritis Res. Ther. 2021, 23, 295. [Google Scholar] [CrossRef]
- Freire, M.; Rivera, A.; Sopeña, B.; Vilella, C.T.; Castillo, A.G.-D.; Argüelles, D.C.; Rubio, J.L.C.; Rivas, M.R.; Martínez, L.T.; Parra, J.A.T.; et al. Clinical and epidemiological differences between men and women with systemic sclerosis: A study in a Spanish systemic sclerosis cohort and literature review. Ann. Rheum. Dis. 2017, 35, 89–97. [Google Scholar]
- Stevens, A.M.; Kanaan, S.B.; Torok, K.S.; Medsger, T.A.; Mayes, M.D.; Reveille, J.D.; Klein-Gitelman, M.; Reed, A.M.; Lee, T.; Li, S.C.; et al. Brief Report: HLA-DRB1, DQA1, and DQB1 in Juvenile-Onset Systemic Sclerosis. Arthritis Rheumatol. 2016, 68, 2772–2777. [Google Scholar] [CrossRef]
- Yang, C.; Tang, S.; Zhu, D.; Ding, Y.; Qiao, J. Classical Disease-Specific Autoantibodies in Systemic Sclerosis: Clinical Features, Gene Susceptibility, and Disease Stratification. Front. Med. 2020, 7, 587773. [Google Scholar] [CrossRef]
- Diaz-Gallo, L.; Gourh, P.; Broen, J.; Simeon, C.; Fonollosa, V.; Ortego-Centeno, N.; Agarwal, S.; Vonk, M.; Coenen, M.; Riemekasten, G.; et al. Analysis of the influence of PTPN22 gene polymorphisms in systemic sclerosis. Ann. Rheum. Dis. 2010, 70, 454–462. [Google Scholar] [CrossRef]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. Classification Criteria for Systemic Sclerosis: An ACR-EULAR Collaborative Initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- Utsunomiya, A.; Oyama, N.; Hasegawa, M. Potential Biomarkers in Systemic Sclerosis: A Literature Review and Update. J. Clin. Med. 2020, 9, 3388. [Google Scholar] [CrossRef]
- Rutka, K.; Garkowski, A.; Karaszewska, K.; Łebkowska, U. Imaging in Diagnosis of Systemic Sclerosis. J. Clin. Med. 2021, 10, 248. [Google Scholar] [CrossRef]
- Gigante, A.; Leodori, G.; Pellicano, C.; Villa, A.; Rosato, E. Assessment of kidney involvement in systemic sclerosis: From scleroderma renal crisis to subclinical renal vasculopathy. Am. J. Med. Sci. 2022, 364, 529–537. [Google Scholar] [CrossRef]
- McMahan, Z.H.; Volkmann, E.R. An update on the pharmacotherapeutic options and treatment strategies for systemic sclerosis. Expert Opin. Pharmacother. 2020, 21, 2041–2056. [Google Scholar] [CrossRef]
- Codina, A.F.; Walker, K.M.; Pope, J.E. The Scleroderma Algorithm Group Treatment Algorithms for Systemic Sclerosis According to Experts. Arthritis Rheumatol. 2018, 70, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Moriana, C.; Moulinet, T.; Jaussaud, R.; Decker, P. JAK inhibitors and systemic sclerosis: A systematic review of the literature. Autoimmun. Rev. 2022, 21, 103168. [Google Scholar] [CrossRef] [PubMed]
- AseBio. Innovative Biological Medicines and Biosimilars. Available online: https://www.asebio.com/areas-de-trabajo/salud/medicamentos-biologicos-biosimilares (accessed on 13 December 2022).
- European Medicines Agency. Information Guide for Healthcare Professionals Produced Jointly by the European Medicines Agency and the European Commission. Available online: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_es.pdf (accessed on 15 December 2022).
- Bruni, C.; Cuomo, G.; Rossi, F.W.; Praino, E.; Bellando-Randone, S. Kidney involvement in systemic sclerosis: From pathogenesis to treatment. J. Scleroderma Relat. Disord. 2018, 3, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Kowal-Bielecka, O.; Landewé, R.; Avouac, J.; Chwiesko, S.; Miniati, I.; Czirják, L.; Clements, P.; Denton, C.; Farge, D.; Fligelstone, K.; et al. EULAR recommendations for the treatment of systemic sclerosis: A report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann. Rheum. Dis. 2009, 68, 620–628. [Google Scholar] [CrossRef]
- Law, M.; Stewart, C.; Pollock, N.; Letts, L.; Bosch, J.; Westmorland, M. Guidelines for Critical Review of Qualitative Studies; McMaster University Occupational Therapy Evidence-Based Practice Research Group: Hamilton, ON, Canada, 1998; pp. 1–9. [Google Scholar]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Allanore, Y.; Wung, P.; Soubrane, C.; Esperet, C.; Marrache, F.; Bejuit, R.; Lahmar, A.; Khanna, D.; Denton, C.P. A randomised, double-blind, placebo-controlled, 24-week, phase II, proof-of-concept study of romilkimab (SAR156597) in early diffuse cutaneous systemic sclerosis. Ann. Rheum. Dis. 2020, 79, 1600–1607. [Google Scholar] [CrossRef]
- Gordon, J.K.; Martyanov, V.; Franks, J.M.; Bernstein, E.J.; Szymonifka, J.; Magro, C.; Wildman, H.F.; Wood, T.A.; Whitfield, M.L.; Spiera, R.F. Belimumab for the Treatment of Early Diffuse Systemic Sclerosis: Results of a Randomized, Double-Blind, Placebo-Controlled, Pilot Trial. Arthritis Rheumatol. 2018, 70, 308–316. [Google Scholar] [CrossRef]
- Khanna, D.; Denton, C.P.; Lin, C.J.F.; Van Laar, J.M.; Frech, T.M.; Anderson, M.E.; Baron, M.; Chung, L.; Fierlbeck, G.; Lakshminarayanan, S.; et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: Results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann. Rheum. Dis. 2017, 77, 212–220. [Google Scholar] [CrossRef]
- Khanna, D.; Spino, C.; Johnson, S.; Chung, L.; Whitfield, M.L.; Denton, C.P.; Berrocal, V.; Franks, J.; Mehta, B.; Molitor, J.; et al. Abatacept in Early Diffuse Cutaneous Systemic Sclerosis: Results of a Phase II Investigator-Initiated, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. Arthritis Rheumatol. 2020, 72, 125–136. [Google Scholar] [CrossRef]
- Khanna, D.; Allanore, Y.; Denton, C.P.; Kuwana, M.; Matucci-Cerinic, M.; Pope, J.E.; Atsumi, T.; Bečvář, R.; Czirják, L.; Hachulla, E.; et al. Riociguat in patients with early diffuse cutaneous systemic sclerosis (RISE-SSc): Randomised, double-blind, placebo-controlled multicentre trial. Ann. Rheum. Dis. 2020, 79, 618–625. [Google Scholar] [CrossRef]
- Shima, Y.; Kawaguchi, Y.; Kuwana, M. Add-on tocilizumab versus conventional treatment for systemic sclerosis, and cytokine analysis to identify an endotype to tocilizumab therapy. Mod. Rheumatol. 2018, 29, 134–139. [Google Scholar] [CrossRef]
- Foti, R.; De Pasquale, R.; Bosco, Y.D.; Visalli, E.; Amato, G.; Gangemi, P.; Foti, R.; Ramondetta, A. Clinical and Histopathological Features of Scleroderma-like Disorders: An Update. Medicina 2021, 57, 1275. [Google Scholar] [CrossRef]
- Fang, D.; Chen, B.; Lescoat, A.; Khanna, D.; Mu, R. Immune cell dysregulation as a mediator of fibrosis in systemic sclerosis. Nat. Rev. Rheumatol. 2022, 18, 683–693. [Google Scholar] [CrossRef]
- Shima, Y. Cytokines Involved in the Pathogenesis of SSc and Problems in the Development of Anti-Cytokine Therapy. Cells 2021, 10, 1104. [Google Scholar] [CrossRef]
- Denton, C.P.; Ong, V.H.; Xu, S.; Chen-Harris, H.; Modrusan, Z.; Lafyatis, R.; Khanna, D.; Jahreis, A.; Siegel, J.; Sornasse, T. Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: Insights from the faSScinate clinical trial in systemic sclerosis. Ann. Rheum. Dis. 2018, 77, 1362–1371. [Google Scholar] [CrossRef]
- Fairley, J.; Oon, S.; Saracino, A.; Nikpour, M. Management of cutaneous manifestations of lupus erythematosus: A systematic review. Semin. Arthritis Rheum. 2020, 50, 95–127. [Google Scholar] [CrossRef]
- Fuschiotti, P. Role of IL-13 in systemic sclerosis. Cytokine 2011, 56, 544–549. [Google Scholar] [CrossRef]
- Navarro, C. Pulmonary involvement in systemic sclerosis. Alveolitis, fibrosis and pulmonary arterial hypertension. Reumatol. Clínica 2006, 2, S16–S19. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Volkmann, E.R.; Hoffmann-Vold, A.M.; Maher, T. Current and future perspectives on management of systemic sclerosis-associated interstitial lung disease. Expert Rev. Clin. Immunol. 2019, 15, 1009–1017. [Google Scholar] [CrossRef]
- Erre, G.L.; Sebastiani, M.; Fenu, M.A.; Zinellu, A.; Floris, A.; Cavagna, L.; Renzoni, E.; Manfredi, A.; Passiu, G.; Woodman, R.J.; et al. Efficacy, Safety, and Tolerability of Treatments for Systemic Sclerosis-Related Interstitial Lung Disease: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2020, 9, 2560. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-N.; Yang, Q.-R.; Zhu, G.-Q.; Pan, L.; Xia, J.-X.; Wang, Q. Comparative efficacy and safety of immunosuppressive therapies for systemic sclerosis related interstitial lung disease: A Bayesian network analysis. Mod. Rheumatol. 2019, 30, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, A.; Cassone, G.; Furini, F.; Gremese, E.; Venerito, V.; Atzeni, F.; Arrigoni, E.; Della Casa, G.; Cerri, S.; Govoni, M.; et al. Tocilizumab therapy in rheumatoid arthritis with interstitial lung disease: A multicentre retrospective study. Intern. Med. J. 2019, 50, 1085–1090. [Google Scholar] [CrossRef]
- Allanore, Y.; Collaborators, O.B.O.T.E.; Bozzi, S.; Terlinden, A.; Huscher, D.; Amand, C.; Soubrane, C.; Siegert, E.; Czirják, L.; Carreira, P.E.; et al. Health Assessment Questionnaire-Disability Index (HAQ-DI) use in modelling disease progression in diffuse cutaneous systemic sclerosis: An analysis from the EUSTAR database. Thromb. Haemost. 2020, 22, 1–11. [Google Scholar] [CrossRef]
- Pope, J. Measures of systemic sclerosis (scleroderma): Health Assessment Questionnaire (HAQ) and Scleroderma HAQ (SHAQ), Physician- and Patient-Rated Global Assessments, Symptom Burden Index (SBI), University of California, Los Angeles, Scleroderma Clinical Trials. Arthritis Care Res. 2011, 63, S98–S111. [Google Scholar] [CrossRef]
- Johnson, S.R.; Hawker, G.A.; Davis, A.M. The health assessment questionnaire disability index and scleroderma health assessment questionnaire in scleroderma trials: An evaluation of their measurement properties. Arthritis Rheum. 2005, 53, 256–262. [Google Scholar] [CrossRef]
- Guerreiro Castro, S.; Isenberg, D.A. Belimumab in systemic lupus erythematosus (SLE): Evidence-to-date and clinical usefulness. Ther. Adv. Musculoskelet. Dis. 2017, 9, 75–85. [Google Scholar] [CrossRef]
- ClinicalTrialsgov. Belimumab and Rituximab Combination Therapy for the Treatment of Diffuse Cutaneous Systemic Sclerosis. Available online: https://clinicaltrials.gov/ct2/show/NCT03844061 (accessed on 14 January 2023).
- Good Clinical Practice. Clinical trials on Systemic Scleroderma: Diffuse Scleroderma: Systemic Scleroderma: Riociguat: Riociguat (Adempas, BAY63-2521). Clinical Trials Registry. Available online: https://ichgcp.net/es/clinical-trials-registry/NCT02283762 (accessed on 15 January 2023).
- Chakravarty, E.F.; Martyanov, V.; Fiorentino, D.; Wood, T.A.; Haddon, D.J.; Jarrell, J.A.; Utz, P.J.; Genovese, M.C.; Whitfield, M.L.; Chung, L. Gene expression changes reflect clinical response in a placebo-controlled randomized trial of abatacept in patients with diffuse cutaneous systemic sclerosis. Thromb. Haemost. 2015, 17, 1–14. [Google Scholar] [CrossRef]
- European Medicines Agency. EU/3/19/2246. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3192246 (accessed on 15 January 2023).
| Involved Organ(s) | Clinical Signs | Prevalence | Symptomatology | |
|---|---|---|---|---|
| Vascular [4,5] | Raynaud’s phenomenon | 100% | Changes in skin coloration | |
| Digital ulcers | 41.6% | Hard-to-heal lesions → infection, osteomyelitis, gangrene | ||
| Cutaneous [6] | Dermal hardening | 100% | ||
| Pigmentation alterations | Patchy areas of depigmentation. | |||
| Calcinosis cutis | Subcutaneous nodules Pain | |||
| Musculoskeletal [5] | Fibrosis | 46–97% | Contractures Morning stiffness | |
| Arthritis | Arthralgias | |||
| Gastrointestinal [7] | Esophageal hypomotility | 90% | Dysphagia | |
| Gastroesophageal reflux | Bacterial overgrowth | |||
| Stomach hypomotility | Delayed emptying → Premature satiety, fullness, bloating, nausea | |||
| Intestinal hypomotility | Chronic intestinal pseudo-obstruction Malabsorption | |||
| Anorectal dysfunction | Incontinence | |||
| Renal [8] | Renal scleroderma crisis | 10% | Malignant high blood pressure Angiopathic hemolytic anemia Thrombocytopenia Proteinuria Macrohematuria Acute renal failure | |
| Cardiac [9] | Arrhythmias | Ventricular | 90% | Fatigue Palpitations Syncope Dizziness |
| Supraventricular | 66% | |||
| Ventricular multiform premature beats | 40% | |||
| Left bundle branch block | 16% | |||
| First-degree atrioventricular blocks | 8% | |||
| Pericardial conditions | Pericardial effusion | 78% | Chest pain Dyspnea Fever | |
| Pericarditis | 77.5% | |||
| Myocardial dysfunction | RV dysfunction | 69% | Fatigue Calf edema Dyspnea Venous congestion | |
| LV dysfunction | 46% | |||
| Cardiac failure | 20–25% | |||
| Valvular dysfunction | Mitral valves prolapse | 20% | Dyspnea Pain Edema Palpitations Fatigue | |
| Pulmonary [2] | Pulmonary interstitial disease | 40% | Dyspnea Dry cough Crackles Pulmonary arterial hypertension Cor pulmonale | |
| Pulmonary arterial hypertension | 15–20% | RV hypertrophy ↓ Cardiac output Heart failure | ||
| Items | Sub-Item(s) | Weight/Score |
|---|---|---|
| Skin thickening of the fingers of both hands extending proximal to the metacarpophalangeal joints (sufficient criterion) | 9 | |
| Skin thickening of the fingers (only count the higher score) | Puffy fingers | 2 |
| Sclerodactyly of the fingers (distal to the metacarpophalangeal joints but proximal to the interphalangeal joints) | 4 | |
| Fingertip lesions (only count the higher score) | Digital tip ulcers | 2 |
| Fingertip pitting scars | 3 | |
| Telangiectasia | 2 | |
| Abnormal nailfold capillaries | 2 | |
| Pulmonary arterial hypertension and/or interstitial lung disease (maximum score is 2) | Pulmonary arterial hypertension | 2 |
| Interstitial lung disease | 2 | |
| Raynaud’s phenomenon | 2 | |
| SSc-related autoantibodies (anticentromere, anti-topoisomerase I [anti–Scl-70], anti-RNA polymerase III) (maximum score is 3) | Anticentromere | 3 |
| Anti–topoisomerase I | 3 | |
| Anti–RNA polymerase III | 3 |
| Affectation | General Measures | 1st Line Treatment | 2nd Line Treatment | 3rd Line Treatment | New Therapeutic Tools | |
|---|---|---|---|---|---|---|
| Peripheral vascular [4,5] | Avoid exposure to cold, sudden changes in temperature, stress, smoking, vasospastic substances. | Calcium channel blockers | Phosphodiesterase-5 inhibitors | If severe RP: Prostanoids | Prostacyclins and prostaglandins (Iloprost, Treprostanil) Statins Topical agents Biologic drugs (Riociguat, Bosentan) Botulinum toxin | |
| If mild RP: Calcium channel blockers or angiotensin receptor blockers. Skin involvement | ||||||
| Skin [5] | mRSS ≤ 32 | Avoid friction and trauma | Methotrexate | Mycophenolate mofetil | Pirfenidone Nintendanib Rituximab | |
| mRSS > 32 | Mycophenolate mofetil | Methotrexate | Cyclophosphamides | Hematopoietic cell transplantation Pirfenidone Nintendanib Rituximab | ||
| DU prevention | Calcium channel blockers | Phosphodiesterase-5 inhibitors | Bosentan Prostaglandins | |||
| DU treatment | Calcium channel blockers | Phosphodiesterase-5 inhibitors | Prostanoids | |||
| Musculoskeletal [5] | Moderate exercise and physiotherapy | Methotrexate | Low dose Corticosteroid | Hydroxychloroquines | Rituximab Tocilizumab Abatacept | |
| Gastrointestinal [7] | ↓ Motility | Prevent malnutrition | Proton pump inhibitors | Agents that high motility | Surgical treatment | |
| Bacterial overgrowth | Antibiotherapy | |||||
| Renal [25] | Angiotensin-converting enzyme inhibitors | Calcium channel blockers Aldosterone II receptor antagonists | Renal replacement therapy Dialysis | |||
| Renal Crisis [25] | Renal replacement therapy Dialysis | |||||
| Cardiac [9] | Arrhythmias | Verapamil Amiodarone | Ablation Pacemaker | |||
| Pericardial involvement | Anti-inflammatory drugs Corticosteroids | Pericardial drainage Pericardiocentesis | ||||
| Myocardial dysfunction | Angiotensin-converting enzyme inhibitors Beta-blockers Diuretics | Cardiac resynchronization | ||||
| Valvular dysfunction | Cardiac transplantation Transplantation of affected valves | |||||
| Pulmonary [2] | Mycophenolate mofetil | Lung transplantation | Rituximab Tocilizumab | |||
| Drug | Function | Mechanism |
|---|---|---|
| Tocilizumab | Immunosuppressant | It binds to soluble and membrane-bound IL-6 receptors and inhibits IL-6-mediated signaling |
| Belimumab | Selective immunosuppressant | IgG1 monoclonal antibody that specifically binds to the soluble form of human B-cell-stimulating protein |
| Riociguat | Anti-hypertension pulmonary | Stimulator of the soluble guanylyl cyclase |
| Abatacept | Selective immunosuppressant | Selectively modulates a key co-stimulatory signal for the full activation of T lymphocytes expressing CD28 |
| Romilkimab | Selective immunosuppressant | Biospecific antibody to IgG4 which neutralizes IL-4 and IL-13 |
| First Author, Year of Publication, Country, and Drug | Study Design | Participants (Baseline Sample Size, Age, Sex, Withdrawals, and Final Group Sample Size) | Intervention | Outcomes | Results |
|---|---|---|---|---|---|
| Allanore et al., 2020 France [30] Romilkimab | Phase II, randomized, double-blind, placebo-controlled clinical study | 20 ♂ and 77 ♀ >18 years Study withdrawals: 10 no = 48 Romilkimab no = 49 PBO | 200 mg Romilkimab or PBO SC/week for 24 weeks. | mRSS FVC% DLCO% HAQ-DI | GI vs. GC: ↓* mRSS ↑ FVC % ↑ DLCO % ↓HAQ-DI score Changes from baseline: ↓* mRSS ↓ FVC % ↓ DLCO % ↓HAQ-DI score |
| Gordon et al., 2018 USA [31] Belimumab | Randomized, double-blind, placebo-controlled pilot study | n = 20 >18 years Study withdrawals: 2 no = 7 Belimumab + MMF no = 10 PBO + MMF n = 6 Belimumab + MMF n = 9 PBO + MMF | 10 mg/kg Belimumab or PBO c/2 weeks first 3 doses and c/4 weeks until week 48 + 1000 mg 2 times/day MMF 48 weeks | mRSS FCV% DLCO% | GI vs. GC: ↓ mRSS ↑ FVC % ↑ DLCO % Changes from baseline: ↓* mRSS ↑* FVC % ↑* DLCO % |
| Khanna et al., 2018 USA [32] Tocilizumab | Phase II, randomized, double-blind, placebo-controlled clinical trial | 20 ♂ and 67 ♀ >18 years Study withdrawals: 36 no = 44 PBO no = 43 TCZ no = 31 PBO-TCZ no = 30 TCZ-TCZ n = 24 PBO-TCZ n = 27 TCZ-TCZ | 162 mg TCZ sc 48 weeks double blind + 162 mg TCZ sc 48 weeks open period | total Mrss HAQ-DI score FCV% DLCO % | GI vs. GC: ↓ total mRSS ↔ HAQ-DI score ↓ FVC% ↓ DLCO % Changes from baseline: ↓ total mRSS ↓ HAQ-DI score ↓ FVC % ↓ DLCO % |
| Khanna et al., 2020 USA [33] Abatacept | Phase II, randomized, double-blind, placebo-controlled clinical study | 22 ♂ and 66 ♀ >18 years Study withdrawals: 12 no = 44 Abatacept no = 44 PBO n = 35 Abatacept n = 34 PBO | 125 mg Abatacept or PBO SC 1 times/week 12 months | mRSS FVC% HAQ-DI | GI vs. GC: ↓ mRSS ↑ FVC % ↓HAQ-DI score Changes from baseline: ↓ mRSS ↓ FVC % ↓ HAQ-DI score |
| Khanna et al., 2020 USA [34] Riociguat | Phase II, randomized, double-blind, placebo-controlled, international, multicenter clinical trial | 29 ♂ and 92 ♀ >18 years Study withdrawals: 33 no = 60 Riociguat no = 61 PBO n = 42 Riociguat n = 46 PBO | 0.5–2.5 mg Riociguat 3 times/day 52 weeks | mRSS FVC% DLCO% HAQ-DI | GI vs. GC: ↓ mRSS ↑ FVC % ↑ DLCO % ↓HAQ-DI score Changes from baseline: ↓ mRSS ↓ FVC % ↓ DLCO % ↑ HAQ-DI score |
| Shima et al., 2018 Japan [35] Tocilizumab | Randomized, open-label clinical trial | 10 ♂ and 3 ♀ 20–65 years Study withdrawals: 0 n = 7 TZC + conventional treatment n = 6 conventional treatment | 8 mg/kg/month TCZ 6 months | DLCO % mRSS | GI vs. GC: ↑DLCO % ↓ mRSS Changes from baseline: ↓ DLCO % ↓ mRSS |
| Adverse Effects | Biological Drugs | ||||
|---|---|---|---|---|---|
| Tocilizumab | Belimumab | Riociguat | Abatacept | Romilkimab | |
| Infections | 8 | 1 | |||
| Cardiac | 1 | 1 | |||
| Gastrointestinal | 1 | ||||
| Respiratory | 1 | ||||
| Renal | |||||
| Vascular | 1 | ||||
| Neoplasms | 1 | ||||
| Musculoskeletal | |||||
| Nervous | |||||
| Endocrinologists | |||||
| Psychiatric | 1 | ||||
| Reproductive | |||||
| Blood and Lymphatics | 1 | ||||
| Dermatological | |||||
| Deaths | 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lázaro, D.; Iglesias-Lázaro, M.; Garrosa, E.; Rodríguez-García, S.; Jerves Donoso, D.; Gutiérrez-Abejón, E.; Jorge-Finnigan, C. Impact of Innovative Treatment Using Biological Drugs for the Modulation of Diffuse Cutaneous Systemic Sclerosis: A Systematic Review. Medicina 2023, 59, 247. https://doi.org/10.3390/medicina59020247
Fernández-Lázaro D, Iglesias-Lázaro M, Garrosa E, Rodríguez-García S, Jerves Donoso D, Gutiérrez-Abejón E, Jorge-Finnigan C. Impact of Innovative Treatment Using Biological Drugs for the Modulation of Diffuse Cutaneous Systemic Sclerosis: A Systematic Review. Medicina. 2023; 59(2):247. https://doi.org/10.3390/medicina59020247
Chicago/Turabian StyleFernández-Lázaro, Diego, María Iglesias-Lázaro, Evelina Garrosa, Saray Rodríguez-García, David Jerves Donoso, Eduardo Gutiérrez-Abejón, and Conrado Jorge-Finnigan. 2023. "Impact of Innovative Treatment Using Biological Drugs for the Modulation of Diffuse Cutaneous Systemic Sclerosis: A Systematic Review" Medicina 59, no. 2: 247. https://doi.org/10.3390/medicina59020247
APA StyleFernández-Lázaro, D., Iglesias-Lázaro, M., Garrosa, E., Rodríguez-García, S., Jerves Donoso, D., Gutiérrez-Abejón, E., & Jorge-Finnigan, C. (2023). Impact of Innovative Treatment Using Biological Drugs for the Modulation of Diffuse Cutaneous Systemic Sclerosis: A Systematic Review. Medicina, 59(2), 247. https://doi.org/10.3390/medicina59020247








