Laser-Induced Porcine Model of Experimental Retinal Vein Occlusion: An Optimized Reproducible Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Housing and Anesthesia
2.2. Experimental Retinal Vein Occlusion
2.2.1. Experimental Branch Retinal Vein Occlusion
2.2.2. Experimental Central Retinal Vein Occlusion
2.3. Imaging
2.3.1. Funduscopic Photography
2.3.2. Fluorescein Angiography
2.3.3. Optical Coherence Tomography
2.4. Sample Collection
2.5. Sample Preparation for Proteomic Analysis
2.6. Western Blotting
2.7. Immunohistochemistry
2.7.1. Fixation Using Formalin
2.7.2. Fixation Using Clarke’s Fixative
2.8. Targeted Mass Spectrometry by Selected Reaction Monitoring (SRM)
3. Results
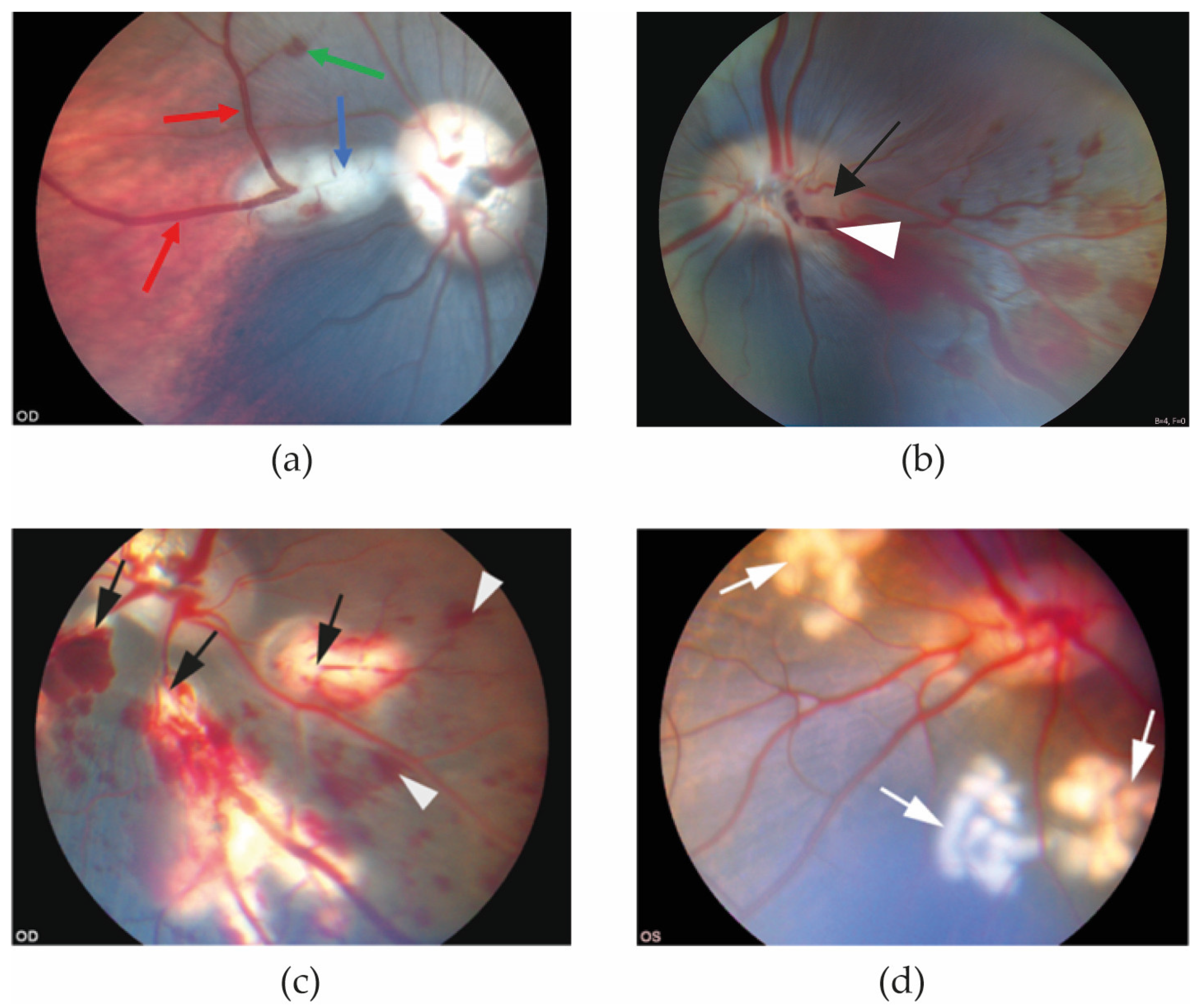
3.1. Funduscopic Photography
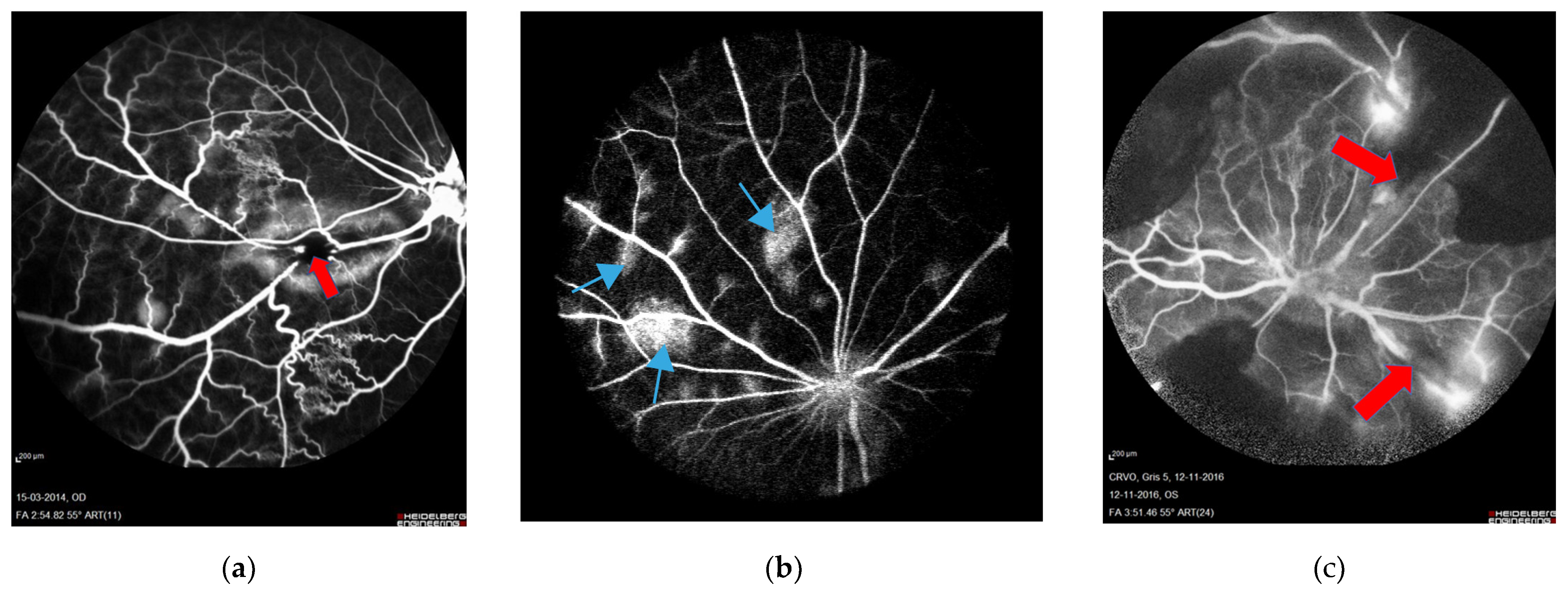
3.2. Fluorescein Angiography
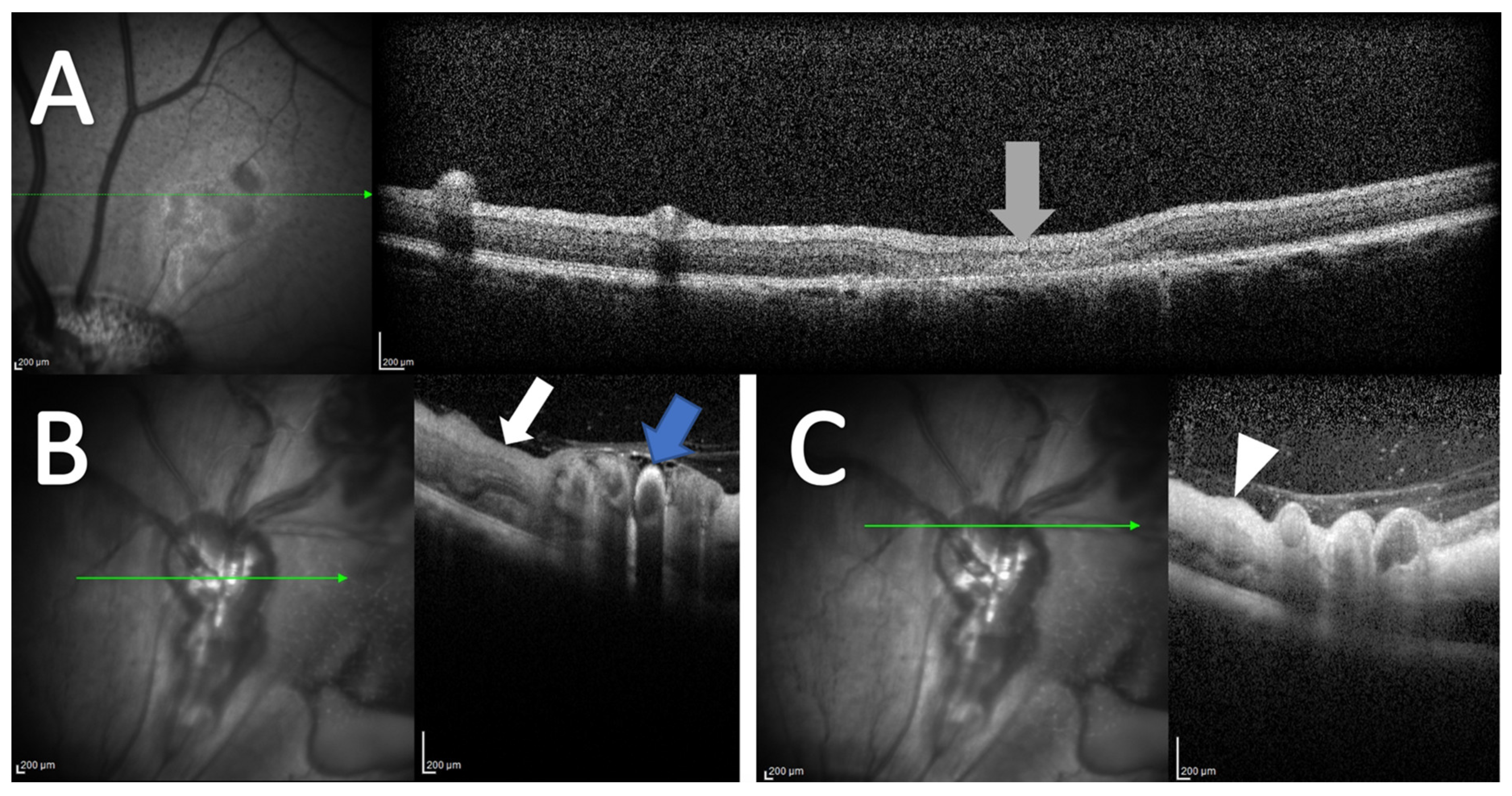
3.3. Optical Coherence Tomography
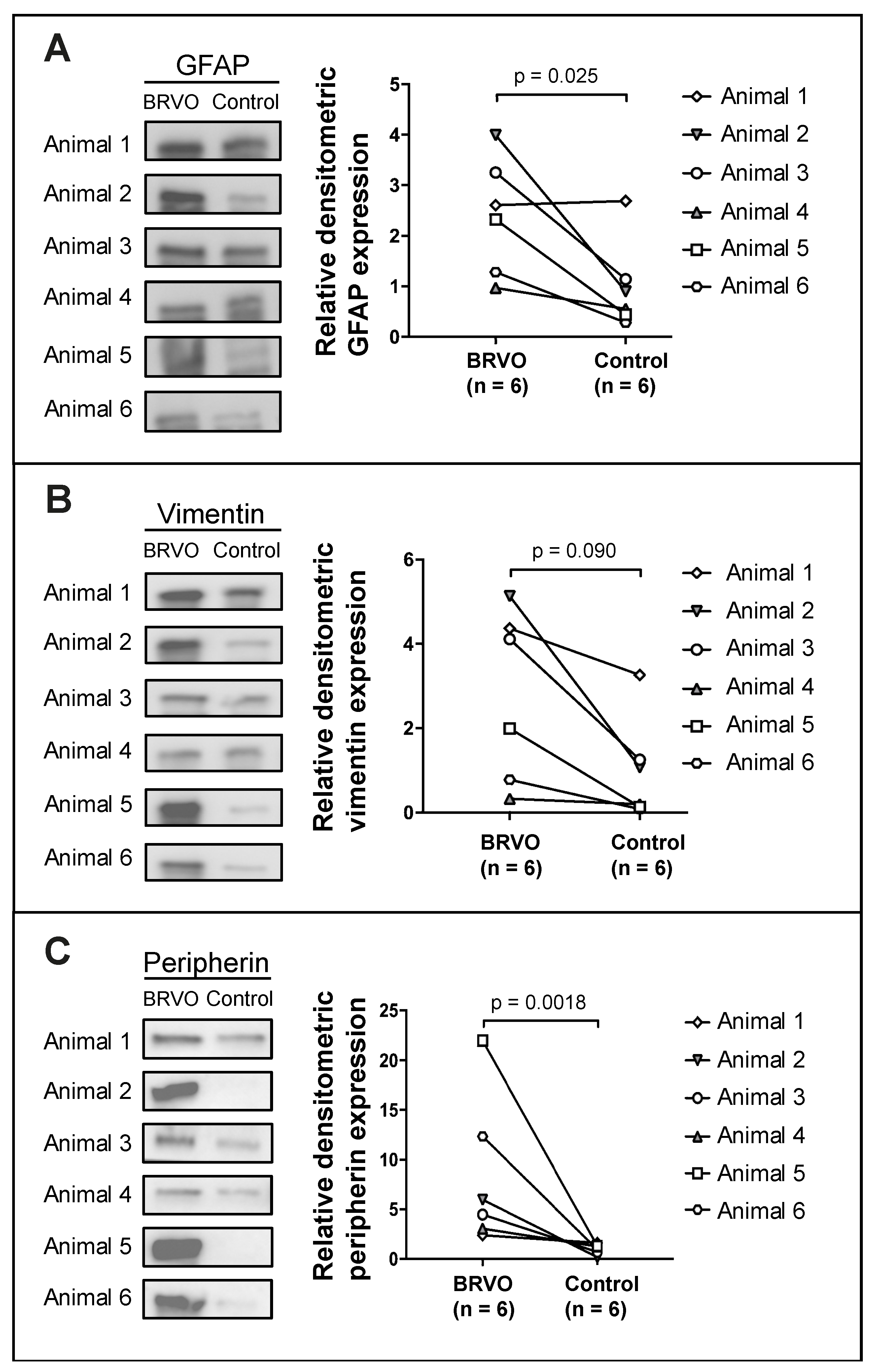
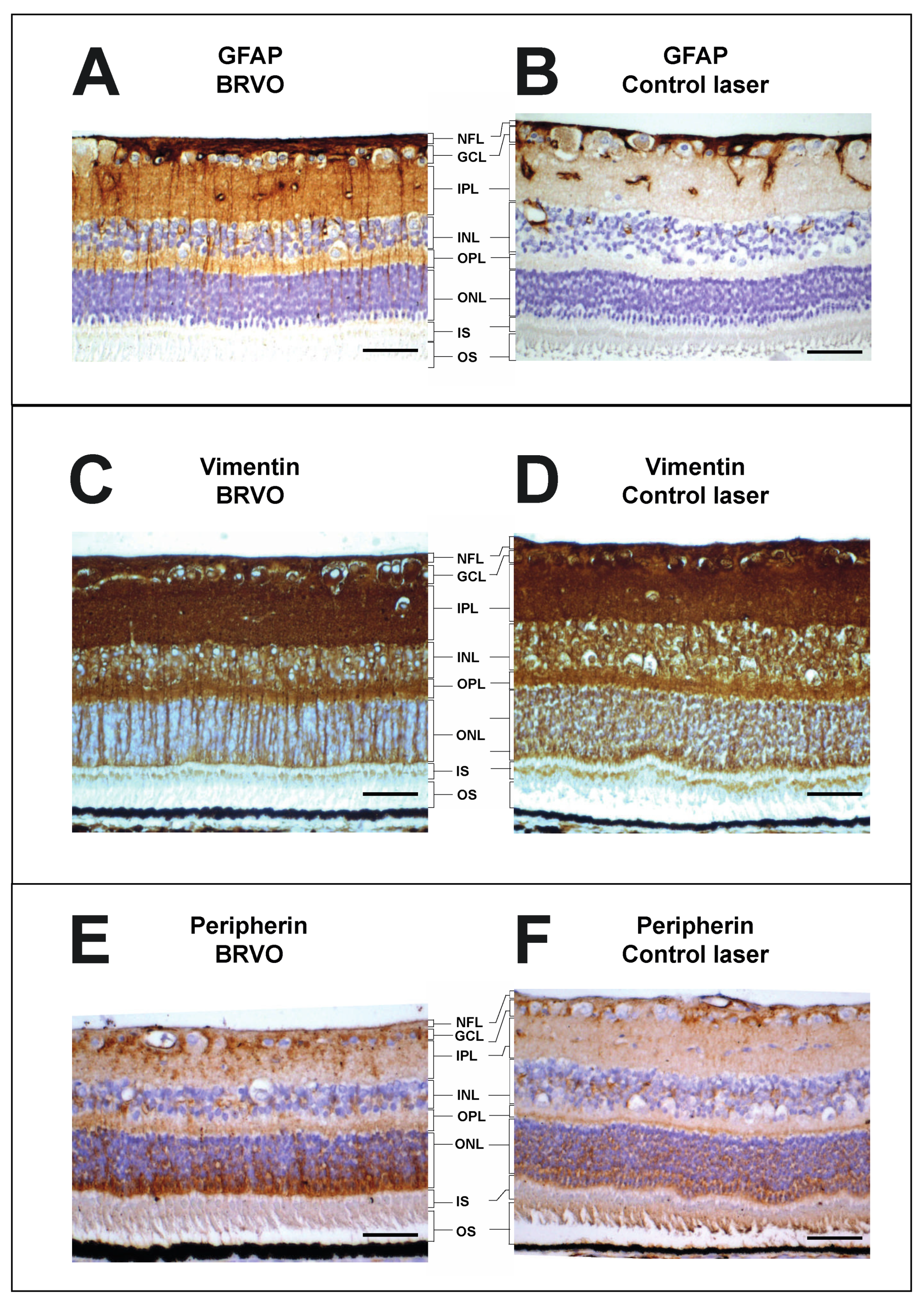
3.4. Assessment of Retinal Stress through Characterization of Retinal Müller Cell Proteins
3.5. Proteomics
4. Discussion
4.1. Strengths of Experimental RVO in the Porcine Eye
4.2. Limitations of Experimental RVO in the Porcine Eye
4.3. Alternative Models of Experimental RVO
4.4. Clinical Future Perspectives of Porcine RVO Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, S.L.; McIntosh, R.L.; Lim, L.; Mitchell, P.; Cheung, N.; Kowalski, J.W.; Nguyen, H.P.; Wang, J.J.; Wong, T.Y. Natural history of branch retinal vein occlusion: An evidence-based systematic review. Ophthalmology 2010, 117, 1094–1101.e5. [Google Scholar] [CrossRef]
- McIntosh, R.L.; Rogers, S.L.; Lim, L.; Cheung, N.; Wang, J.J.; Mitchell, P.; Kowalski, J.W.; Nguyen, H.P.; Wong, T.Y. Natural history of central retinal vein occlusion: An evidence-based systematic review. Ophthalmology 2010, 117, 1113–1123.e15. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B. Branch retinal vein occlusion: Natural history of visual outcome. JAMA Ophthalmol. 2014, 132, 13–22. [Google Scholar] [CrossRef]
- Bertelsen, M.; Linneberg, A.; Rosenberg, T.; Christoffersen, N.; Vorum, H.; Gade, E.; Larsen, M. Comorbidity in patients with branch retinal vein occlusion: Case-control study. BMJ Br. Med. J. 2012, 345, e7885. [Google Scholar] [CrossRef]
- Newman-Casey, P.A.; Stem, M.; Talwar, N.; Musch, D.C.; Besirli, C.G.; Stein, J.D. Risk factors associated with developing branch retinal vein occlusion among enrollees in a United States managed care plan. Ophthalmology 2014, 121, 1939–1948. [Google Scholar] [CrossRef]
- Buehl, W.; Sacu, S.; Schmidt-Erfurth, U. Retinal vein occlusions. Dev. Ophthalmol. 2010, 46, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.; Talks, S.J.; Amoaku, W.; Talks, K.; Sivaprasad, S. Retinal vein occlusion (RVO) guideline: Executive summary. Eye 2022, 36, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Sophie, R.; Pearlman, J.; Brown, D.M.; Boyer, D.S.; Heier, J.S.; Marcus, D.M.; Feiner, L.; Patel, A. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: The RETAIN study. Ophthalmology 2014, 121, 209–219. [Google Scholar] [CrossRef]
- Callizo, J.; Ziemssen, F.; Bertelmann, T.; Feltgen, N.; Voegeler, J.; Koch, M.; Eter, N.; Liakopoulos, S.; Schmitz-Valckenberg, S.; Spital, G. Real-World Data: Ranibizumab Treatment For Retinal Vein Occlusion In The OCEAN Study. Clin. Ophthalmol. 2019, 13, 2167–2179. [Google Scholar] [CrossRef]
- Hogg, H.D.J.; Talks, S.J.; Pearce, M.; di Simplicio, S. Real-World Visual and Neovascularisation Outcomes from anti-VEGF in Central Retinal Vein Occlusion. Ophthalmic Epidemiol. 2021, 28, 70–76. [Google Scholar] [CrossRef]
- Ang, J.L.; Ah-Moye, S.; Kim, L.N.; Nguyen, V.; Hunt, A.; Barthelmes, D.; Gillies, M.C.; Mehta, H. A systematic review of real-world evidence of the management of macular oedema secondary to branch retinal vein occlusion. Eye 2020, 34, 1770–1796. [Google Scholar] [CrossRef]
- Frederiksen, K.H.; Stokholm, L.; Frederiksen, P.H.; Jørgensen, C.M.; Möller, S.; Kawasaki, R.; Peto, T.; Grauslund, J. Cardiovascular morbidity and all-cause mortality in patients with retinal vein occlusion: A Danish nationwide cohort study. Br. J. Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Martel, A.; Nahon-Esteve, S.; Martini, K.; Almairac, F.; Baillif, S. Feelings, preoperative anxiety, and need for information in patients undergoing intravitreal injections. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Senra, H.; Balaskas, K.; Mahmoodi, N.; Aslam, T. Experience of Anti-VEGF Treatment and Clinical Levels of Depression and Anxiety in Patients With Wet Age-Related Macular Degeneration. Am. J. Ophthalmol. 2017, 177, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.; Vukicevic, M.; Koklanis, K.; Itsiopoulos, C. Experiences of patients undergoing anti-VEGF treatment for neovascular age-related macular degeneration: A systematic review. Psychol. Health Med. 2015, 20, 296–310. [Google Scholar] [CrossRef]
- Cehofski, L.J.; Mandal, N.; Honoré, B.; Vorum, H. Analytical platforms in vitreoretinal proteomics. Bioanalysis 2014, 6, 3051–3066. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Yasuda, K.; Mimura, T.; Suganuma, N.; Shimura, M. Retinal Microcirculation and Cytokines as Predictors for Recurrence of Macular Edema after Intravitreal Ranibizumab Injection in Branch Retinal Vein Occlusion. J. Clin. Med. 2020, 10, 58. [Google Scholar] [CrossRef]
- Noma, H.; Yasuda, K.; Shimura, M. Change of cytokines after intravitreal ranibizumab in patients with recurrent branch retinal vein occlusion and macular edema. Eur. J. Ophthalmol. 2021, 31, 204–210. [Google Scholar] [CrossRef]
- Noma, H.; Yasuda, K.; Mimura, T.; Ofusa, A.; Shimura, M. Relationship between retinal blood flow and cytokines in central retinal vein occlusion. BMC Ophthalmol. 2020, 20, 215. [Google Scholar] [CrossRef]
- Noma, H.; Yasuda, K.; Shimura, M. Cytokines and the Pathogenesis of Macular Edema in Branch Retinal Vein Occlusion. J. Ophthalmol. 2019, 2019, 5185128. [Google Scholar] [CrossRef]
- Noma, H.; Yasuda, K.; Shimura, M. Cytokines and Pathogenesis of Central Retinal Vein Occlusion. J. Clin. Med. 2020, 9, 3457. [Google Scholar] [CrossRef] [PubMed]
- Cehofski, L.J.; Honoré, B.; Vorum, H. A Review: Proteomics in Retinal Artery Occlusion, Retinal Vein Occlusion, Diabetic Retinopathy and Acquired Macular Disorders. Int. J. Mol. Sci. 2017, 18, 907. [Google Scholar] [CrossRef] [PubMed]
- Cehofski, L.J.; Kojima, K.; Terao, N.; Kitazawa, K.; Thineshkumar, S.; Grauslund, J.; Vorum, H.; Honoré, B. Aqueous Fibronectin Correlates With Severity of Macular Edema and Visual Acuity in Patients With Branch Retinal Vein Occlusion: A Proteome Study. Investig. Ophthalmol. Vis. Sci. 2020, 61, 6. [Google Scholar] [CrossRef]
- Hansen, M.S.; Rasmussen, M.; Grauslund, J.; Subhi, Y.; Cehofski, L.J. Proteomic analysis of vitreous humour of eyes with diabetic macular oedema: A systematic review. Acta Ophthalmol. 2022, 100, e1043–e1051. [Google Scholar] [CrossRef] [PubMed]
- Cehofski, L.J.; Kruse, A.; Alsing, A.N.; Sejergaard, B.F.; Nielsen, J.E.; Pedersen, S.; Muttuvelu, D.V.; Kirkeby, S.; Honoré, B.; Vorum, H. Aflibercept Intervention in Experimental Branch Retinal Vein Occlusion Results in Upregulation of DnaJ Homolog Subfamily C Member 17. J. Ophthalmol. 2021, 2021, 6690260. [Google Scholar] [CrossRef]
- Cehofski, L.J.; Kruse, A.; Alsing, A.N.; Sejergaard, B.F.; Nielsen, J.E.; Schlosser, A.; Sorensen, G.L.; Grauslund, J.; Honoré, B.; Vorum, H. Proteome Analysis of Aflibercept Intervention in Experimental Central Retinal Vein Occlusion. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2022, 27, 3360. [Google Scholar] [CrossRef]
- Khayat, M.; Lois, N.; Williams, M.; Stitt, A.W. Animal Models of Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6175–6192. [Google Scholar] [CrossRef]
- Cehofski, L.J.; Kruse, A.; Mæng, M.O.; Sejergaard, B.F.; Schlosser, A.; Sorensen, G.L.; Grauslund, J.; Honoré, B.; Vorum, H. Dexamethasone Intravitreal Implant Is Active at the Molecular Level Eight Weeks after Implantation in Experimental Central Retinal Vein Occlusion. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2022, 27, 5687. [Google Scholar] [CrossRef]
- Cehofski, L.J.; Kruse, A.; Alsing, A.N.; Nielsen, J.E.; Pedersen, S.; Kirkeby, S.; Honoré, B.; Vorum, H. Intravitreal bevacizumab upregulates transthyretin in experimental branch retinal vein occlusion. Mol. Vis. 2018, 24, 759–766. [Google Scholar] [PubMed]
- Cehofski, L.J.; Kruse, A.; Magnusdottir, S.O.; Alsing, A.N.; Nielsen, J.E.; Kirkeby, S.; Honoré, B.; Vorum, H. Dexamethasone intravitreal implant downregulates PDGFR-α and upregulates caveolin-1 in experimental branch retinal vein occlusion. Exp. Eye Res. 2018, 171, 174–182. [Google Scholar] [CrossRef]
- Cehofski, L.J.; Kruse, A.; Kjærgaard, B.; Stensballe, A.; Honoré, B.; Vorum, H. Proteins involved in focal adhesion signaling pathways are differentially regulated in experimental branch retinal vein occlusion. Exp. Eye Res. 2015, 138, 87–95. [Google Scholar] [CrossRef]
- Cehofski, L.J.; Kruse, A.; Kirkeby, S.; Alsing, A.N.; Nielsen, J.E.; Kojima, K.; Honoré, B.; Vorum, H. IL-18 and S100A12 Are Upregulated in Experimental Central Retinal Vein Occlusion. Int. J. Mol. Sci. 2018, 19, 3328. [Google Scholar] [CrossRef]
- Cehofski, L.J.; Kruse, A.; Bøgsted, M.; Magnusdottir, S.O.; Stensballe, A.; Honoré, B.; Vorum, H. Retinal proteome changes following experimental branch retinal vein occlusion and intervention with ranibizumab. Exp. Eye Res. 2016, 152, 49–56. [Google Scholar] [CrossRef]
- Cehofski, L.J.; Kruse, A.; Kjærgaard, B.; Stensballe, A.; Honoré, B.; Vorum, H. Dye-Free Porcine Model of Experimental Branch Retinal Vein Occlusion: A Suitable Approach for Retinal Proteomics. J. Ophthalmol. 2015, 2015, 839137. [Google Scholar] [CrossRef]
- Christakopoulos, C.; Cehofski, L.J.; Christensen, S.R.; Vorum, H.; Honoré, B. Proteomics reveals a set of highly enriched proteins in epiretinal membrane compared with inner limiting membrane. Exp. Eye Res. 2019, 186, 107722. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.P.; Fisher, S.K. Up-regulation of glial fibrillary acidic protein in response to retinal injury: Its potential role in glial remodeling and a comparison to vimentin expression. Int. Rev. Cytol. 2003, 230, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Verardo, M.R.; Lewis, G.P.; Takeda, M.; Linberg, K.A.; Byun, J.; Luna, G.; Wilhelmsson, U.; Pekny, M.; Chen, N.-F.; Fisher, S.K. Abnormal reactivity of muller cells after retinal detachment in mice deficient in GFAP and vimentin. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3659–3665. [Google Scholar] [CrossRef]
- Hippert, C.; Graca, A.B.; Basche, M.; Kalargyrou, A.A.; Georgiadis, A.; Ribeiro, J.; Matsuyama, A.; Aghaizu, N.; Bainbridge, J.W.; Smith, A.J.; et al. RNAi-mediated suppression of vimentin or glial fibrillary acidic protein prevents the establishment of Müller glial cell hypertrophy in progressive retinal degeneration. Glia 2021, 69, 2272–2290. [Google Scholar] [CrossRef]
- Hol, E.M.; Capetanaki, Y. Type III Intermediate Filaments Desmin, Glial Fibrillary Acidic Protein (GFAP), Vimentin, and Peripherin. Cold Spring Harb. Perspect. Biol. 2017, 9, a021642. [Google Scholar] [CrossRef] [PubMed]
- Ejstrup, R.; Scherfig, E.; la Cour, M. Electrophysiological consequences of experimental branch retinal vein occlusion in pigs and the effect of dorzolamide. Investig. Ophthalmol. Vis. Sci. 2011, 52, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.P.; Charteris, D.G.; Sethi, C.S.; Fisher, S.K. Animal models of retinal detachment and reattachment: Identifying cellular events that may affect visual recovery. Eye 2002, 16, 375–387. [Google Scholar] [CrossRef]
- Rootman, J. Vascular system of the optic nerve head and retina in the pig. Br. J. Ophthalmol. 1971, 55, 808–819. [Google Scholar] [CrossRef]
- de Almeida, A.M.; Bendixen, E. Pig proteomics: A review of a species in the crossroad between biomedical and food sciences. J. Proteom. 2012, 75, 4296–4314. [Google Scholar] [CrossRef]
- Barone, F.; Nannoni, E.; Elmi, A.; Lambertini, C.; Scorpio, D.G.; Ventrella, D.; Vitali, M.; Maya-Vetencourt, J.F.; Martelli, G.; Benfenati, F.; et al. Behavioral Assessment of Vision in Pigs. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 350–356. [Google Scholar] [CrossRef]
- Lassota, N. Clinical and histological aspects of CNV formation: Studies in an animal model. Acta Ophthalmol. 2008, 86, 1–24. [Google Scholar] [CrossRef]
- Choi, K.-E.; Anh, V.T.Q.; Kim, J.T.; Yun, C.; Cha, S.; Ahn, J.; Goo, Y.S.; Kim, S.-W. An experimental pig model with outer retinal degeneration induced by temporary intravitreal loading of N-methyl-N-nitrosourea during vitrectomy. Sci. Rep. 2021, 11, 258. [Google Scholar] [CrossRef]
- Vrolyk, V.; Desmarais, M.-J.; Lambert, D.; Haruna, J.; Benoit-Biancamano, M.-O. Neonatal and Juvenile Ocular Development in Göttingen Minipigs and Domestic Pigs: A Histomorphological and Immunohistochemical Study. Vet. Pathol. 2020, 57, 889–914. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Fekrat, S. Retinal vein occlusion: Beyond the acute event. Surv. Ophthalmol. 2011, 56, 281–299. [Google Scholar] [CrossRef]
- Rehak, M.; Wiedemann, P. Retinal vein thrombosis: Pathogenesis and management. J. Thromb. Haemost. 2010, 8, 1886–1894. [Google Scholar] [CrossRef]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal models in burn research. Cell. Mol. Life Sci. 2014, 71, 3241–3255. [Google Scholar] [CrossRef]
| Eyes (n) | Animals (n) | References | |
|---|---|---|---|
| BRVO | 65 | 38 * | [25,29,30,31,33] |
| CRVO | 43 | 27 ** | [26,30,32] |
| Controls | 22 | [25,26,31,32] | |
| Total | 130 | 65 | 8 |
| Animal | Pros | Cons |
|---|---|---|
| Danish Landrace pigs | Eyes are easily enucleated. | Adult animals weigh up to 200 kg. |
| Relatively cheap (10% of cost of a Göttingen minipig). | Not well suited for long-term studies > 2 months. | |
| Expenses per animal are high compared with rodents. | ||
| Göttingen minipigs | Well suited for long-term studies. Adult animals weigh up to 40 kg. | Deep orbital cavities which can make enucleation challenging. |
| Expensive. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mæng, M.O.; Roshanth, N.; Kruse, A.; Nielsen, J.E.; Kjærgaard, B.; Honoré, B.; Vorum, H.; Cehofski, L.J. Laser-Induced Porcine Model of Experimental Retinal Vein Occlusion: An Optimized Reproducible Approach. Medicina 2023, 59, 243. https://doi.org/10.3390/medicina59020243
Mæng MO, Roshanth N, Kruse A, Nielsen JE, Kjærgaard B, Honoré B, Vorum H, Cehofski LJ. Laser-Induced Porcine Model of Experimental Retinal Vein Occlusion: An Optimized Reproducible Approach. Medicina. 2023; 59(2):243. https://doi.org/10.3390/medicina59020243
Chicago/Turabian StyleMæng, Mads Odgaard, Nirrooja Roshanth, Anders Kruse, Jonas Ellegaard Nielsen, Benedict Kjærgaard, Bent Honoré, Henrik Vorum, and Lasse Jørgensen Cehofski. 2023. "Laser-Induced Porcine Model of Experimental Retinal Vein Occlusion: An Optimized Reproducible Approach" Medicina 59, no. 2: 243. https://doi.org/10.3390/medicina59020243
APA StyleMæng, M. O., Roshanth, N., Kruse, A., Nielsen, J. E., Kjærgaard, B., Honoré, B., Vorum, H., & Cehofski, L. J. (2023). Laser-Induced Porcine Model of Experimental Retinal Vein Occlusion: An Optimized Reproducible Approach. Medicina, 59(2), 243. https://doi.org/10.3390/medicina59020243







