Sevoflurane Exposure of Clinical Doses in Pregnant Rats Induces Vcan Changes without Significant Neural Apoptosis in the Offspring
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Experimental Protocol
2.3. Sample Preparation and Ultra-Performance Liquid Chromatography (UPLC) Analysis
2.4. Pattern Recognition Analysis Based on PLS-DA
2.5. Hematoxylin and Eosin (HE) Staining
2.6. TUNEL Staining
2.7. RNA-Seq and Real-Time PCR (RT-PCR) Verification
2.8. Statistical Analysis
3. Results
3.1. UPLC/TOF-MS Analysis of Sevoflurane-Induced Potential Neurotoxicity to Acquire Metabolomic Profiles
3.2. Partial Least Squares-Discriminate Analysis (PLS-DA) and Lipidic Metabolic Pathway Analysis of Sevoflurane Treatment
3.3. HE Staining and Tunel Staining
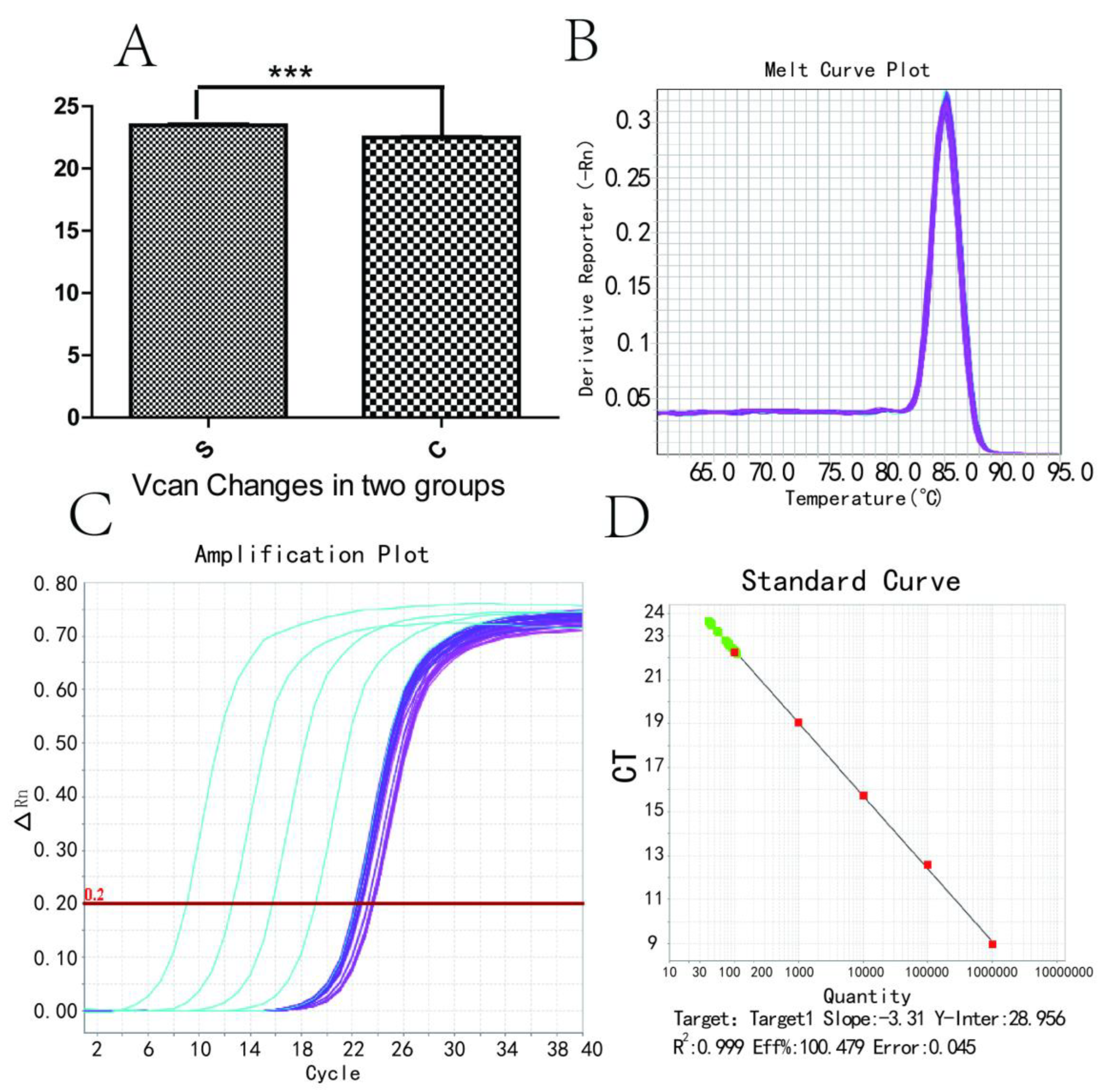
3.4. Vcan Gene RT-PCR Changes and Melting and Amplification Curves
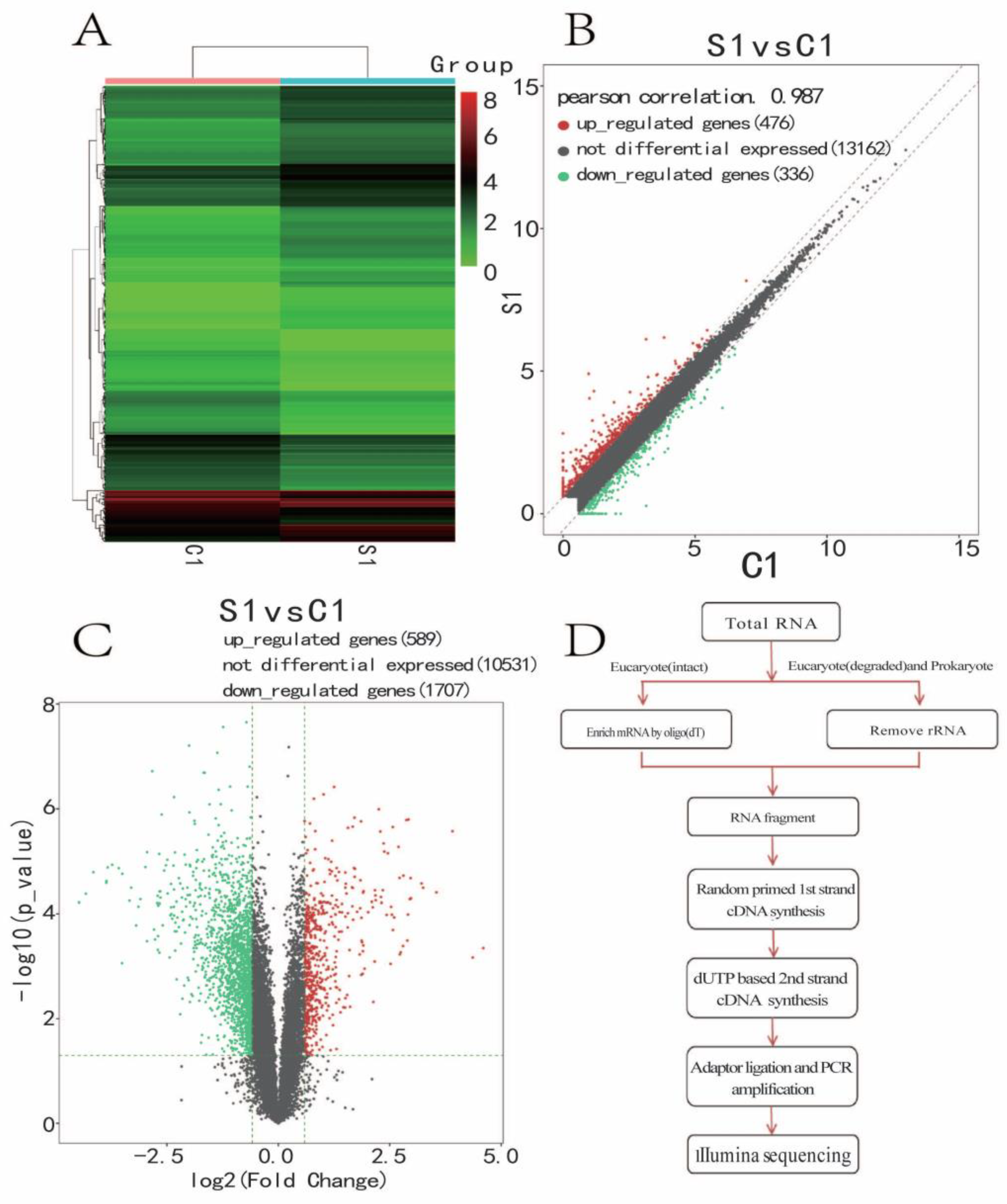
3.5. Significantly Differentially Expressed Gene Cluster Thermogram
3.6. Enrichment Analysis of the KEGG Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Ethics Approval and Consent to Participate
References
- Cattano, D.; Valleggi, S.; Abramo, A.; Forfori, F.; Maze, M.; Giunta, F. Nitrous oxide discretely up-regulates nNOS and p53 in neonatal rat brain. Minerva Anestesiol. 2010, 76, 420–424. [Google Scholar] [PubMed]
- Akbar, M.; Calderon, F.; Wen, Z.; Kim, H.Y. Docosahexaenoic acid: A positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. USA 2005, 102, 10858–10863. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.X.; Akbar, M.; Kevala, K.; Kim, H.Y. Phosphatidylserine is a critical modulator for Akt activation. J. Cell Biol. 2011, 192, 979–992. [Google Scholar] [CrossRef]
- Kim, H.Y.; Akbar, M.; Lau, A.; Edsall, L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3). Role of phosphatidylserine in antiapoptotic effect. J. Biol. Chem. 2000, 275, 35215–35223. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y. Novel metabolism of docosahexaenoic acid in neural cells. J. Biol. Chem. 2007, 282, 18661–18665. [Google Scholar] [CrossRef]
- Kim, H.Y.; Akbar, M.; Kim, Y.S. Phosphatidylserinedependent neuroprotective signaling promoted by docosahexaenoic acid. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 165–172. [Google Scholar] [CrossRef]
- Ma, R.; Wang, X.; Peng, P.; Xiong, J.; Dong, H.; Wang, L.; Ding, Z. α-Lipoic acid inhibits sevoflurane-induced neuronal apoptosis through PI3K/Akt signalling pathway. Cell Biochem. Funct. 2016, 34, 42–47. [Google Scholar] [CrossRef]
- Liu, B.; Gu, Y.; Xiao, H.; Lei, X.; Liang, W.; Zhang, J. Altered metabolomic profiles may be associated with sevoflurane-induced neurotoxicity in neonatal rats. Neurochem. Res. 2015, 40, 788–799. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Mao, T.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Mao, T. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.D. The positive false discovery rate: A bayesian interpretation and the q-value. Ann. Stat. 2003, 31, 2013–2035. [Google Scholar] [CrossRef]
- van Meer, G.; de Kroon, A.I. Lipid map of the mammalian cell. J. Cell Sci. 2011, 124, 5–8. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F. Role of Lipids in Brain Injury and Diseases. Future Lipidol. 2007, 2, 403–422. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lv, J.; Li, X.; Meng, Q.; Yang, Q.; Ma, W.; Li, Y.; Ke, Z.J. Sevoflurane decreases self-renewal capacity and causes c-Jun N-terminal kinase-mediated damage of rat fetal neural stem cells. Sci. Rep. 2017, 7, 46304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Z.; Joseph, D.J.; Inan, S.; Wei, H. Dual Effects of Isoflurane on Proliferation, Differentiation, and Survival in Human Neuroprogenitor Cells. J. Am. Soc. Anesthesiol. 2013, 118, 537–549. [Google Scholar] [CrossRef]
- Zheng, H.; Dong, Y.; Xu, Z.; Crosby, G.; Culley, D.; Zhang, Y.; Xie, Z. Sevoflurane anesthesia in pregnant mice induces neurotoxicity in fetal and offspring mice. Obstet. Anesth. Dig. 2013, 118, 516–526. [Google Scholar] [CrossRef]
- Suehara, T.; Morishita, J.; Ueki, M.; Ueno, M.; Maekawa, N.; Mizobuchi, S. Effects of sevoflurane exposure during late pregnancy on brain development of offspring mice. Pediatr. Anesth. 2016, 26, 52–59. [Google Scholar] [CrossRef]
- Fang, F.; Song, R.; Ling, X.; Peng, M.; Xue, Z.; Cang, J. Multiple Sevoflurane Anesthesia in Pregnant Mice Inhibits Neurogenesis of Fetal Hippocampus via Repressing Transcription Factor Pax6. Life Sci. 2017, 175, 16–22. [Google Scholar] [CrossRef]
- Hong, S.; Ostaszewski, B.L.; Yang, T.; O’Malley, T.T.; Jin, M.; Yanagisawa, K.; Li, S.; Bartels, T.; Selkoe, D.J. Soluble Abeta oligomers are rapidly sequestered from brain ISF in vivo and bind GM1 ganglioside on cellular membranes. Neuron 2014, 82, 308–319. [Google Scholar] [CrossRef]
- Ruiz, A.; Joshi, P.; Mastrangelo, R.; Francolini, M.; Verderio, C.; Matteoli, M. Testing Abeta toxicity on primary CNS cultures using drug-screening microfluidic chips. Lab Chip 2014, 14, 2860–2866. [Google Scholar] [CrossRef]
- Shevchenko, A.; Simons, K. Lipidomics: Coming to grips with lipid diversity. Nat. Rev. Mol. Cell Biol. 2010, 11, 593–598. [Google Scholar] [CrossRef]
- de Vries, R.; Kappelle, P.J.; Dallinga-Thie, G.M.; Dullaart, R.P. Plasma phospholipid transfer protein activity is independently determined by obesity and insulin resistance in non-diabetic subjects. Atherosclerosis 2011, 217, 253–259. [Google Scholar] [CrossRef]
- Igarashi, M.; Ma, K.; Gao, F.; Kim, H.; Rapoport, S.I.; Rao, J.S. Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer’s disease prefrontal cortex. J. Alzheimer’s Dis. 2011, 24, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.V.; Westaway, S.K.; Morton, J.E.V.; Gregory, A.; Gissen, P.; Sonek, S.; Cangul, H.; Coryell, J.; Canham, N.; Nardocci, N.; et al. PLA2G6, encoding a phospholipase A(2), is mutated in neurodegenerative disorders with high brain iron. Nat. Genet. 2006, 38, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Hattori, T.; Abdullah, M.; Gong, J.S.; Yamane, T.; Michikawa, M. Phosphatidylcholine protects neurons from toxic effects of amyloid β-protein in culture. Brain Res. 2016, 1642, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Jenner, A.M.; Shui, G.; Cheong, W.F.; Mitchell, T.W.; Nealon, J.R.; Kim, W.S.; McCann, H.; Wenk, M.R.; Halliday, G.M.; et al. Lipid pathway alterations in Parkinson’s disease primary visual cortex. PLoS ONE 2011, 6, e17299. [Google Scholar] [CrossRef] [PubMed]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Van Echten-Deckert, G.; Walter, J. Sphingolipids: Critical players in Alzheimer’s disease. Prog. Lipid Res. 2012, 51, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Hla, T.; Dannenberg, A.J. Sphingolipid signaling in metabolic disorders. Cell Metab. 2012, 16, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sheng, W.; Chen, L.; Dong, H.; Lee, V.; Lu, F.; Wong, C.S.; Lu, W.-Y.; Yang, B.B. Versican V1 isoform induces neuronal differentiation and promotes neurite outgrowth. Mol. Biol. Cell 2004, 15, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, N.; Carini, G.; Zoppi, N.; Ritelli, M.; Colombi, M. Transcriptome analysis of skin fibroblasts with dominant negative COL3A1 mutations provides molecular insights into the etiopathology of vascular Ehlers-Danlos syndrome. PLoS ONE 2018, 13, e0191220. [Google Scholar] [CrossRef]




| C Group (n = 8) | S Group (n = 8) | |
|---|---|---|
| PH | 7.37 ± 0.03 | 7.41 ± 0.01 |
| PaCO2 (mmHg) | 43.80 ± 3.50 | 46.97 ± 4.25 |
| PaO2 (mmHg) | 116.0 ± 12.66 | 124.3 ± 19.59 |
| BE | −0.33 ± 1.53 | 4 ± 1.0 |
| HCO3 | 25.57 ± 1.75 | 29.90 ± 1.90 |
| (SpO2) | 98.33 ± 0.58 | 100 ± 0 |
| No. | M/Z | RT (min) | Ion | Metabolite | Related Pathways | Related Diseases | |
|---|---|---|---|---|---|---|---|
| 1 | 1687 | 496.341 | 2.27 | [M+H]+ | LysoPC(16:0) | glycerophospholipid metabolism ** | pancreatic cancer, Barth sgl, atherosclerosis |
| 2 | 2918 | 707.4991 | 2.96 | [M+K]+ | DG(20:1n9/0:0/20:5n3) | ||
| 3 | 3488 | 774.5638 | 7.86 | [M+H]+ | PE(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/P-18:1(11Z)) | glycerophospholipid metabolism ** | pancreatic cancer, Barth sgl |
| 4 | 3037 | 728.5217 | 8.57 | [M+Na]+ | SM(d18:0/16:0) | sphingolipid metabolism ** | atherosclerosis |
| 5 | 3929 | 808.5897 | 8.85 | [M+H]+ | PC(16:0/22:5(7Z,10Z,13Z,16Z,19Z)) | glycerophospholipid metabolism ** | |
| 6 | 3897 | 806.5729 | 9.08 | [M+Na]+ | Galactosylceramide (d18:1/22:0) | sphingolipid metabolism ** | Hidradenitis suppurativa |
| 7 | 3535 | 780.5505 | 9.13 | [M+Na]+ | CerP(d18:1/26:0) | ||
| 8 | 3238 | 756.5559 | 9.8 | [M+Na]+ | PC(16:0/16:0) | glycerophospholipid metabolism ** | Adolescent idiopathic scoliosis |
| 9 | 5586 | 923.7476 | 22.92 | [M+H]+ | TG(20:5(5Z,8Z,11Z,14Z,17Z)/18:2(9Z,12Z)/20:5(5Z,8Z,11Z,14Z,17Z)) | ||
| 10 | 4776 | 857.7568 | 23.98 | [M+K]+ | TG(14:0/20:1(11Z)/15:0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Hu, X.; Meng, F.; Luo, Q.; Liu, H.; Yang, Z. Sevoflurane Exposure of Clinical Doses in Pregnant Rats Induces Vcan Changes without Significant Neural Apoptosis in the Offspring. Medicina 2023, 59, 190. https://doi.org/10.3390/medicina59020190
Jin Y, Hu X, Meng F, Luo Q, Liu H, Yang Z. Sevoflurane Exposure of Clinical Doses in Pregnant Rats Induces Vcan Changes without Significant Neural Apoptosis in the Offspring. Medicina. 2023; 59(2):190. https://doi.org/10.3390/medicina59020190
Chicago/Turabian StyleJin, Yi, Xiaoxue Hu, Fanhua Meng, Qing Luo, Henry Liu, and Zeyong Yang. 2023. "Sevoflurane Exposure of Clinical Doses in Pregnant Rats Induces Vcan Changes without Significant Neural Apoptosis in the Offspring" Medicina 59, no. 2: 190. https://doi.org/10.3390/medicina59020190
APA StyleJin, Y., Hu, X., Meng, F., Luo, Q., Liu, H., & Yang, Z. (2023). Sevoflurane Exposure of Clinical Doses in Pregnant Rats Induces Vcan Changes without Significant Neural Apoptosis in the Offspring. Medicina, 59(2), 190. https://doi.org/10.3390/medicina59020190






