Utility of Combining High-Sensitive Cardiac Troponin I and PESI Score for Risk Management in Patients with Pulmonary Embolism in the Emergency Department
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Phase I
3.2. Phase II
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Pollack, C.V.; Schreiber, D.; Goldhaber, S.Z.; Slattery, D.; Fanikos, J.; O’Neil, B.J.; Thompson, J.R.; Hiestand, B.; Briese, B.A.; Pendleton, R.C.; et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: Initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J. Am. Coll. Cardiol. 2011, 57, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Aujesky, D.; Roy, P.M.; Verschuren, F.; Righini, M.; Osterwalder, J.; Egloff, M.; Renaud, B.; Verhamme, P.; Stone, R.A.; Legall, C.; et al. Yealy DM Outpatient versus inpatient treatment for patients with acute pulmonary embolism: An international, open-label, randomised, non-inferiority trial. Lancet 2011, 378, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Aujesky, D.; Obrosky, D.S.; Stone, R.A.; Auble, T.E.; Perrier, A.; Cornuz, J.; Roy, P.M.; Fine, M.J. Derivation and validation of a prognostic model for pulmonary embolism. Am. J. Respir. Crit. Care Med. 2005, 172, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.; Mallett, S.; Daoud-Elias, M.; Poggi, J.-N.; Clarke, M. Prognostic models in acute pulmonary embolism: A systematic review and meta-analysis. BMJ Open 2016, 6, e010324. [Google Scholar] [CrossRef]
- Morillo, R.; Moores, L.; Jiménez, D. Prognostic Scores for Acute Pulmonary Embolism. Semin. Thromb. Hemost. 2017, 43, 486–492. [Google Scholar] [CrossRef]
- Kohn, C.G.; Mearns, E.S.; Parker, M.W.; Hernandez, A.V.; Coleman, C.I. Prognostic accuracy of clinical prediction rules for early post-pulmonary embolism all-cause mortality: A bivariate meta-analysis. Chest 2015, 147, 1043–1062. [Google Scholar] [CrossRef]
- Hobohm, L.; Hellenkamp, K.; Hasenfuß, G.; Münzel, T.; Konstantinides, S.; Lankeit, M. Comparison of risk assessment strategies for not-high-risk pulmonary embolism. Eur. Respir. J. 2016, 47, 1170–1178. [Google Scholar] [CrossRef]
- Quezada, C.A.; Bikdeli, B.; Barrios, D.; Barbero, E.; Chiluiza, D.; Muriel, A.; Casazza, F.; Monreal, M.; Yusen, R.D.; Jiménez, D. Meta-Analysis of Prevalence and Short-Term Prognosis of Hemodynamically Unstable Patients With Symptomatic Acute Pulmonary Embolism. Am. J. Cardiol. 2019, 123, 684–689. [Google Scholar] [CrossRef]
- Bova, C.; Vanni, S.; Prandoni, P.; Morello, F.; Dentali, F.; Bernardi, E.; Mumoli, N.; Bucherini, E.; Barbar, S.; Picariello, C.; et al. A prospective validation of the Bova score in normotensive patients with acute pulmonary embolism. Thromb. Res. 2018, 165, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Hobohm, L.; Becattini, C.; Konstantinides, S.V.; Casazza, F.; Lankeit, M. Validation of a fast prognostic score for risk stratification of normotensive patients with acute pulmonary embolism. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2020, 109, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Donzé, J.; Le Gal, G.; Fine, M.J.; Roy, P.M.; Sanchez, O.; Verschuren, F.; Cornuz, J.; Meyer, G.; Perrier, A.; Righini, M.; et al. Prospective validation of the Pulmonary Embolism Severity Index. A clinical prognostic model for pulmonary embolism. Thromb. Haemost. 2008, 100, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Woods, C.; Shorr, A.F. The validation and reproducibility of the pulmonary embolism severity index. J. Thromb. Haemost. 2010, 8, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.; Aujesky, D.; Moores, L.; Gómez, V.; Lobo, J.L.; Uresandi, F.; Otero, R.; Monreal, M.; Muriel, A.; Yusen, R.D.; et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch. Intern. Med. 2010, 170, 1383–1389. [Google Scholar] [CrossRef]
- Singanayagam, A.; Scally, C.; Al-Khairalla, M.Z.; Leitch, L.; Hill, L.E.; Chalmers, J.D.; Hill, A.T. Are biomarkers additive to pulmonary embolism severity index for severity assessment in normotensive patients with acute pulmonary embolism? QJM Int. J. Med. 2011, 104, 125–131. [Google Scholar] [CrossRef]
- Sanchez, O.; Trinquart, L.; Colombet, I.; Durieux, P.; Huisman, M.V.; Chatellier, G.; Meyer, G. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: A systematic review. Eur. Heart J. 2008, 29, 1569–1577. [Google Scholar] [CrossRef]
- Erkens, P.M.; Gandara, E.; Wells, P.S.; Shen, A.Y.; Bose, G.; Le Gal, G.; Rodger, M.; Prins, M.H.; Carrier, M. Does the Pulmonary Embolism Severity Index accurately identify low risk patients eligible for outpatient treatment? Thromb. Res. 2012, 129, 710–714. [Google Scholar] [CrossRef]
- Barco, S.; Mahmoudpour, S.H.; Planquette, B.; Sanchez, O.; Konstantinides, S.V.; Meyer, G. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: A systematic review and meta-analysis. Eur. Heart J. 2019, 40, 902–910. [Google Scholar] [CrossRef]
- Welsh, P.; Preiss, D.; Hayward, C.; Shah, A.S.V.; McAllister, D.; Briggs, A.; Boachie, C.; McConnachie, A.; Padmanabhan, S.; Welsh, C.; et al. Cardiac Troponin T and Troponin I in the General Population. Circulation 2019, 139, 2754–2764. [Google Scholar] [CrossRef]
- Fry, M.; Burr, G. Review of the triage literature: Past, present, future? Aust. Emerg. Nurs. J. 2002, 5, 33–38. [Google Scholar] [CrossRef]
- Wells, P.S.; Anderson, D.R.; Bormanis, J.; Guy, F.; Mitchell, M.; Gray, L.; Clement, C.; Robinson, K.S.; Lewandowski, B. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997, 350, 1795–1798. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, G.; Righini, M.; Roy, P.M.; Sanchez, O.; Aujesky, D.; Bounameaux, H.; Perrier, A. Prediction of pulmonary embolism in the emergency department: The revised Geneva score. Ann. Intern. Med. 2006, 144, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.A.; Courtney, D.M.; Kabrhel, C.; Moore, C.L.; Smithline, H.A.; Plewa, M.C.; Richman, P.B.; O’Neil, B.J.; Nordenholz, K. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J. Thromb. Haemost. 2008, 6, 772–780. [Google Scholar] [CrossRef]
- Krintus, M.; Kozinski, M.; Boudry, P.; Capell, N.E.; Köller, U.; Lackner, K.; Lefèvre, G.; Lennartz, L.; Lotz, J.; Herranz, A.M.; et al. European multicenter analytical evaluation of the Abbott ARCHITECT STAT high sensitive troponin I immunoassay. Clin. Chem. Lab. Med. 2014, 52, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Becattini, C.; Vedovati, M.C.; Agnelli, G. Prognostic value of troponins in acute pulmonary embolism: A meta-analysis. Circulation 2007, 116, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Coutance, G.; Cauderlier, E.; Ehtisham, J.; Hamon, M.; Hamon, M. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: A meta-analysis. Crit. Care 2011, 15, R103. [Google Scholar] [CrossRef]
- Konstantinides, S.; Geibel, A.; Olschewski, M.; Kasper, W.; Hruska, N.; Jäckle, S.; Binder, L. Importance of cardiac troponins I and T in risk stratification of patients with acute pulmonary embolism. Circulation 2002, 106, 1263–1268. [Google Scholar] [CrossRef]
- Müller-Bardorff, M.; Weidtmann, B.; Giannitsis, E.; Kurowski, V.; Katus, H.A. Release kinetics of cardiac troponin T in survivors of confirmed severe pulmonary embolism. Clin. Chem. 2002, 48, 673–675. [Google Scholar] [CrossRef]
- Musani, S.K.; Fox, E.R.; Kraja, A.; Bidulescu, A.; Lieb, W.; Lin, H.; Beecham, A.; Chen, M.H.; Felix, J.F.; Fox, C.S.; et al. Genome-wide association analysis of plasma B-type natriuretic peptide in blacks: The Jackson Heart Study. Circ. Cardiovasc. Genet. 2015, 8, 122–130. [Google Scholar] [CrossRef]
- Verweij, N.; Mahmud, H.; Mateo Leach, I.; de Boer, R.A.; Brouwers, F.P.; Yu, H.; Asselbergs, F.W.; Struck, J.; Bakker, S.J.; Gansevoort, R.T.; et al. Genome-wide association study on plasma levels of midregional-proadrenomedullin and C-terminal-pro-endothelin-1. Hypertension 2013, 61, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Dellas, C.; Tschepe, M.; Seeber, V.; Zwiener, I.; Kuhnert, K.; Schäfer, K.; Hasenfuß, G.; Konstantinides, S.; Lankeit, M. A novel H-FABP assay and a fast prognostic score for risk assessment of normotensive pulmonary embolism. Thromb. Haemost. 2014, 111, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Bova, C.; Sanchez, O.; Prandoni, P.; Lankeit, M.; Konstantinides, S.; Vanni, S.; Fernández-Golfín, C.; Yusen, R.D.; Jiménez, D. Validation of a Model for Identification of Patients at Intermediate to High Risk for Complications Associated With Acute Symptomatic Pulmonary Embolism. Chest 2015, 148, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lankeit, M.; Friesen, D.; Aschoff, J.; Dellas, C.; Hasenfuss, G.; Katus, H.; Konstantinides, S.; Giannitsis, E. Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur. Heart J. 2010, 31, 1836–1844. [Google Scholar] [CrossRef]
| Phase I | Phase II | P | |
|---|---|---|---|
| n. | 291 | 83 | |
| Age, yrs (mean ± se) | 70 ± 15 | 71 ± 16 | n.s. |
| Gender, n (Female %) | 149 (53%) | 50 (61%) | n.s. |
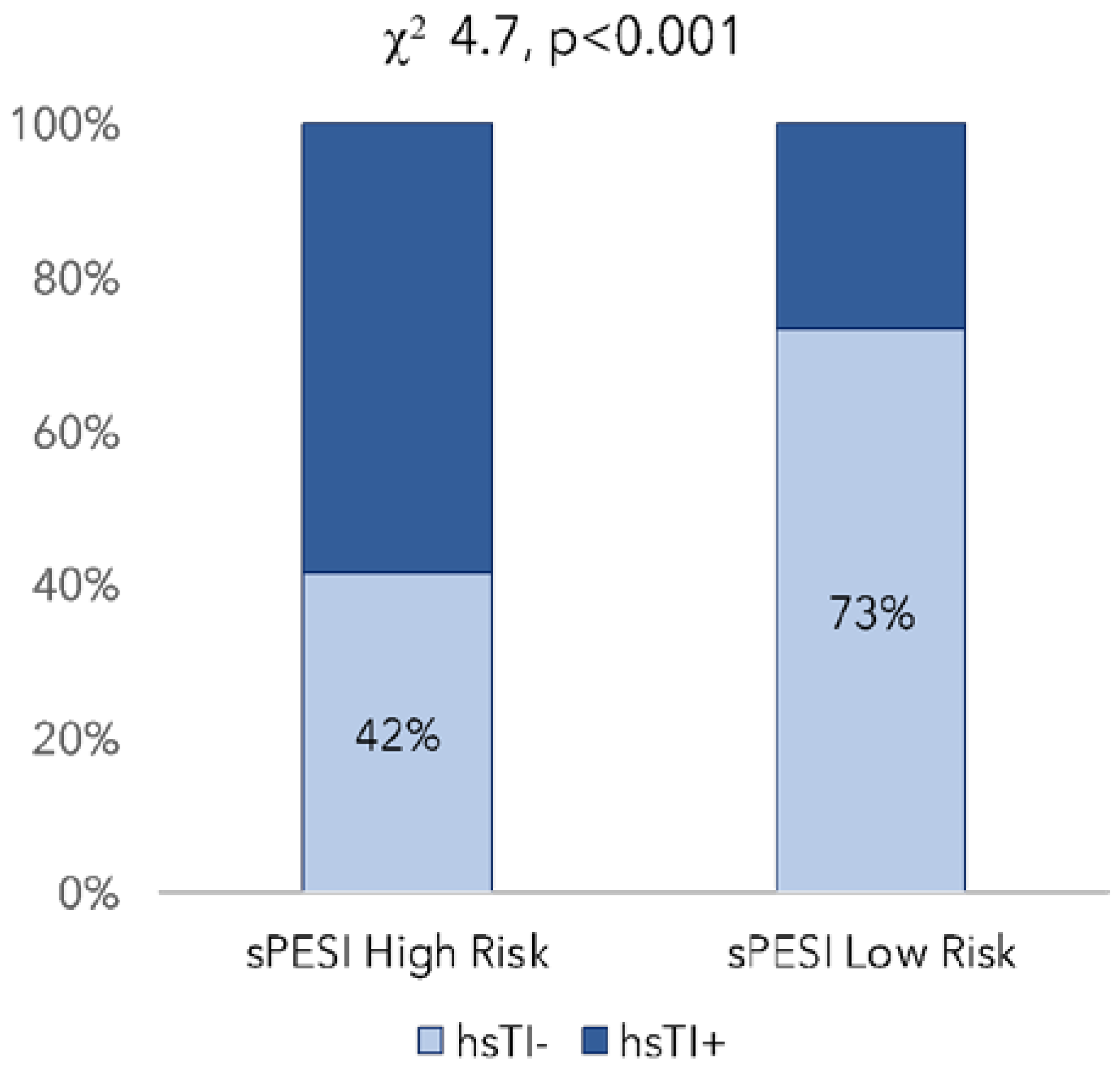
| sPESI, % (high risk) | 207 (71%) | 54 (64%) | n.s. |
| SBP, mmHg (mean ± se) | 129 ± 2 | 132 ± 3 | n.s. |
| Hr, beat/min (mean ± se) | 94 ± 1 | 92 ± 2 | n.s. |
| RR, breath/min (mean + se) | 21 ± 1 | 21 ± 1 | n.s. |
| SpO2, % (mean ± se) | 93.7 ± 0.3 | 94.1 ± 0.6 | n.s. |
| MEWS, (mean ± se) | 2.3 ± 0.3 | 2.9 ± 02 | n.s. |
| RV diam, mm (mean ± se) | 3.81 ± 0.04 | 3.85 ± 0.10 | n.s. |
| LV diam, mm (mean ± se) | 3.45 ± 0.05 | 3.75 ± 0.10 | 0.01 |
| Ratio RV/LV, % (mean ± se) | 1.16 ± 0.03 | 1.06 ± 0.03 | n.s. |
| hs-cTnI, ng/L (mean ± se) | 187 ± 45 | 89 ± 20 | n.s. |
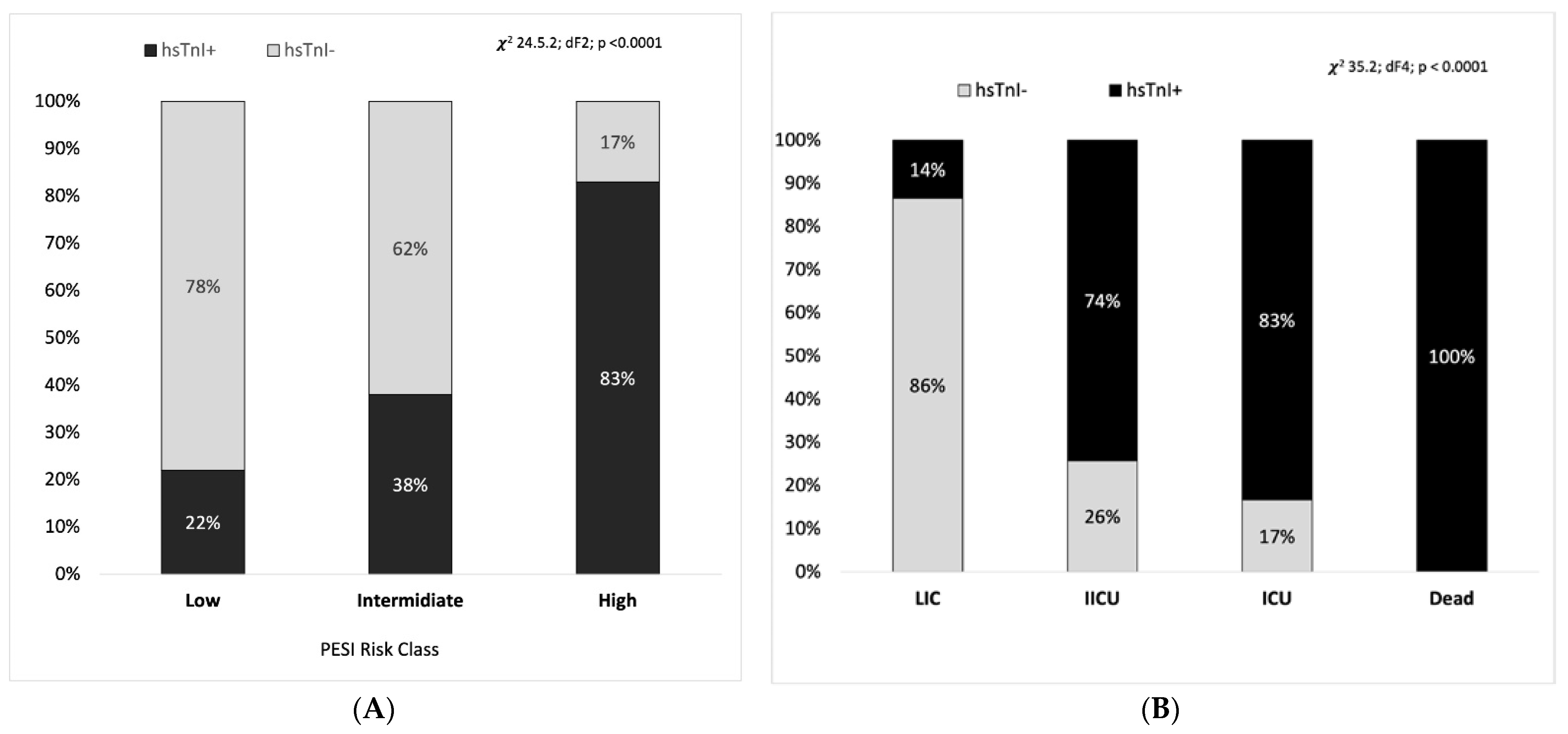
| ICU, n (%) | 60 (21%) | 11 (14%) | n.s. |
| Death, n (%) | 5 (2%) | 3 (3%) | n.s. |
| HS-TnI− | HS-TnI+ | 1w-ANOVA | |
|---|---|---|---|
| Number of patients | 148 | 143 | - |
| Age, yrs ±SD | 69 ± 1 | 70 ± 1 | n.s. |
| MEWS | 1.7 ± 0.3 | 3.0 ± 0.5 | 0.02 |
| RV diam, mm | 3.59 ± 0.06 | 4.03 ± 0.07 | <0.001 |
| LV diam, mm | 3.68 ± 0.05 | 3.22 ± 0.07 | <0.001 |
| Ratio RV/LV | 0.99 ± 0.02 | 1.34 ± 0.04 | <0.001 |
| Ratio < 1, % | 46% | 18% | <0.001 |
| ICU, n (%) | 17% | 25% | n.s. |
| Death, n (%) | - | 4% | 0.02 |
| Phase 1 | Phase 2 | p Value | |
|---|---|---|---|
| Hb, gr/dL (mean + se) | 13.4 ± 0.020 | 12.8 ± 0.2 | n.s. |
| Creatinine, g/dL (mean + se) | 0.986 ± 0.001 | 0.96 ± 0.04 | n.s. |
| Hs-cTnI, ng/L (mean + se) | 187.4 ± 0.481 | 89 ± 20 | n.s. |
| D-dimer, μg/mL (mean + se) | 5.4 ± 0.018 | 5.7 ± 0.8 | n.s. |
| P/F ratio (mean + se) | n.a. | 319 ± 10 | n.s. |
| pH (mean + se) | n.a. | 7.458 ± 0.007 | n.s. |
| WELLS (mean + se) | 3.7 ± 0.018 | 3.5 ± 0.3 | n.s. |
| GENEVE (mean + se) | 6.4 ± 0.012 | 7.4 ± 0.4 | n.s. |
| hs-cTnI− | hs-cTnI+ | p Value | |
|---|---|---|---|
| Age, yrs ±SD | 61.1 ± 16.3 | 76.9 ± 13.8 | 0.01 |
| MEWS | 1.50 ± 0.22 | 4.05 ± 0.29 | 0.0000001 |
| SBP, mmHg | 138 ± 4 | 126 ± 4 | 0.04 |
| HR, beat/min | 88 ± 2 | 96 ± 4 | ns |
| RR, breath/min | 19 ± 1 | 24 ± 1 | 0.00001 |
| D-dimer, μg/mL | 3.7 ± 0,8 | 7.7 ± 1,3 | 0.008 |
| BNP, μg/mL | 81 ± 23 | 437 ± 116 | 0.002 |
| RV diam, mm | 3.75 ± 0.17 | 3.92 ± 0.12 | n.s. |
| LV diam, mm | 3.48 ± 0.14 | 3.90 ± 0.12 | <0.05 |
| Ratio RV/LV | 126 ± 0.05 | 0.99 ± 0.04 | <0.001 |
| Ratio < 1, % | 18% | 46% | <0.001 |
| ICU, n (%) | 15% | 16% | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cennamo, E.; Valli, G.; Riead, E.K.M.; Casalboni, S.; Papasidero, I.D.; De Marco, F.; Mariani, A.; Pepe, P.; Santangelo, G.; Mastracchi, M.; et al. Utility of Combining High-Sensitive Cardiac Troponin I and PESI Score for Risk Management in Patients with Pulmonary Embolism in the Emergency Department. Medicina 2023, 59, 185. https://doi.org/10.3390/medicina59020185
Cennamo E, Valli G, Riead EKM, Casalboni S, Papasidero ID, De Marco F, Mariani A, Pepe P, Santangelo G, Mastracchi M, et al. Utility of Combining High-Sensitive Cardiac Troponin I and PESI Score for Risk Management in Patients with Pulmonary Embolism in the Emergency Department. Medicina. 2023; 59(2):185. https://doi.org/10.3390/medicina59020185
Chicago/Turabian StyleCennamo, Elisa, Gabriele Valli, Engy Khaled Mohamed Riead, Silvia Casalboni, Ilaria Dafne Papasidero, Francesca De Marco, Anna Mariani, Paola Pepe, Giuseppe Santangelo, Marina Mastracchi, and et al. 2023. "Utility of Combining High-Sensitive Cardiac Troponin I and PESI Score for Risk Management in Patients with Pulmonary Embolism in the Emergency Department" Medicina 59, no. 2: 185. https://doi.org/10.3390/medicina59020185
APA StyleCennamo, E., Valli, G., Riead, E. K. M., Casalboni, S., Papasidero, I. D., De Marco, F., Mariani, A., Pepe, P., Santangelo, G., Mastracchi, M., Fratini, P., Pistilli, G., Pignatelli, P., Ruggieri, M. P., & Di Somma, S. (2023). Utility of Combining High-Sensitive Cardiac Troponin I and PESI Score for Risk Management in Patients with Pulmonary Embolism in the Emergency Department. Medicina, 59(2), 185. https://doi.org/10.3390/medicina59020185







