Amentoflavone Mitigates Cyclophosphamide-Induced Pulmonary Toxicity: Involvement of -SIRT-1/Nrf2/Keap1 Axis, JAK-2/STAT-3 Signaling, and Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
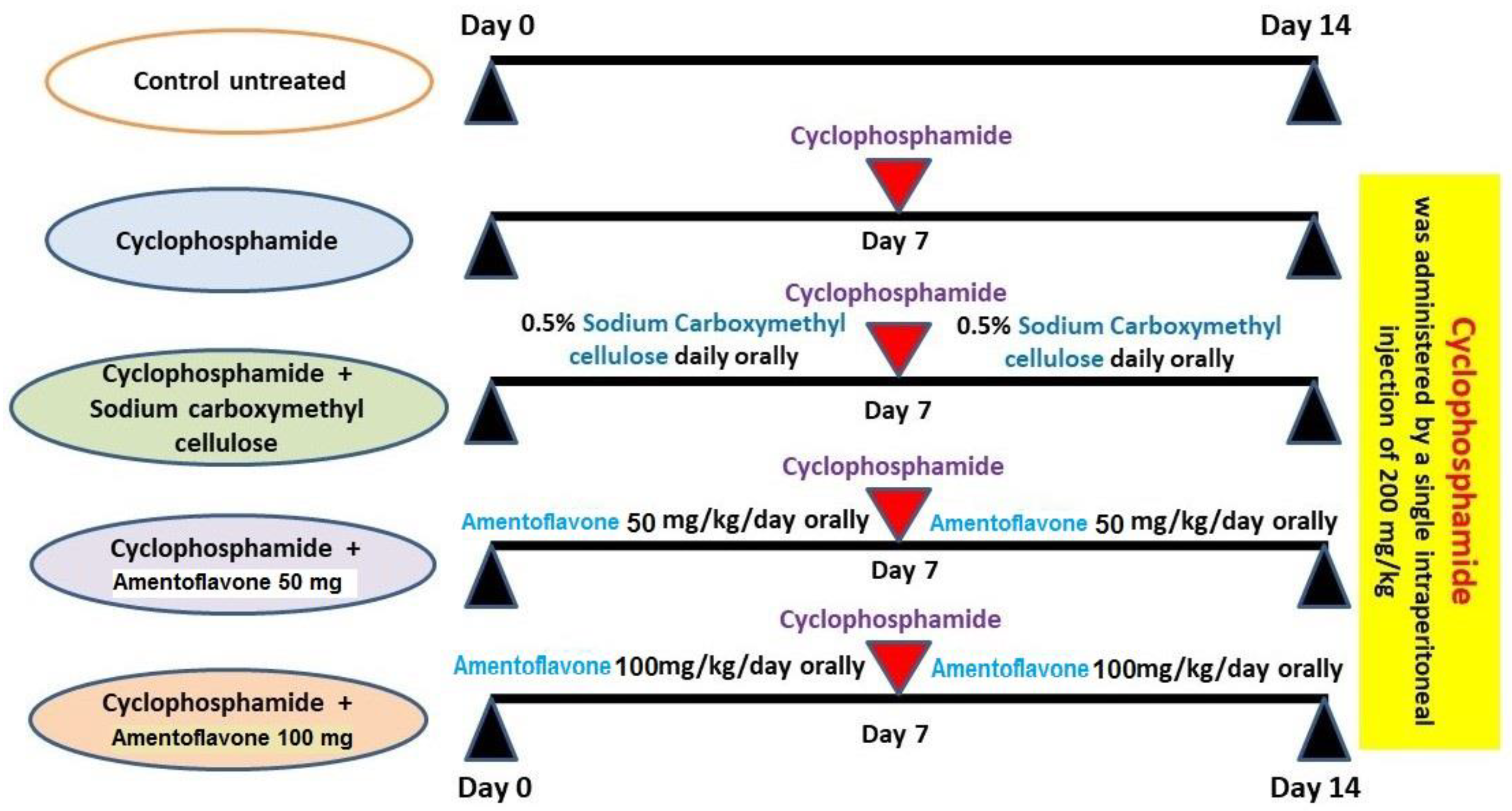
2.2. Experimental Protocol
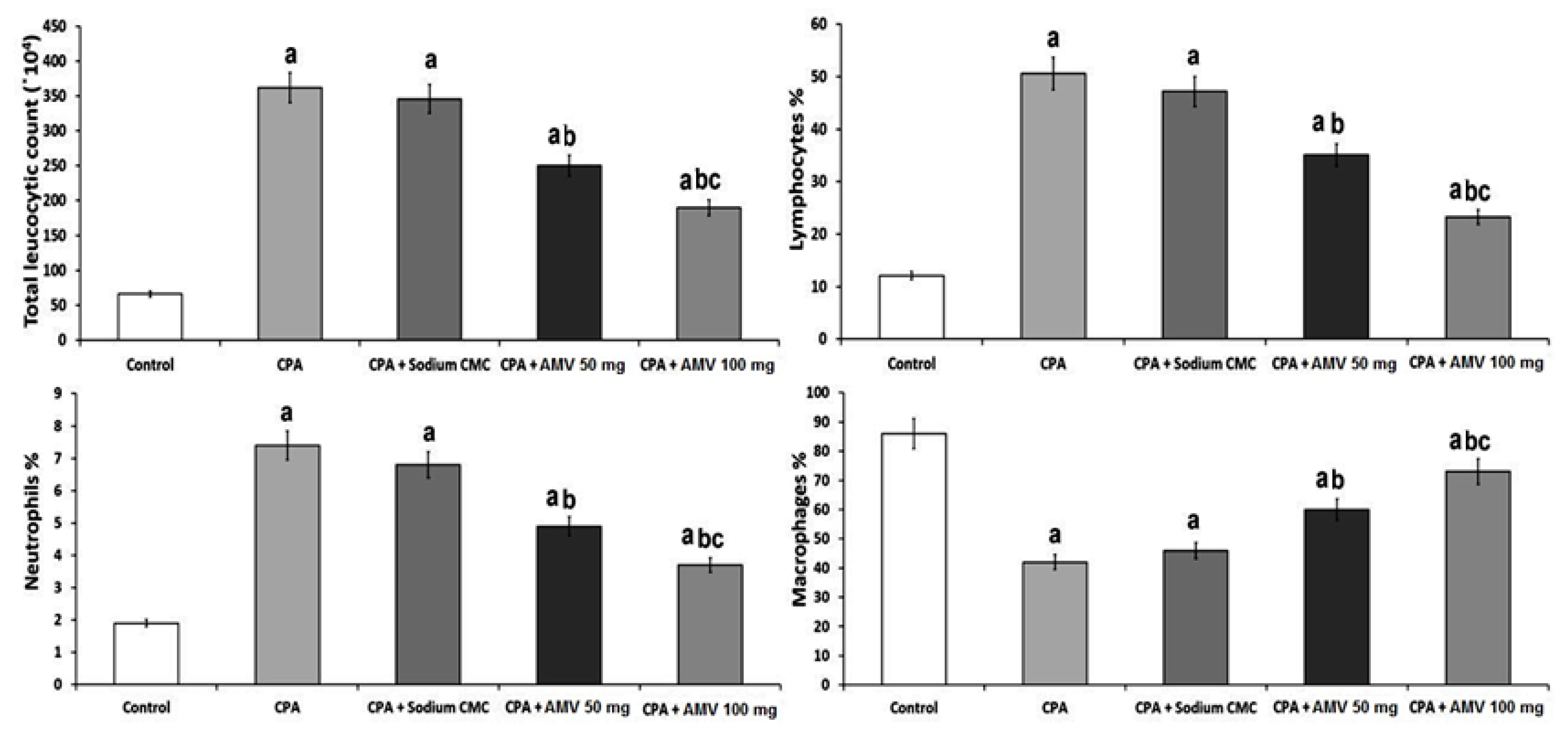
2.3. Quantification of the Changes in the Total and Differential Leucocytic Counts in BALF in the Studied Groups
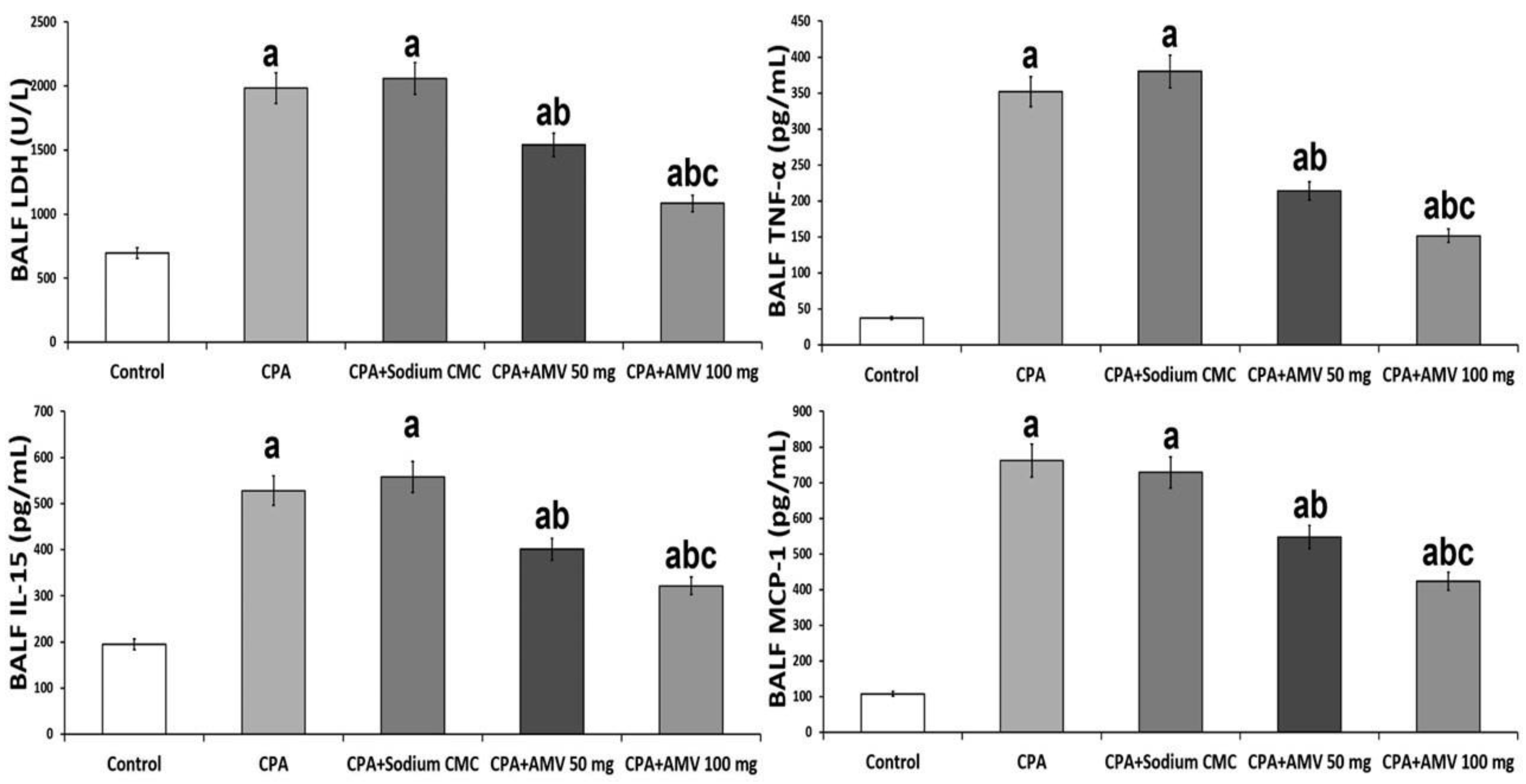
2.4. Assessment of Lactate Dehydrogenase (LDH), Tumor Necrosis Factor-Alpha (TNF-α), Interleukin 15 (IL-15), and Monocyte Chemotactic Protein 1 (MCP-1) in BALF
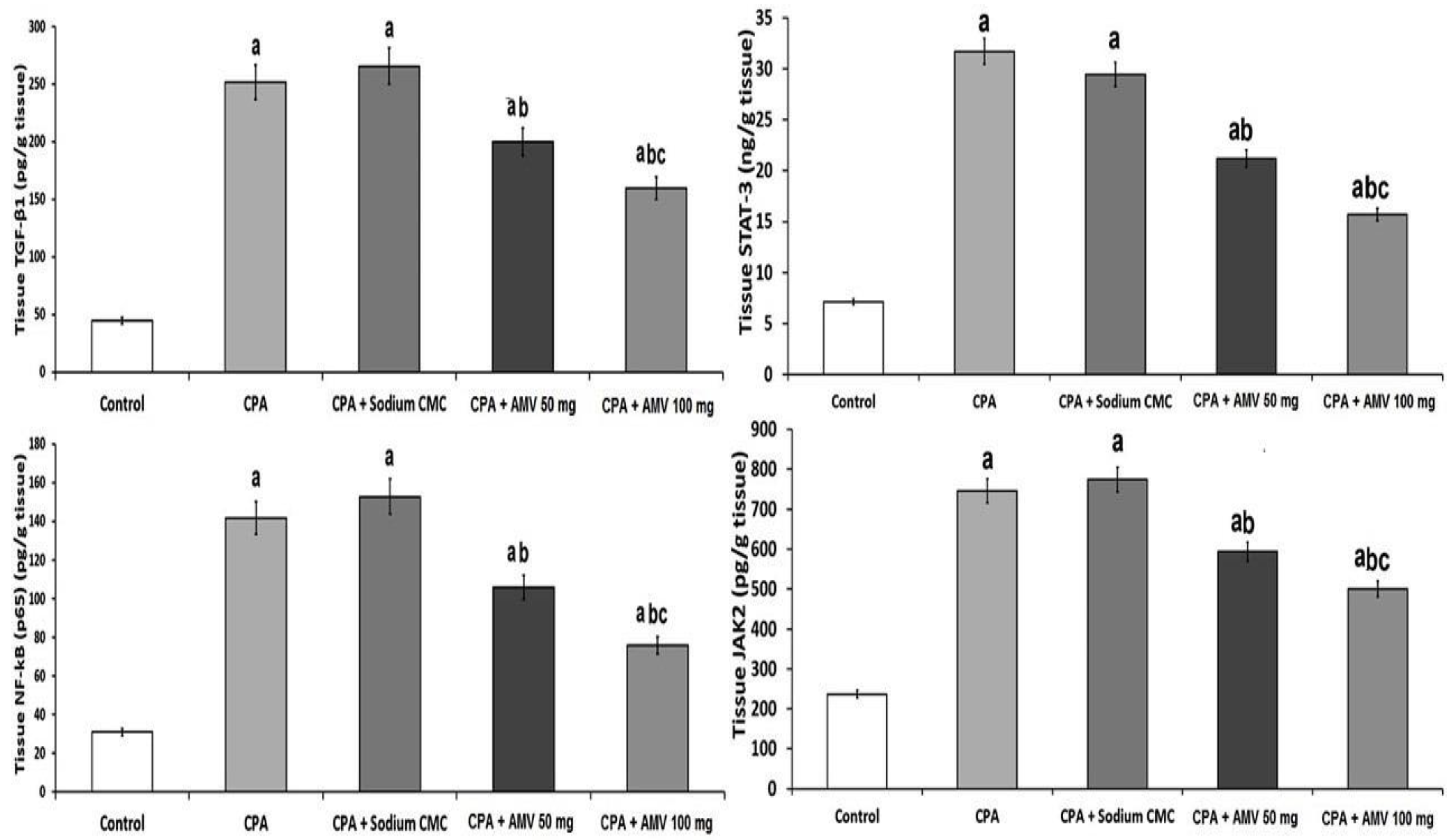
2.5. Detection of the Levels of TGF-β1 and Nuclear Factor Kappa B (NF-κB) p65 in the Lung Tissues
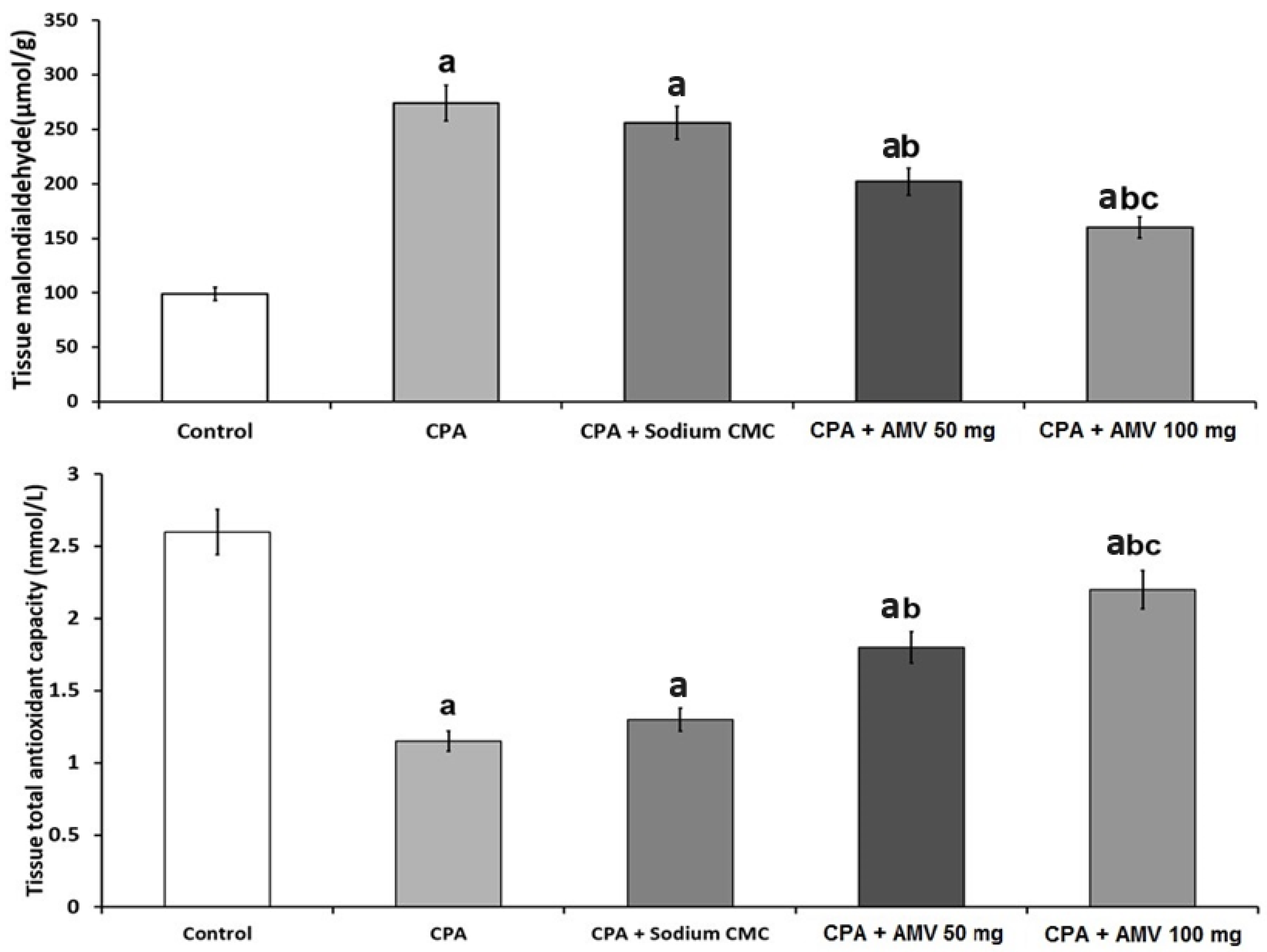
2.6. Assay of the Tissue Content of Malondialdehyde (MDA) and Total Antioxidant Capacity (TAC) in the Lung Specimens
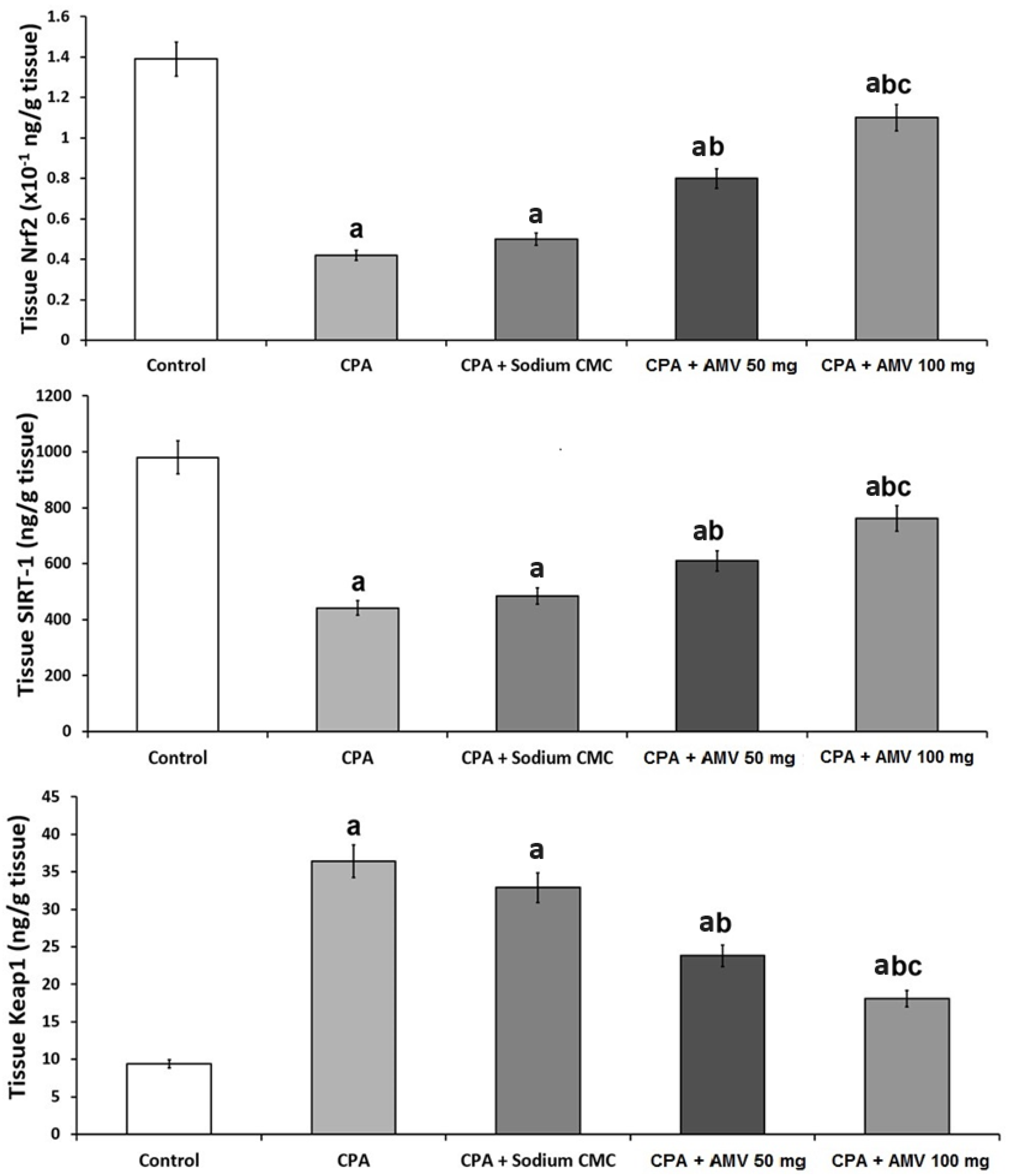
2.7. Quantification of Nuclear Factor (Erythroid-Derived 2)-like 2 (Nrf2), SIRT-1, and Kelch-like ECH-Associated Protein 1 (Keap1) Contents in the Lung Tissues
2.8. Assessment of Janus Kinase 2 (JAK2) and Signal Transducer and Activator of Transcription 3 (STAT3) in the Pulmonary Tissues
2.9. Determination of Tissue Hydroxyproline and Matrix Metalloproteinase (MMP)-3 and -9 in the Pulmonary Tissues
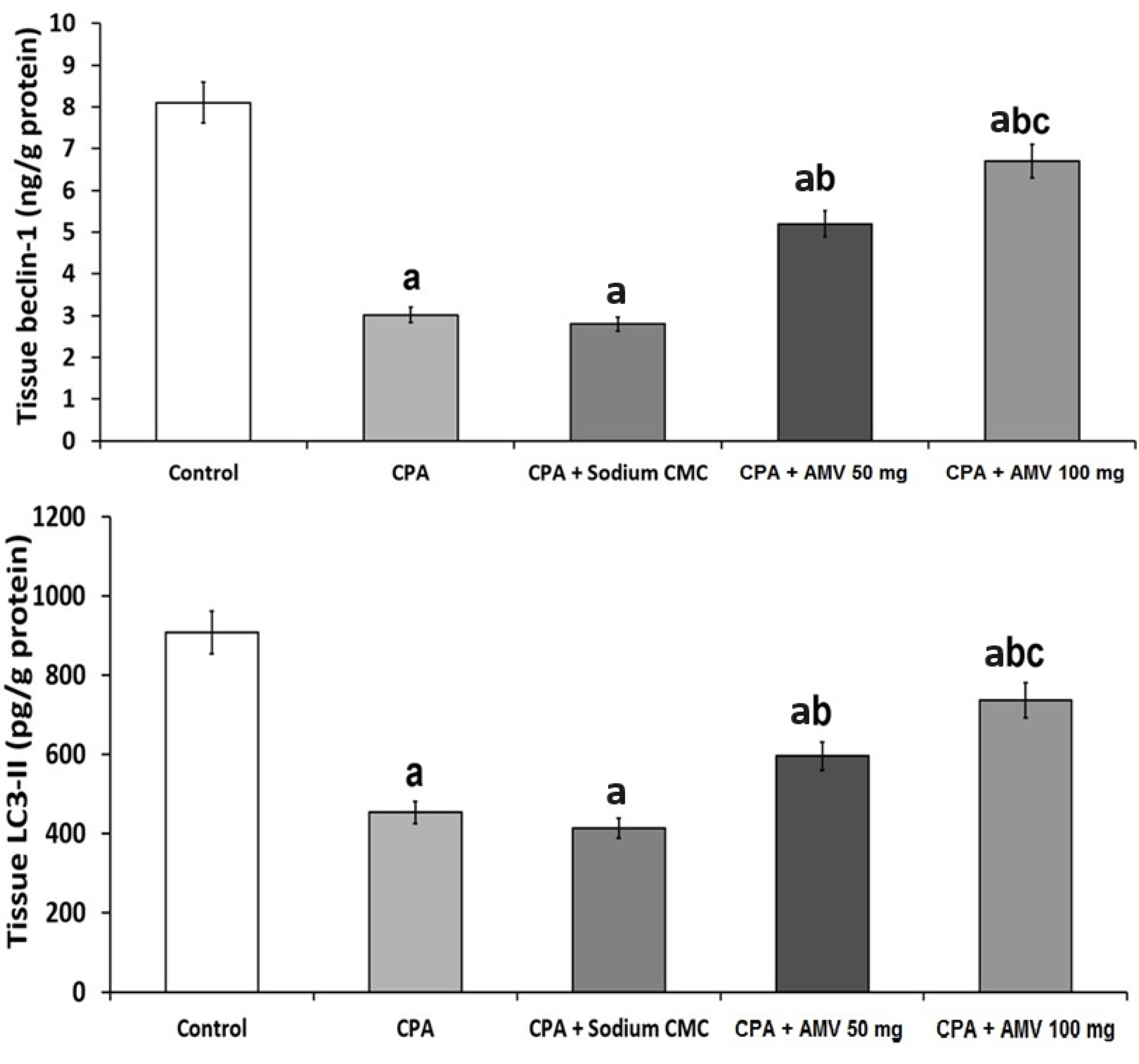
2.10. Measurement of the Lung Tissue Content of Beclin 1 and LC3-II as Markers of Autophagy
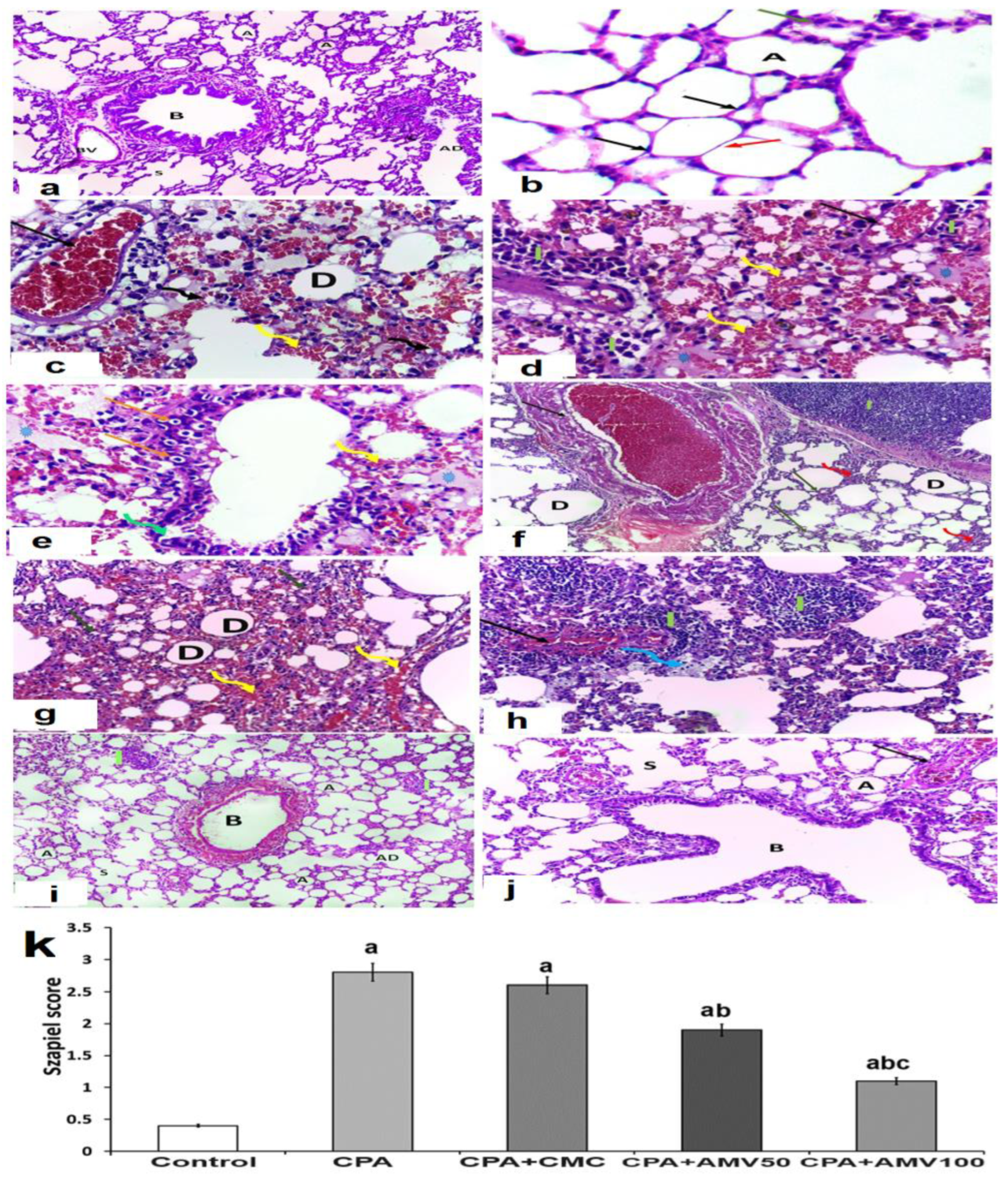
2.11. Analysis of the Histopathological Changes Induced by the Different Treatments in the Lung Tissue Specimens
2.12. Detection and Quantification of the Immunoreactivity to Cleaved Caspase 3 Antibodies in the Pulmonary Tissues
2.13. Assessment of the Effect of CPA with or without AMV on the Electron Microscopic Picture of the Pulmonary Tissues
2.14. Analysis of the Statistical Data
3. Results
3.1. AMV Dose-Dependently Ameliorated the Effect of CPA Administration on the Total and Differential Leucocytic Counts in BALF
3.2. AMV Administration, in a Dose-Dependent Manner, Abrogated the Perturbations in BALF LDH, TNF-α, IL-15, and MCP-1 Levels Induced by CPA Injection
3.3. AMV Dose-Dependently Reversed the Changes Elicited by CPA Injection in TGF-β1, NF-κB (p65), JAK2, and STAT3 Levels in the Pulmonary Tissues
3.4. AMV Dose-Dependently Mitigated the Changes in MDA and TAC Levels in the Pulmonary Tissues Induced via CPA Injection
3.5. AMV Administration Impeded the Changes in Nrf2, SIRT-1, and Keap1 Levels in the Pulmonary Tissues Induced via CPA Injection
3.6. AMV Reversed the Changes Induced by CPA Injection in Hydroxyproline, MMP-3, and MMP-9 Levels in the Pulmonary Tissues
3.7. Effect of the Different Treatments on the Markers of Autophagy
3.8. Effect of the Administration of the Different Doses of AMV on the Histopathological Changes in the Pulmonary Tissues Induced via CPA Injection
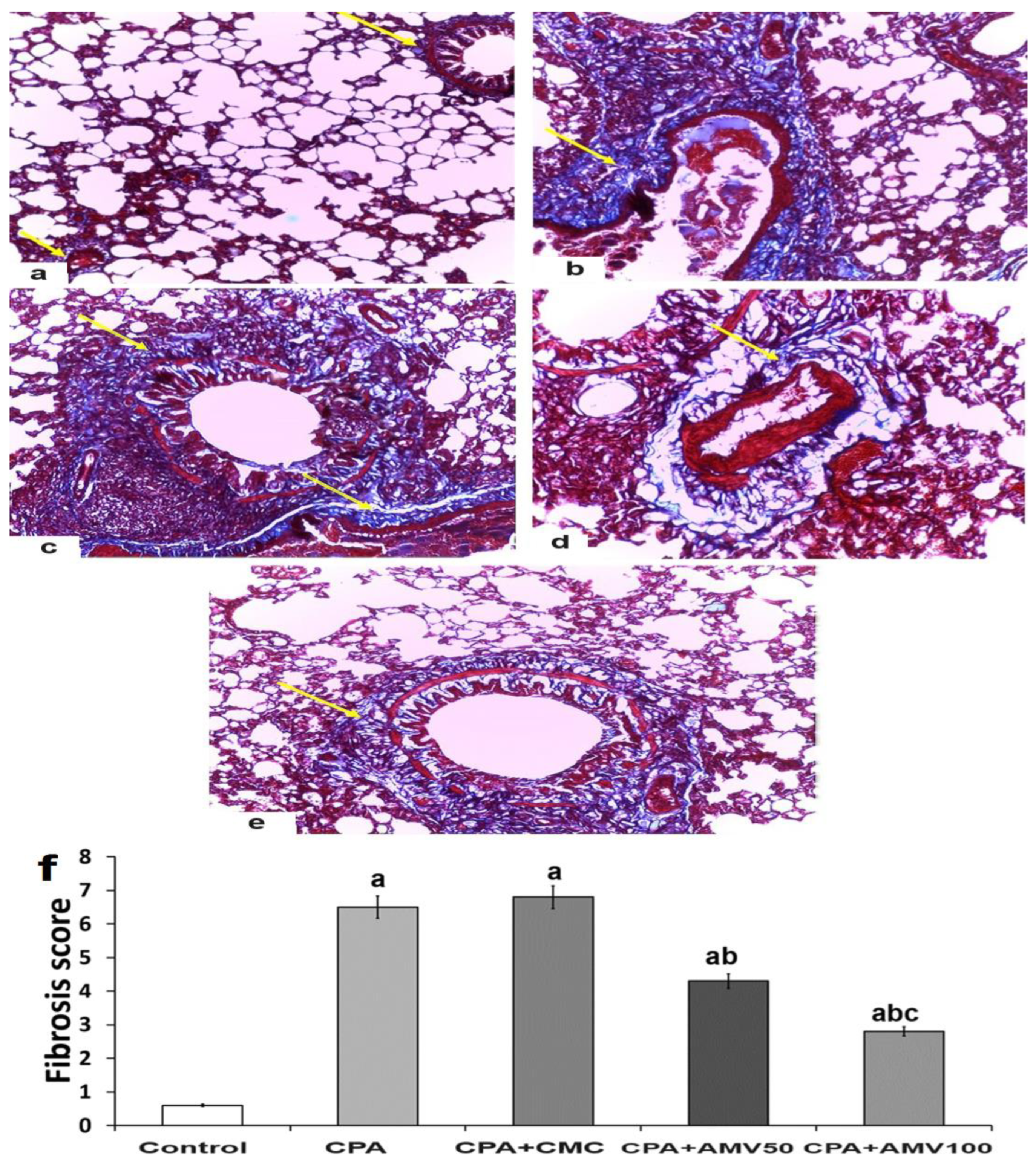
3.9. Effect of the Administration of the Different Doses of AMV on the Amount of Collagen Fibers Deposited in the Pulmonary Tissues Induced by CPA Injection
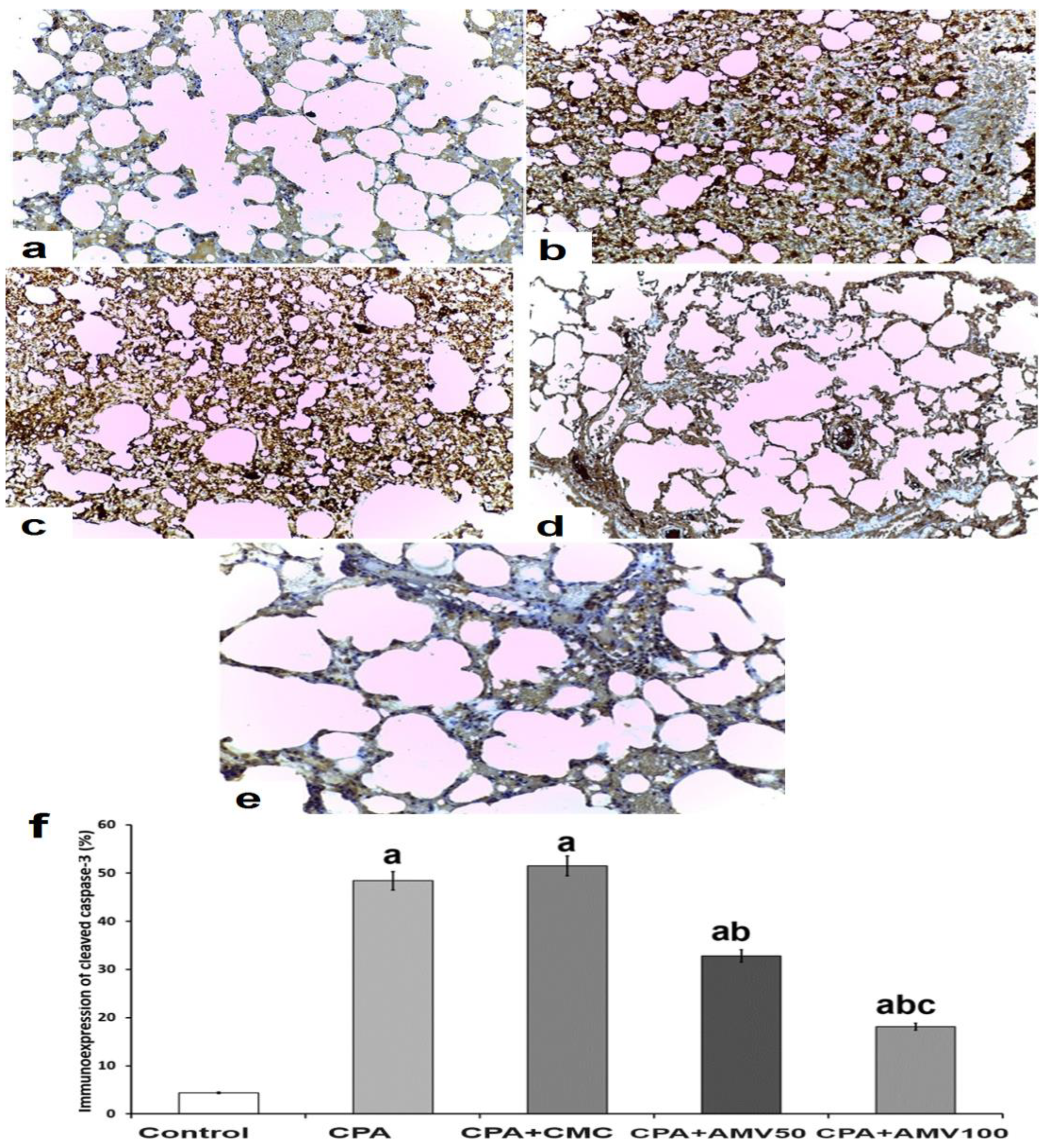
3.10. Effect of the Different Doses of AMV on the Changes in the Immunoexpression of Cleaved Caspase 3 in the Pulmonary Tissues of Rats Injected with CPA
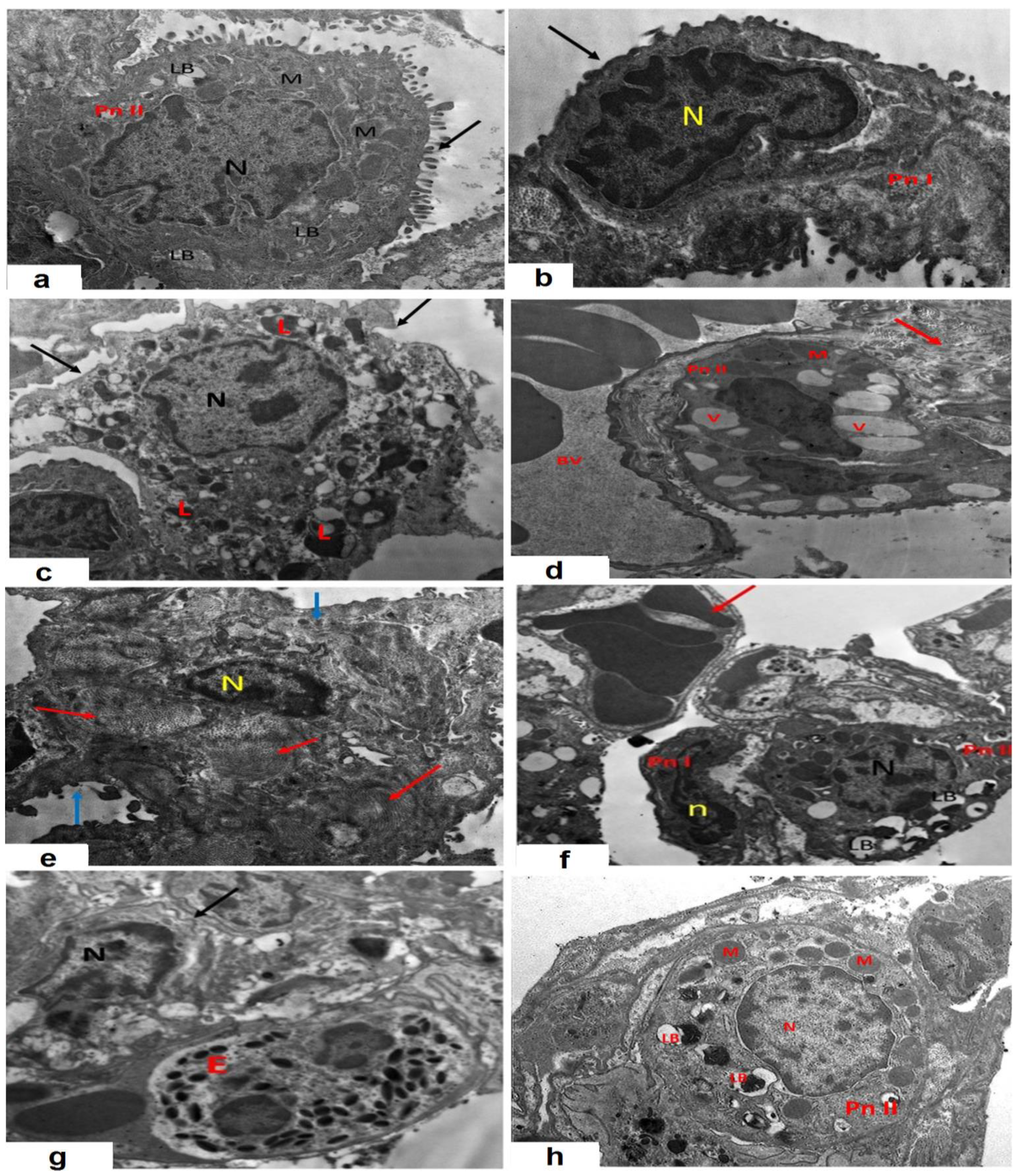
3.11. AMV Combatted the Effects of CPA Injection on the Electron Microscopic Picture of the Specimens Extracted from the Pulmonary Tissues
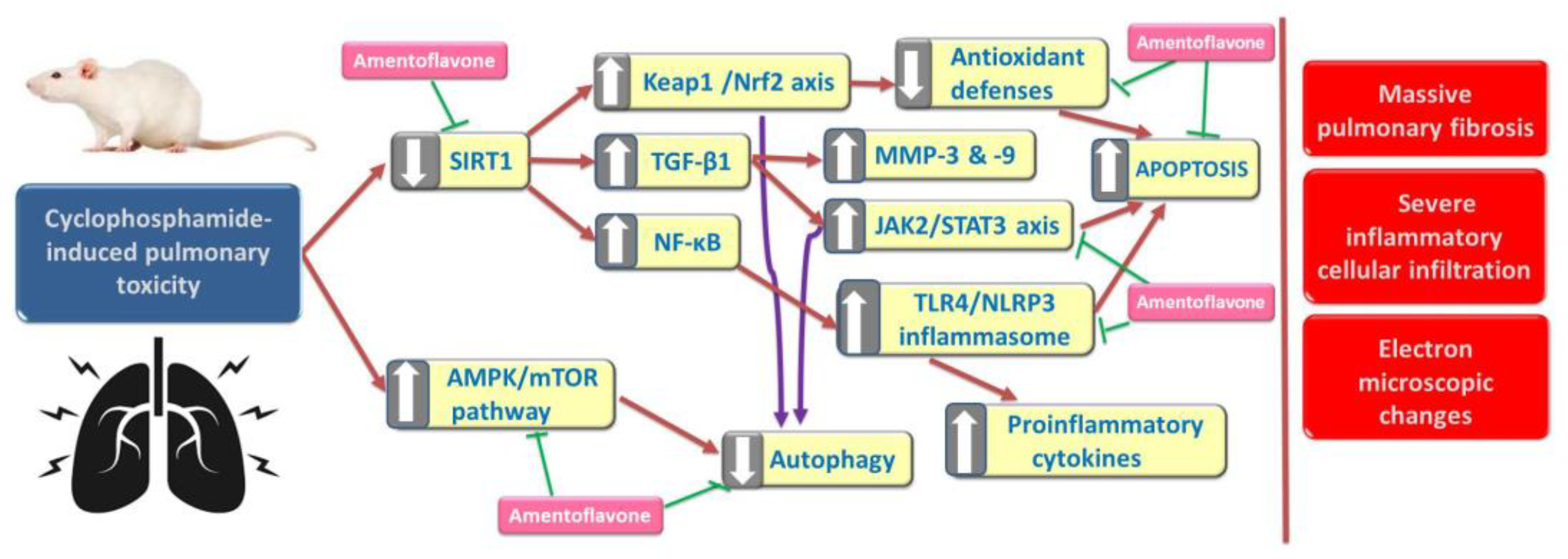
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, Y.K.; Si, Y.R.; An, G.Y.; Yuan, P. Efficacy and safety of cyclophosphamide in anthracycline- and taxane-based neoadjuvant chemotherapy in breast cancer: A meta-analysis. Gland Surg. 2021, 10, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Pugh, D.; Farrah, T.E.; Gallacher, P.J.; Kluth, D.C.; Dhaun, N. Cyclophosphamide-Induced Lung Injury. Kidney Int. Rep. 2018, 4, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Alsemeh, A.E.; Abdullah, D.M. Protective effect of alogliptin against cyclophosphamide-induced lung toxicity in rats: Impact on PI3K/Akt/FoxO1 pathway and downstream inflammatory cascades. Cell Tissue Res. 2022, 388, 417–438. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, S.Z. Sesamol Alleviates the Cytotoxic Effect of Cyclophosphamide on Normal Human Lung WI-38 Cells via Suppressing RAGE/NF-κB/Autophagy Signaling. Nat. Prod. Bioprospect. 2021, 11, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Dong, H.; Huang, N.; Fang, J. Oxidative stress and inflammation regulation of sirtuins: New insights into common oral diseases. Front. Physiol. 2022, 13, 953078. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Gao, X.; Wei, W. The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-β1 expressions in rat glomerular mesangial cells. Exp. Cell Res. 2017, 361, 63–72. [Google Scholar] [CrossRef]
- Salama, R.M.; Mohamed, A.M.; Hamed, N.S.; Ata, R.M.; NourelDeen, A.S.; Hassan, M.A. Alogliptin: A novel approach against cyclophosphamide-induced hepatic injury via modulating SIRT1/FoxO1 pathway. Toxicol. Res. 2020, 9, 561–568. [Google Scholar] [CrossRef]
- Huo, R.; Guo, Q.; Hu, J.; Li, N.; Gao, R.; Mi, L.; Zhang, Z.; Liu, H.; Guo, Z.; Zhao, H.; et al. Therapeutic Potential of Janus Kinase Inhibitors for the Management of Interstitial Lung Disease. Drug Des. Dev. Ther. 2022, 16, 991–998. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, H.; Li, J.; Xue, R.; Liu, H.; Zhu, Z.; Pan, C.; Lin, Y.; Hu, A.; Gou, P.; et al. Elevated HMGB1 expression induced by hepatitis B virus X protein promotes epithelial-mesenchymal transition and angiogenesis through STAT3/miR-34a/NF-κB in primary liver cancer. Am. J. Cancer Res. 2021, 11, 479–494. [Google Scholar]
- You, X.; Jiang, X.; Zhang, C.; Jiang, K.; Zhao, X.; Guo, T.; Zhu, X.; Bao, J.; Dou, H. Dihydroartemisinin attenuates pulmonary inflammation and fibrosis in rats by suppressing JAK2/STAT3 signaling. Aging 2022, 14, 1110–1127. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Li, D.; Qin, Y.; Sun, C.X.; Wang, Y.L.; Gao, L.; Ling-Hu, L.; Zhang, F.; Cai, W.; Zhu, L.; et al. Cardioprotective effects of Amentoflavone by suppression of apoptosis and inflammation on an in vitro and vivo model of myocardial ischemia-reperfusion injury. Int. Immunopharmacol. 2021, 101 Pt B, 108296. [Google Scholar] [CrossRef]
- Balaha, M.F.; Almalki, Z.S.; Alahmari, A.K.; Ahmed, N.J.; Balaha, M.F. AMPK/mTOR-driven autophagy & Nrf2/HO-1 cascade modulation by amentoflavone ameliorates indomethacin-induced gastric ulcer. Biomed. Pharmacother. 2022, 151, 113200. [Google Scholar] [CrossRef]
- Xiong, X.; Tang, N.; Lai, X.; Zhang, J.; Wen, W.; Li, X.; Li, A.; Wu, Y.; Liu, Z. Insights Into Amentoflavone: A Natural Multifunctional Biflavonoid. Front. Pharmacol. 2021, 12, 768708. [Google Scholar] [CrossRef] [PubMed]
- Dejani, N.N.; Elshabrawy, H.A.; Bezerra Filho CD, S.M.; de Sousa, D.P. Anticoronavirus and Immunomodulatory Phenolic Compounds: Opportunities and Pharmacotherapeutic Perspectives. Biomolecules 2021, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, M.M. Amentoflavone Induces Autophagy and Modulates p53. Cell J. 2019, 21, 27–34. [Google Scholar] [CrossRef]
- Rabidas, S.S.; Prakash, C.; Tyagi, J.; Suryavanshi, J.; Kumar, P.; Bhattacharya, J.; Sharma, D. A Comprehensive Review on Anti-Inflammatory Response of Flavonoids in Experimentally-Induced Epileptic Seizures. Brain Sci. 2023, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Viall, A.K.; LeVine, D.N. Performance of a Nageottehemocytometer method and a flow cytometric assay for residual leukocyte quantification in leukoreduced canine packed red blood cells. J. Vet. Emerg. Crit. Care 2020, 30, 272–278. [Google Scholar] [CrossRef]
- Hübner, R.H.; Gitter, W.; El Mokhtari, N.E.; Mathiak, M.; Both, M.; Bolte, H.; Freitag-Wolf, S.; Bewig, B. Standardized quantification of pulmonary fibrosis in histological samples. BioTechniques 2008, 44, 507–517. [Google Scholar] [CrossRef]
- Sharma, P.; Alizadeh, J.; Juarez, M.; Samali, A.; Halayko, A.J.; Kenyon, N.J.; Ghavami, S.; Zeki, A.A. Autophagy, Apoptosis, the Unfolded Protein Response, and Lung Function in Idiopathic Pulmonary Fibrosis. Cells 2021, 10, 1642. [Google Scholar] [CrossRef]
- Mohamed, M.T.; Zaitone, S.A.; Ahmed, A.; Mehanna, E.T.; El-Sayed, N.M. Raspberry Ketones Attenuate Cyclophosphamide-Induced Pulmonary Toxicity in Mice through Inhibition of Oxidative Stress and NF-ΚB Pathway. Antioxidants 2020, 9, 1168. [Google Scholar] [CrossRef]
- Qian, P.; Peng, C.H.; Ye, X. Interstitial pneumonia induced by cyclophosphamide: A case report and review of the literature. Respir. Med. Case Rep. 2019, 26, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Elgohary, R.; Amin, M.M.; Elwahab, S.A. Impact of protocatechuic acid on alleviation of pulmonary damage induced by cyclophosphamide targeting peroxisome proliferator activator receptor, silent information regulator type-1, and fork head box protein in rats. Inflammopharmacology 2023, 31, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.M.; Kamel, E.O.; Gad-Elrab, W.M.; Ahmed, M.A.; Mohammedsaleh, Z.M.; Ali, F.E.M. Lansoprazole attenuates cyclophosphamide-induced cardiopulmonary injury by modulating redox-sensitive pathways and inflammation. Mol. Cell. Biochem. 2023, 478, 2319–2335. [Google Scholar] [CrossRef]
- Wang, J.; Shao, W.; Niu, H.; Yang, T.; Wang, Y.; Cai, Y. Immunomodulatory Effects of Colistin on Macrophages in Rats by Activating the p38/MAPK Pathway. Front. Pharmacol. 2019, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Hickman-Davis, J.M.; Lindsey, J.R.; Matalon, S. Cyclophosphamide decreases nitrotyrosine formation and inhibits nitric oxide production by alveolar macrophages in mycoplasmosis. Infect. Immun. 2001, 69, 6401–6410. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, C.; Du, Y.; Huang, Y.; Zhao, Q. Amentoflavone ameliorates cold stress-induced inflammation in lung by suppression of C3/BCR/NF-κB pathways. BMC Immunol. 2019, 20, 49. [Google Scholar] [CrossRef]
- Abdallah, H.M.I.; Abdel-Rahman, R.F.; El Awdan, S.A.; Allam, R.M.; El-Mosallamy, A.E.M.K.; Selim, M.S.; Mohamed, S.S.; Arbid, M.S.; Farrag, A.R.H. Protective effect of some natural products against chemotherapy-induced toxicity in rats. Heliyon 2019, 5, e01590. [Google Scholar] [CrossRef]
- Boutanquoi, P.M.; Burgy, O.; Beltramo, G.; Bellaye, P.S.; Dondaine, L.; Marcion, G.; Pommerolle, L.; Vadel, A.; Spanjaard, M.; Demidov, O.; et al. TRIM33 prevents pulmonary fibrosis by impairing TGF-β1 signalling. Eur. Respir. J. 2020, 55, 1901346. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Abdel-Latif, G.A.; Elwahab, A.H.A.; Hasan, R.A.; ElMongy, N.F.; Ramzy, M.M.; Louka, M.L.; Schaalan, M.F. A novel protective role of sacubitril/valsartan in cyclophosphamide induced lung injury in rats: Impact of miRNA-150-3p on NF-κB/MAPK signaling trajectories. Sci. Rep. 2020, 10, 13045. [Google Scholar] [CrossRef] [PubMed]
- Afshari, H.; Nourbakhsh, M.; Salehi, N.; Mahboubi-Rabbani, M.; Zarghi, A.; Noori, S. STAT3-mediated Apoptotic-enhancing Function of Sclareol against Breast Cancer Cells and Cell Sensitization to Cyclophosphamide. Iran. J. Pharm. Res. IJPR 2020, 19, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, Y.; Wang, S.; Shen, Q.; Zhou, X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018, 415, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Luan, J.; Gao, S.; Li, S.; Jiang, Q.; Liu, R.; Liang, Q.; Zhang, R.; Zhang, F.; Li, X.; et al. Fedratinib Attenuates Bleomycin-Induced Pulmonary Fibrosis via the JAK2/STAT3 and TGF-β1 Signaling Pathway. Molecules 2021, 26, 4491. [Google Scholar] [CrossRef]
- Taverna, S.; Tonacci, A.; Ferraro, M.; Cammarata, G.; Cuttitta, G.; Bucchieri, S.; Pace, E.; Gangemi, S. High Mobility Group Box 1: Biological Functions and Relevance in Oxidative Stress Related Chronic Diseases. Cells 2022, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, F.K.; Ali, S.I.; Elgohary, R.; Salama, A. Natural β-carotene prevents acute lung injury induced by cyclophosphamide in mice. PLoS ONE 2023, 18, e0283779. [Google Scholar] [CrossRef] [PubMed]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Yang, K.; Dong, W. SIRT1-Related Signaling Pathways and Their Association with Bronchopulmonary Dysplasia. Front. Med. 2021, 8, 595634. [Google Scholar] [CrossRef]
- Mansour, H.H.; Omran, M.M.; Hasan, H.F.; El Kiki, S.M. Modulation of bleomycin-induced oxidative stress and pulmonary fibrosis by N-acetylcysteine in rats via AMPK/SIRT1/NF-κβ. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1943–1952. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Yang, M.; Yan, K.; Fan, X.; Ci, X.; Peng, L. Amentoflavone Ameliorates Carrageenan-Induced Pleurisy and Lung Injury by Inhibiting the NF-κB/STAT3 Pathways via Nrf2 Activation. Front. Pharmacol. 2022, 13, 763608. [Google Scholar] [CrossRef] [PubMed]
- Deniz, F.S.Ş.; Eren, G.; Orhan, I.E. Flavonoids as Sirtuin Modulators. Curr. Top. Med. Chem. 2022, 22, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Sun, J.; Zhang, S.; Nie, Y.; Zhou, S.; Zeng, Y. Progress in understanding and treating idiopathic pulmonary fibrosis: Recent insights and emerging therapies. Front. Pharmacol. 2023, 14, 1205948. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Martínez, G.; Jiménez-Álvarez, L.A.; Cruz-Lagunas, A.; Ignacio-Cortés, S.; Gómez-García, I.A.; Rodríguez-Reyna, T.S.; Choreño-Parra, J.A.; Zúñiga, J. Possible Role of Matrix Metalloproteinases and TGF-β in COVID-19 Severity and Sequelae. J. Interferon Cytokine Res. 2022, 42, 352–368. [Google Scholar] [CrossRef]
- Elkington, P.T.; Friedland, J.S. Matrix metalloproteinases in destructive pulmonary pathology. Thorax 2006, 61, 259–266. [Google Scholar] [CrossRef]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef]
- Sun, Q.; Zhen, P.; Li, D.; Liu, X.; Ding, X.; Liu, H. Amentoflavone promotes ferroptosis by regulating reactive oxygen species (ROS)/5′AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) to inhibit the malignant progression of endometrial carcinoma cells. Bioengineered 2022, 13, 13269–13279. [Google Scholar] [CrossRef]
- Elkhoely, A.; Estfanous, R.S.; Alrobaian, M.; Borg, H.M.; Kabel, A.M. Repositioning itraconazole for amelioration of bleomycin-induced pulmonary fibrosis: Targeting HMGB1/TLR4 Axis, NLRP3 inflammasome/NF-κB signaling, and autophagy. Life Sci. 2023, 313, 121288. [Google Scholar] [CrossRef]
- Al-Amarat, W.; Abukhalil, M.H.; Alruhaimi, R.S.; Alqhtani, H.A.; Aldawood, N.; Alfwuaires, M.A.; Althunibat, O.Y.; Aladaileh, S.H.; Algefare, A.I.; Alanezi, A.A.; et al. Upregulation of Nrf2/HO-1 Signaling and Attenuation of Oxidative Stress, Inflammation, and Cell Death Mediate the Protective Effect of Apigenin against Cyclophosphamide Hepatotoxicity. Metabolites 2022, 12, 648. [Google Scholar] [CrossRef]
- Yue, Y.L.; Zhang, M.Y.; Liu, J.Y.; Fang, L.J.; Qu, Y.Q. The role of autophagy in idiopathic pulmonary fibrosis: From mechanisms to therapies. Ther. Adv. Respir. Dis. 2022, 16, 17534666221140972. [Google Scholar] [CrossRef] [PubMed]
- Saghir, S.A.M.; Alharbi, S.A.; Al-Garadi, M.A.; Al-Gabri, N.; Rady, H.Y.; Olama, N.K.; Abdulghani, M.A.M.; Al Hroob, A.M.; Almaiman, A.A.; Bin-Jumah, M.; et al. Curcumin Prevents Cyclophosphamide-Induced Lung Injury in Rats by Suppressing Oxidative Stress and Apoptosis. Processes 2020, 8, 127. [Google Scholar] [CrossRef]
- Jung, S.; Jeong, H.; Yu, S.W. Autophagy as a decisive process for cell death. Exp. Mol. Med. 2020, 52, 921–930. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaha, M.F.; Alamer, A.A.; Aldossari, R.M.; Aodah, A.H.; Helal, A.I.; Kabel, A.M. Amentoflavone Mitigates Cyclophosphamide-Induced Pulmonary Toxicity: Involvement of -SIRT-1/Nrf2/Keap1 Axis, JAK-2/STAT-3 Signaling, and Apoptosis. Medicina 2023, 59, 2119. https://doi.org/10.3390/medicina59122119
Balaha MF, Alamer AA, Aldossari RM, Aodah AH, Helal AI, Kabel AM. Amentoflavone Mitigates Cyclophosphamide-Induced Pulmonary Toxicity: Involvement of -SIRT-1/Nrf2/Keap1 Axis, JAK-2/STAT-3 Signaling, and Apoptosis. Medicina. 2023; 59(12):2119. https://doi.org/10.3390/medicina59122119
Chicago/Turabian StyleBalaha, Mohamed F., Ahmed A. Alamer, Rana M. Aldossari, Alhussain H. Aodah, Azza I. Helal, and Ahmed M. Kabel. 2023. "Amentoflavone Mitigates Cyclophosphamide-Induced Pulmonary Toxicity: Involvement of -SIRT-1/Nrf2/Keap1 Axis, JAK-2/STAT-3 Signaling, and Apoptosis" Medicina 59, no. 12: 2119. https://doi.org/10.3390/medicina59122119
APA StyleBalaha, M. F., Alamer, A. A., Aldossari, R. M., Aodah, A. H., Helal, A. I., & Kabel, A. M. (2023). Amentoflavone Mitigates Cyclophosphamide-Induced Pulmonary Toxicity: Involvement of -SIRT-1/Nrf2/Keap1 Axis, JAK-2/STAT-3 Signaling, and Apoptosis. Medicina, 59(12), 2119. https://doi.org/10.3390/medicina59122119







