Role of Magnetic Resonance Imaging in Pelvic Organ Prolapse Evaluation
Abstract
1. Introduction
2. Materials and Methods
- Presence of prolapse (cystocele and/or hysterocele and/or rectocele) ≥ stage 2 POP-Q;
- Indication for vaginal surgery (vaginal hysterectomy in case of hysterocele; anterior and posterior fascial repair for cystocele and rectocele, respectively).
2.1. Exclusion Criterion was Previous Vaginal Surgery
2.2. MRI Imaging Protocol
2.3. Preparation
2.4. Sequences
- -
- High-spatial-resolution static sequences for studying the morphology of the levator ani;
- -
- Dynamic sequences used to assess abnormalities of the pelvic organs during contraction, at rest, during straining, and during defecation.
- -
- SSFSE (TR/TE, 708/90; flip angle, 90°; section thickness, 8 mm; bandwidth, 83.3 kHz; FOV, 34 cm; matrix, 384 × 224; several averages, 0.5; acquisition time for each image, 0.3 s) in the midsagittal plane, with sequential acquisition during contraction, rest, and straining;
- -
- FIESTA (TR/TE, 3.3/1.4; flip angle, 45°; section thickness, 8 mm; bandwidth, 125 kHz; FOV, 35 cm; matrix, 224 × 224; a number of averages, 1; number of images, 20; acquisition time, 20 s) in the midsagittal plane, with continuous multiphase acquisition during contraction, rest, straining, and defecation.
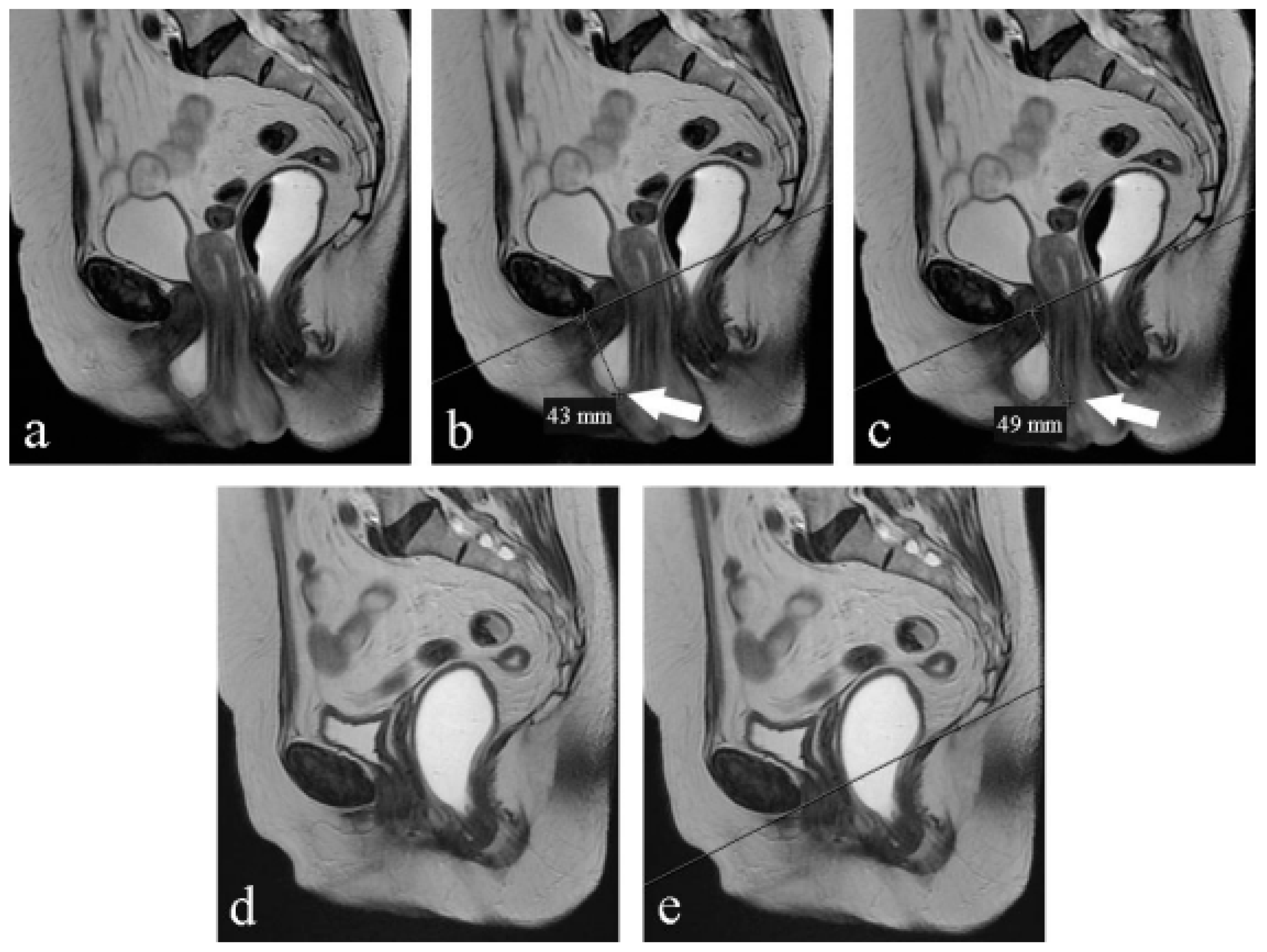
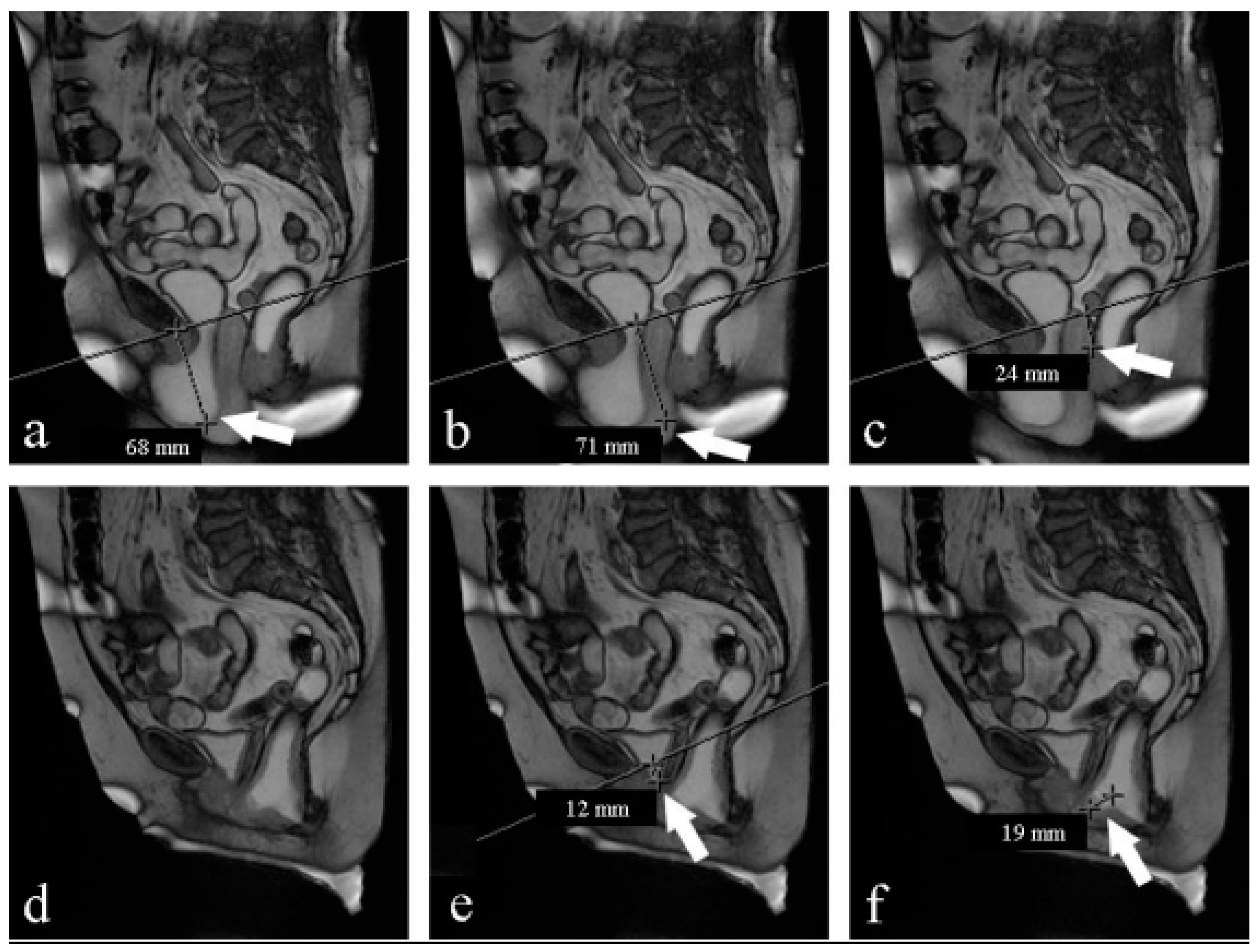
- Pubococcygeal line (PCL): a line drawn from the inferior margin of the pubic symphysis to the last coccygeal joint [16];
- Levator plate angle (LPA): enclosed between the levator plate and the PCL where the ileo-coccygeus touches the coccyx. It is usually 11.7° ± 4.8 in healthy women under the Valsalva maneuver [16].
- Posterior vesicoureteral angle (VUA): the angle between the axis of the urethra and the bladder base. In normal conditions, it is about 90°.
- The H-line: extends from the inferior aspect of the pubic symphysis to the anorectal junction, represents the genital hiatus: on effort, it is 5.8 cm ± 0.5 [19].
- The M-line: dropped as a perpendicular line from the PCL to the posterior aspect of the H-line: normally on effort, it is 1.3 cm ± 0.5 [19].
- The total vaginal length (TVL): distance from the posterior part of the introitus to the proximal vagina; if the cervix is present, it corresponds to the posterior fornix; if the woman had a hysterectomy, it corresponds to the vaginal vault.
- Funneling: the opening of the proximal urethra on effort.
- The thickness of the mid urethra.
2.5. Surgery
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelleher, C.J.; Cardozo, L.D.; Khullar, V.; Salvatore, S. A new questionnaire to assess the quality of life of urinary incontinent women. Br. J. Obstet. Gynaecol. 1997, 104, 1374–1379. [Google Scholar] [CrossRef]
- Tinelli, A.; Malvasi, A.; Rahimi, S.; Negro, R.; Vergara, D.; Martignago, R.; Pellegrino, M.; Cavallotti, C. Age-related pelvic floor modifications and prolapse risk factors in postmenopausal women. Menopause 2010, 17, 204–212. [Google Scholar] [CrossRef]
- Morley, G.W. Treatment of uterine and vaginal prolapse. Clin. Obstet. Gynecol. 1996, 39, 959–969. [Google Scholar] [CrossRef]
- Olsen, A.L.; Smith, V.J.; Bergstrom, J.O.; Colling, J.C.; Clark, A.L. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet. Gynecol. 1997, 89, 501–506. [Google Scholar] [CrossRef]
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int. Urogynecol. J. 2010, 21, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Mostwin, J.L.; Rosenshein, N.B.; Zerhouni, E. Pelvic floor descent in women: Dynamic evaluation with fast MR imaging and cinematic display. Radiology 1991, 179, 25–33. [Google Scholar] [CrossRef]
- Mengert, W.F. Mechanics of uterine support and position. Am. J. Obstet. Gynecol. 1936, 31, 775–782. [Google Scholar] [CrossRef]
- DeLancey, J.O. Anatomy and biomechanics of genital prolapse. Clin. Obstet. Gynecol. 1993, 36, 897–909. [Google Scholar] [CrossRef]
- DeLancey, J.O. Functional anatomy of the pelvic floor. In Imaging Pelvic Floor Disorders; Bartram, C.I., DeLancey, J.O., Halligan, S., Eds.; Springer: New York, NY, USA, 2003; pp. 27–38. [Google Scholar]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; van Kerrebroeck, P.; Victor, A.; Wein, A.J. The standardization of terminology of lower urinary tract function. Report from the standardization subcommittee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef]
- Bump, R.C.; Cundiff, G.W. Pelvic organ prolapse. In Clinical Urogynaecology; Stanton, S.L., Monga, A.K., Eds.; Churchill Livingstone: London, UK, 2000; pp. 357–372. [Google Scholar]
- Bump, R.C.; Mattiasson, A.; Bø, K.; Brubaker, L.P.; DeLancey, J.O.; Klarskov, P.; Shull, B.L.; Smith, A.R. The standardization of female pelvic organ prolapse and pelvic floor dysfunction. Am. J. Obstet. Gynecol. 1996, 175, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Kelvin, F.M.; Maglinte, D.D. Dynamic cystoproctography of female pelvic floor defects and their interrelationships. AJR Am. J. Roentgenol. 1997, 169, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Fielding, J.R. MR imaging of pelvic floor relaxation. Radiol. Clin. N. Am. 2003, 41, 747–756. [Google Scholar] [CrossRef]
- Kruyt, R.H.; Delemarre, J.B.; Doornbos, J.; Vogel, H.J. Normal anorectum: Dynamic MR imaging anatomy. Radiology 1991, 179, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.; Lewis, M.; Yalamanchili, S.; Lim, R.; Wong, S.; Bennett, G. Prevalence of pelvic organ prolapse detected at dynamic MRI in women without history of pelvic floor dysfunction: Comparison of two reference lines. Clin. Radiol. 2014, 69, e71–e77. [Google Scholar] [CrossRef]
- Lienemann, A.; Anthuber, C.; Baron, A.; Kohz, P.; Reiser, M. Dynamic MR colpocystorectography assessing pelvic floor descent. Eur. Radiol. 1997, 7, 1309–1317. [Google Scholar] [CrossRef]
- Goh, V.; Halligan, S.; Kaplan, G.; Healy, J.C.; Bartram, C.I. Dynamic MRI of the pelvic floor in asymptomatic subjects. AJR Am. J. Roentgenol. 2000, 174, 661–666. [Google Scholar] [CrossRef]
- El Sayed, R.F.; Fielding, J.R.; El Mashed, S.; Morsy, M.M.; Abd El Azim, M.S. Preoperative and postoperative magnetic resonance imaging of female pelvic floor dysfunction: Correlation with clinical findings. J. Women’s Imaging 2005, 7, 163–180. [Google Scholar] [CrossRef][Green Version]
- Wein, A.J. FDA Safety communication: Urogynecologic Surgical Mesh: Update on the Safety and Effectiveness of Trans-vaginal Placement for Pelvic Organ Prolapse. J. Urol. 2011, 186, 2328–2330. [Google Scholar] [CrossRef]
- Kaufman, H.S.; Buller, J.L.; Thompson, J.R.; Pannu, H.K.; DeMeester, S.L.; Genadry, R.R.; Bluemke, D.A.; Jones, B.; Rychcik, J.L.; Cundiff, G.W. Dynamic pelvic magnetic resonance imaging and cystocolpoproctography alter surgical management of pelvic floor disorders. Dis. Colon. Rectum. 2001, 44, 1575–1584. [Google Scholar] [CrossRef]
- Altringer, W.E.; Saclarides, T.J.; Dominguez, J.M.; Brubaker, L.T.; Smith, C.S. Fourcontrast defecography: Pelvic “floor-oscopy”. Dis. Colon. Rectum. 1995, 38, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Wyman, A.M.; Salemi, J.L.; Mikhail, E.; Bassaly, R.; Greene, K.A.; Hart, S.; Lai-Yuen, S. Cost-effectiveness of a preoperative pelvic MRI in pelvic organ prolapse surgery. Int. Urogynecol. J. 2020, 31, 1443–1449. [Google Scholar] [CrossRef]
- Foti, P.V.; Farina, R.; Riva, G.; Coronella, M.; Fisichella, E.; Palmucci, S.; Racalbuto, A.; Politi, G.; Ettorre, G.C. Pelvic floor imaging: Comparison between magnetic resonance imaging and conventional defecography in studying outlet obstruction syndrome. Radiol. Med. 2013, 118, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.C.L.; Peng, Q.; Stokes, M.; Humphrey, V.F.; Payne, C.; Constantinou, C.E. Mechanisms of pelvic floor muscle function and the effect on the urethra during a cough. Eur. Urol. 2010, 57, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H. Cystourethrography in women. Brit. J. Radiol. 1952, 25, 253–259. [Google Scholar] [CrossRef]
- Jeffcoate, T.N.; Roberts, H. Observations on stress incontinence of urine. Am. J. Obst. Gynecol. 1952, 64, 721–738. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, A.; Jeffcoate, T.N.; Roberts, H. Stress incontinence of urine. J. Obstet. Gynaecol. Br. Emp. 1952, 59, 681–720. [Google Scholar] [CrossRef] [PubMed]
- Dutton, W.A. The urethrovesical angle and stress incontinence. Can. Med. Assoc. J. 1960, 83, 1242–1245. [Google Scholar]
| General Characteristics | |
|---|---|
| Age | 60 ± 7 |
| Menopause | 90% |
| Vaginal delivery | 2.5 (average) |
| Forceps or vacuum | 40% |
| Macrosoma | 40% |
| Preoperative symptoms | |
| Heaviness | 100% |
| SUI | 50% |
| Urgency | 50% |
| Frequency | 30% |
| Retention | 70% |
| GRADE | 2 | 3 | 4 | |||
|---|---|---|---|---|---|---|
| Clinic | MR | Clinic | MR | Clinic | MR | |
| Cystocele | 50% | 70% | 50% | 30% | 0% | 0% |
| Hysterocele or apex | 40% | 80% | 10% | 10% | 30% | 0% |
| Rectocele | 10% | 10% | 0% | 0% | 0% | 0% |
| GRADE | 0 | 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinic | MR | Clinic | MR | Clinic | MR | Clinic | MR | Clinic | MR | |
| Cystocele | 80% | 40% | 10% | 40% | 10% | 20% | 0% | 0% | 0% | 0% |
| Hysterocele or apex | 100% | 80% | 0% | 20% | 0% | 0% | 0% | 0% | 0% | 0% |
| Rectocele | 70% | 30% | 20% | 40% | 10% | 30% | 0% | 0% | 0% | 0% |
| At Rest | Pre Average ± DS | Post Average ± DS | p |
|---|---|---|---|
| PCL (mm) | 100 ± 12.22 | 100.2 ± 12.44 | 0.76 |
| H-line (mm) | 58.4 ± 8.96 | 57.4 ± 6.69 | 0.43 |
| M-line (mm) | 55.9 ± 8.03 | 52.3 ± 6.67 | 0.06 |
| TVL (mm) | 66.3 ± 26.33 | 59.4 ± 21.67 | 0.27 |
| Urethra (mm) | 5.1 ± 0.57 | 5.2 ± 0.41 | 0.55 |
| LPA (°) | 22.6 ± 9.05 | 21.2 ± 8.52 | 0.38 |
| VUA (°) | 156.7 ± 48.05 | 161 ± 11.67 | 0.77 |
| Valsalva | Pre Average ± DS | Post Average ± DS | p |
|---|---|---|---|
| PCL (mm) | 103.3 ± 11.26 | 102.6 ± 11.96 | 0 |
| H-line (mm) | 82.2 ± 11.75 | 77.6 ± 12.75 | 0.008 |
| M-line (mm) | 69.8 ± 6.42 | 65 ± 9.64 | 0.001 |
| LPA (°) | 50.7 ± 9.5 | 44.7 ± 11.12 | 0.001 |
| VUA (°) | 130 ± 68 | 187.4 ± 53.32 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarpietro, G.; Foti, P.V.; Conte, C.; Matarazzo, M.G. Role of Magnetic Resonance Imaging in Pelvic Organ Prolapse Evaluation. Medicina 2023, 59, 2074. https://doi.org/10.3390/medicina59122074
Sarpietro G, Foti PV, Conte C, Matarazzo MG. Role of Magnetic Resonance Imaging in Pelvic Organ Prolapse Evaluation. Medicina. 2023; 59(12):2074. https://doi.org/10.3390/medicina59122074
Chicago/Turabian StyleSarpietro, Giuseppe, Pietro Valerio Foti, Carmine Conte, and Maria Grazia Matarazzo. 2023. "Role of Magnetic Resonance Imaging in Pelvic Organ Prolapse Evaluation" Medicina 59, no. 12: 2074. https://doi.org/10.3390/medicina59122074
APA StyleSarpietro, G., Foti, P. V., Conte, C., & Matarazzo, M. G. (2023). Role of Magnetic Resonance Imaging in Pelvic Organ Prolapse Evaluation. Medicina, 59(12), 2074. https://doi.org/10.3390/medicina59122074







