In Vitro Assessment of Anti-Adipogenic and Anti-Inflammatory Properties of Black Cumin (Nigella sativa L.) Seeds Extract on 3T3-L1 Adipocytes and Raw264.7 Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Substance
2.2. HPLC Analyses
2.3. Measurement of DPPH Radical Scavenging Activity
2.4. Cell Culture Conditions and Treatment of Drugs
2.5. Cell Viability Assay
2.6. Nitric Oxide Assay
2.7. ELISA Assay
2.8. RNA Isolation and qPCR Analysis
2.9. Immunochemical Analysis
2.10. Oil Red O Staining
2.11. Statistical Analysis
3. Results
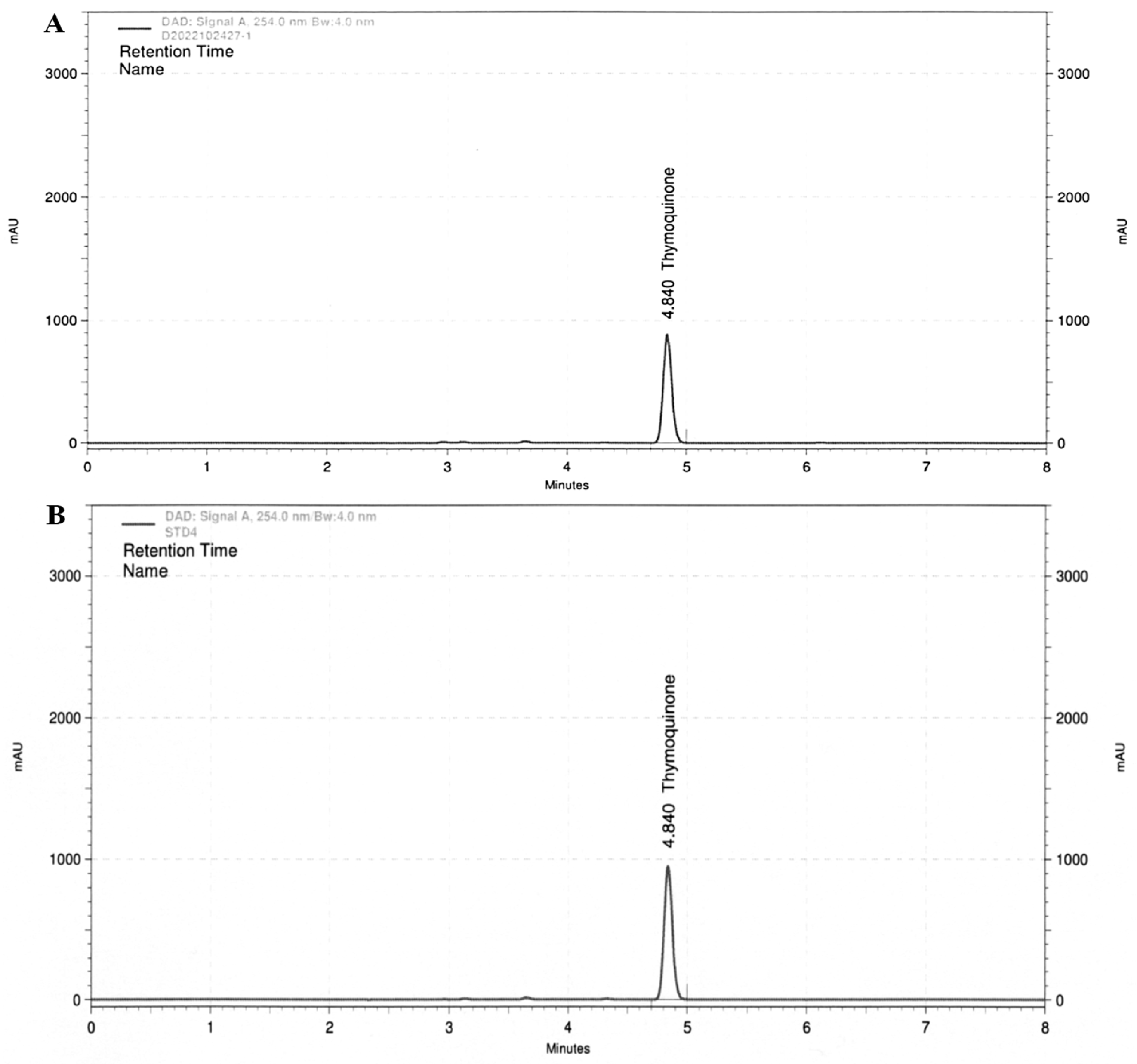
3.1. Detection of Thymoquinone in BCS Extract
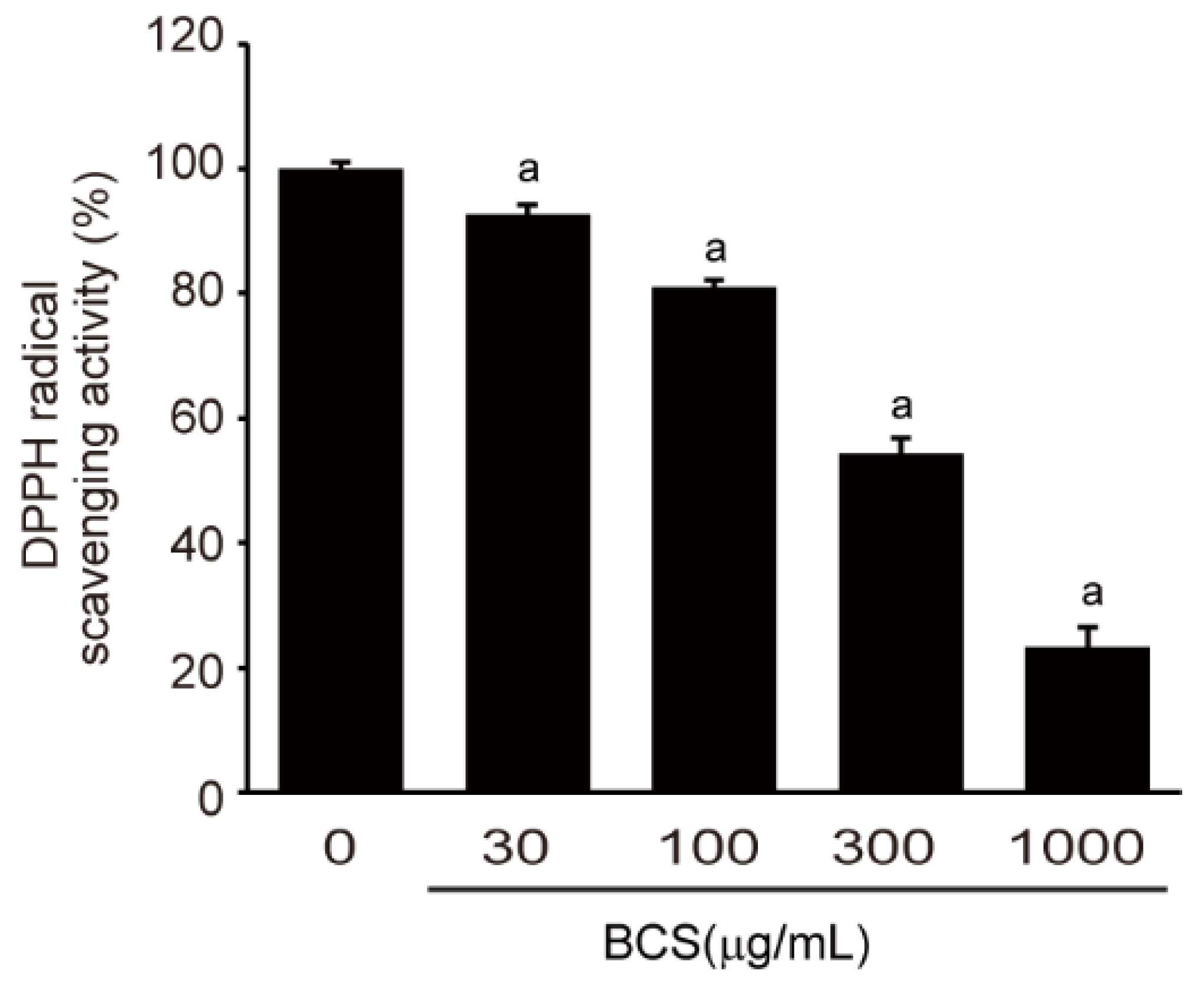
3.2. Free Radical Scavenging Activity Analysis
3.3. Anti-Inflammatory Activity of BCS Extract
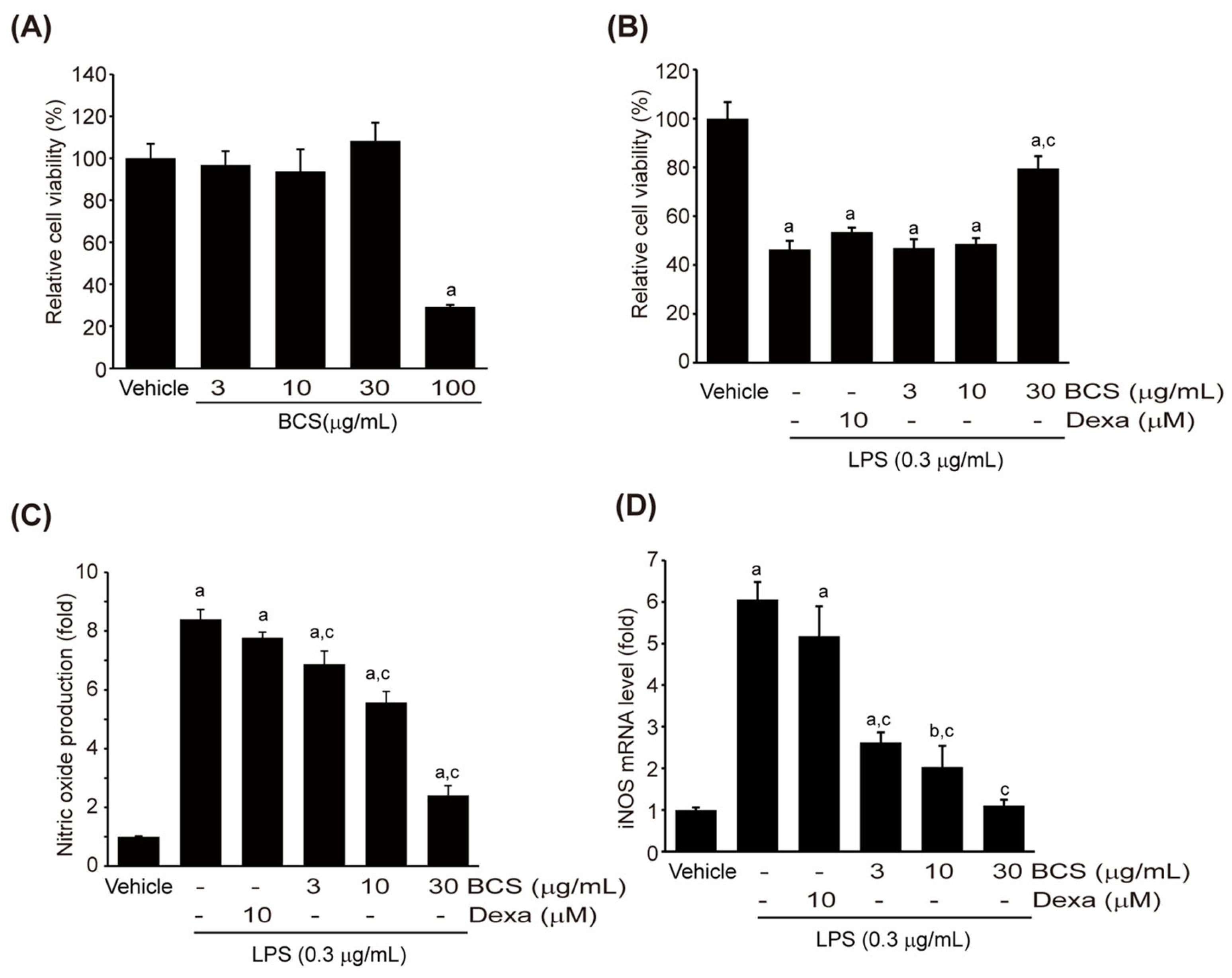
3.3.1. Viability of Raw264.7 Cells
3.3.2. LPS-Induced NO Production
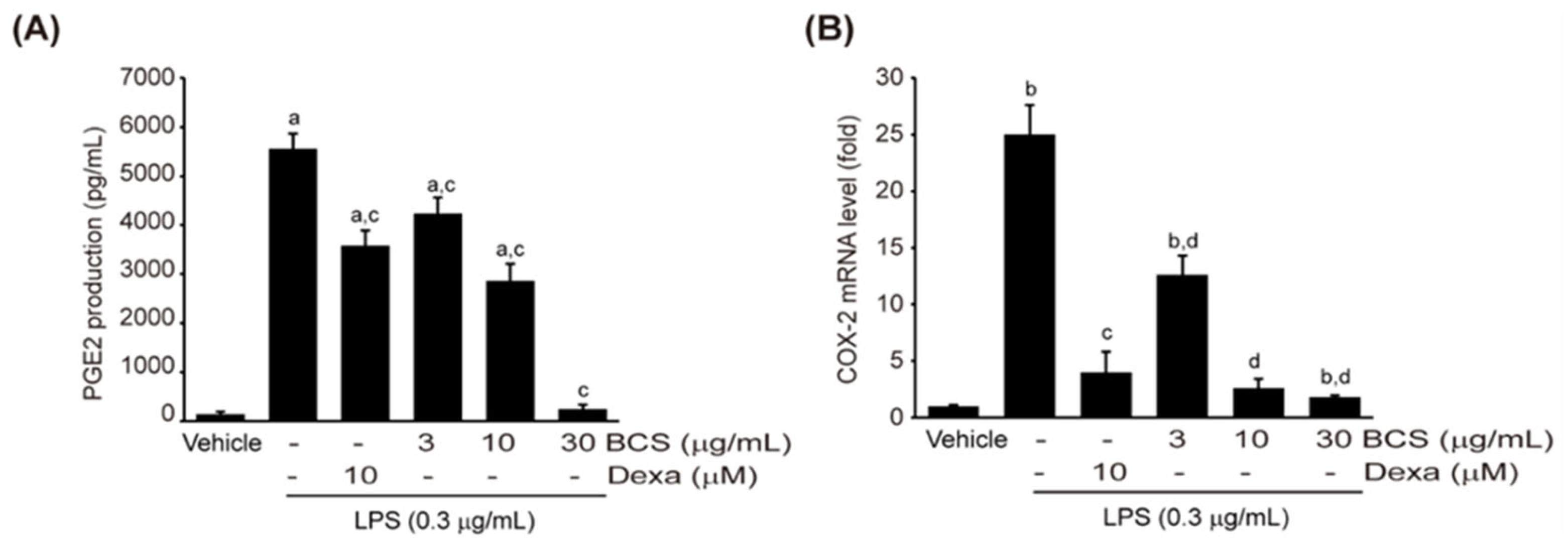
3.3.3. LPS-Induced PGE2 Production
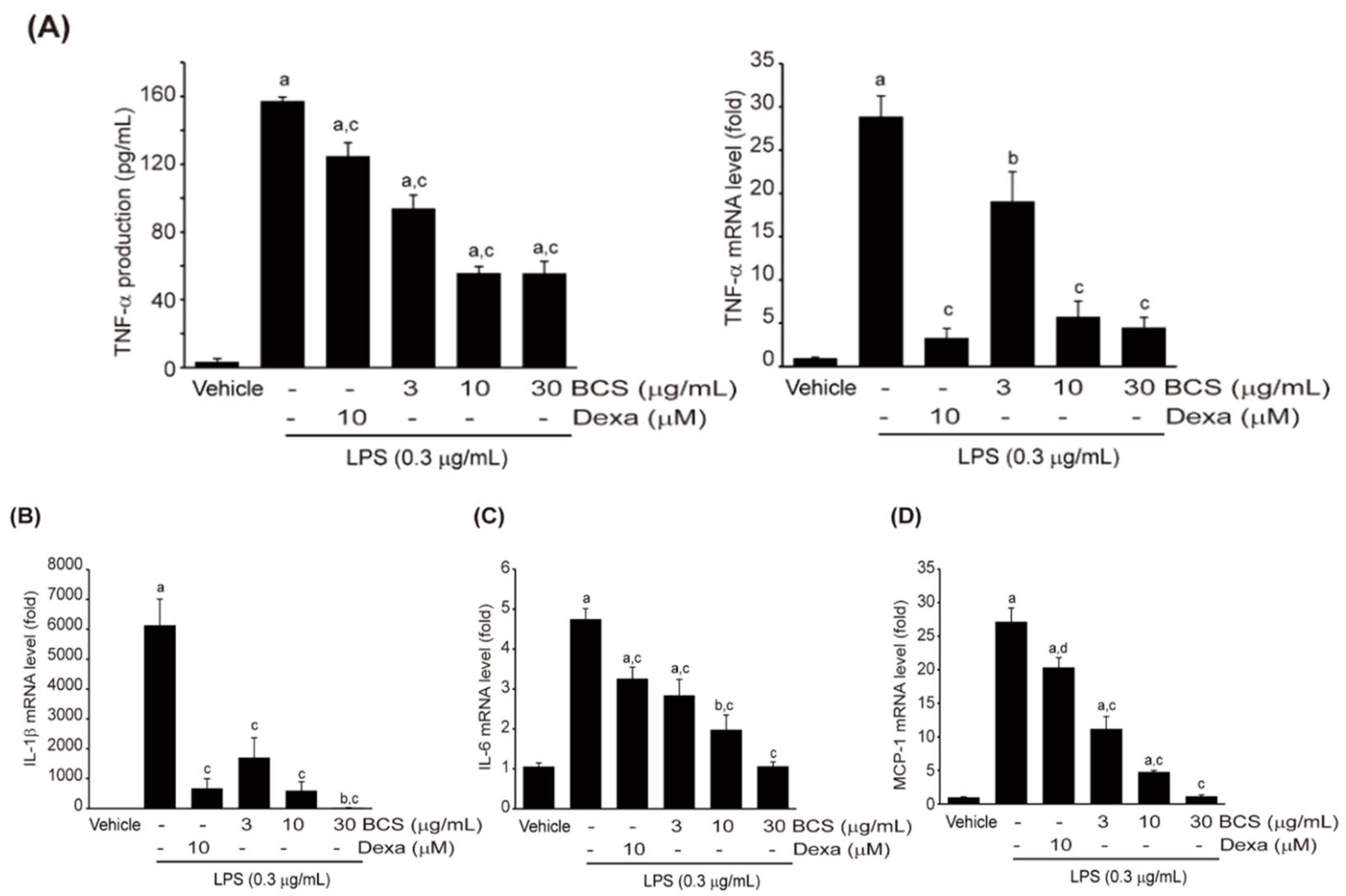
3.3.4. Effect of BCS Extract on LPS-Induced Pro-Inflammatory Cytokine Production
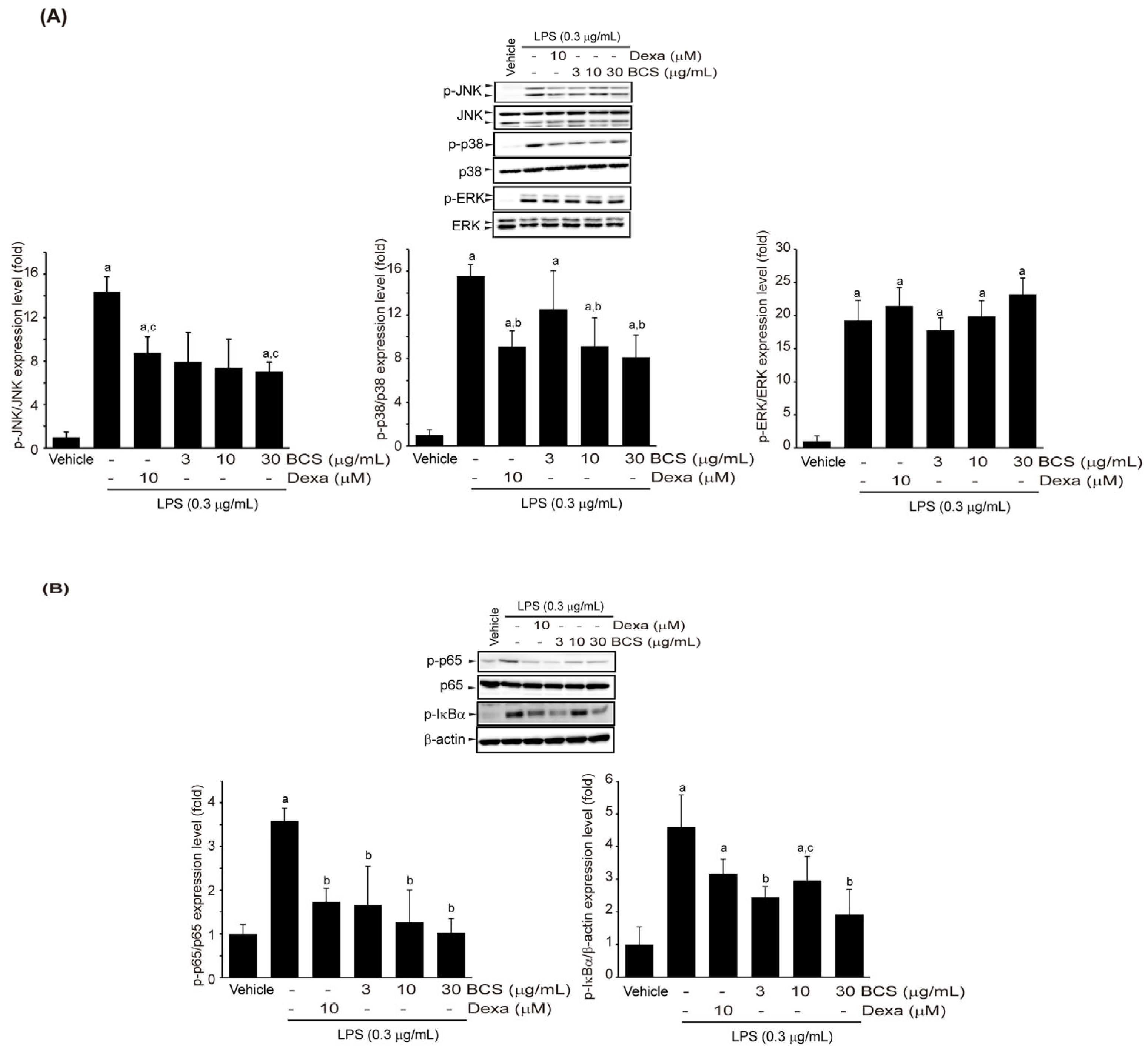
3.3.5. Effect of BCS Extract on LPS-Induced MAPK and NF-κB Phosphorylation
3.4. Adipocyte Differentiation Regulatory Activity of BCS Extract
3.4.1. Effect of BCS Extract on Lipid Accumulation in 3T3-L1 Cells
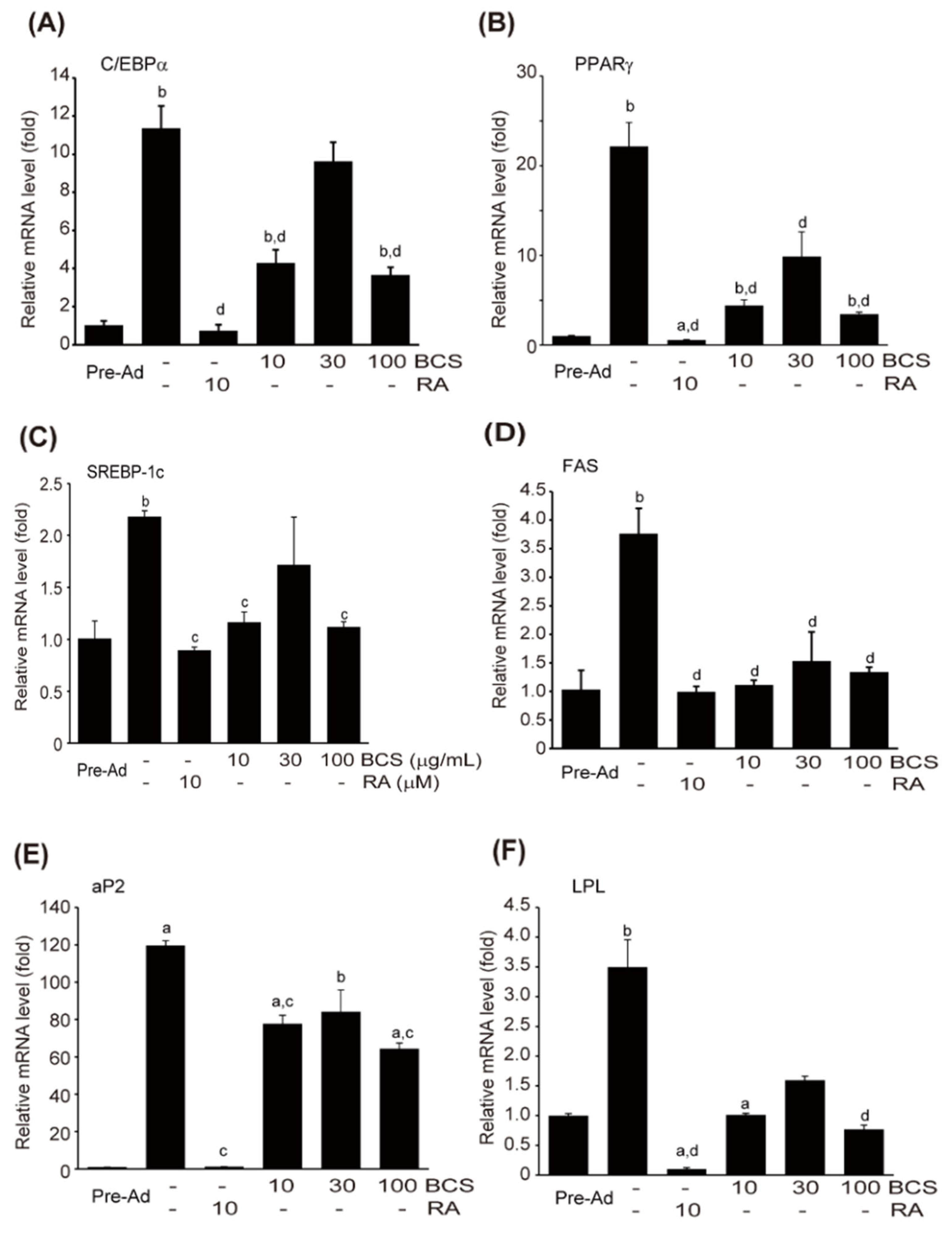
3.4.2. Expression of Genes Related to Adipocyte Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wendel, A.A.; Purushotham, A.; Liu, L.F.; Belury, M.A. Conjugated linoleic acid fails to worsen insulin resistance but induces hepatic steatosis in the presence of leptin in ob/ob mice. J. Lipid Res. 2008, 49, 98–106. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- James, P.T.; Leach, R.; Kalamara, E.; Shayeghi, M. The worldwide obesity epidemic. Obes. Res. 2001, 9, 228S–233S. [Google Scholar] [CrossRef] [PubMed]
- Kunitomi, M.; Wada, J.; Takahashi, K.; Tsuchiyama, Y.; Mimura, Y.; Hida, K.; Miyatake, N.; Fujii, M.; Kira, S.; Shikata, K.; et al. Relationship between reduced serum IGF-I levels and accumulation of visceral fat in Japanese men. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Hashimoto, I.; Okada, T.; Yasuhara, A.; Nakatsuka, A.; et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Inzucchi, S.E. Oral antihyperglycemic therapy for type 2 diabetes: Scientific review. J. Am. Med. Assoc. 2002, 287, 360–372. [Google Scholar] [CrossRef]
- Choi, J.-S.; Kim, J.W.; Park, J.B.; Pyo, S.E.; Hong, Y.K.; Ku, S.-K.; Kim, M.R. Blood glycemia-modulating effects of melanian snail protein hydrolysates in mice with type II diabetes. Int. J. Mol. Med. 2017, 39, 1437–1451. [Google Scholar] [CrossRef][Green Version]
- Choi, B.R.; Cho, I.J.; Jung, S.J.; Kim, J.K.; Park, S.M.; Lee, D.G.; Ku, S.-K.; Park, K.M. Lemon balm and dandelion leaf extract synergistically alleviate ethanol-induced hepatotoxicity by enhancing antioxidant and anti-inflammatory activity. J. Food Biochem. 2020, 44, e13232. [Google Scholar] [CrossRef]
- Choi, E.H.; Chun, Y.S.; Kim, J.K.; Ku, S.-K.; Jeon, S.W.; Park, T.S.; Shim, S.M. Modulating lipid and glucose metabolism by glycosylated kaempferol rich roasted leaves of Lycium chinense via upregulating adiponectin and AMPK activation in obese mice-induced type 2 diabetes. J. Funct. Foods 2020, 72, 104072. [Google Scholar] [CrossRef]
- Shabana, A.; El-Menyar, A.; Asim, M.; Al-Azzeh, H.; Al Thani, H. Cardiovascular benefits of black cumin (Nigella sativa). Cardiovasc. Toxicol. 2013, 13, 9–21. [Google Scholar] [CrossRef]
- Tiji, S.; Benayad, O.; Berrabah, M.; El Mounsi, I.; Mimouni, M. Phytochemical profile and antioxidant activity of Nigella sativa L. growing in Morocco. Sci. World J. 2021, 2021, 6623609. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Iqbal, Z.; Hashem, A.; Al-Arjani, A.F.; Abd-Allah, E.F.; Jafri, A.; Ansari, S.A.; Ansari, M.I. Nigella sativa callus treated with sodium azide exhibit augmented antioxidant activity and DNA damage inhibition. Sci. Rep. 2021, 11, 13954. [Google Scholar] [CrossRef] [PubMed]
- Malekian, S.; Ghassab-Abdollahi, N.; Mirghafourvand, M.; Farshbaf-Khalili, A. The effect of Nigella sativa on oxidative stress and inflammatory biomarkers: A systematic review and meta-analysis. J. Complement. Integr. Med. 2021, 18, 235–259. [Google Scholar] [CrossRef]
- Koshak, A.E.; Yousif, N.M.; Fiebich, B.L.; Koshak, E.A.; Heinrich, M. Comparative immunomodulatory activity of Nigella sativa L. preparations on proinflammatory mediators: A focus on asthma. Front. Pharmacol. 2018, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Fayyad, M.W. Recent advances on the anti-cancer properties of Nigella sativa, a widely used food additive. J. Ayurveda Integr. Med. 2016, 7, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Chen, H.C.; Tania, M.; Zhang, D.Z. Anticancer activities of Nigella sativa (black cumin). Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 226–232. [Google Scholar] [CrossRef]
- Dera, A.A.; Ahmad, I.; Rajagopalan, P.; Shahrani, M.A.; Saif, A.; Alshahrani, M.Y.; Alraey, Y.; Alamri, A.M.; Alasmari, S.; Makkawi, M.; et al. Synergistic efficacies of thymoquinone and standard antibiotics against multi-drug resistant isolates. Saudi Med. J. 2021, 42, 196–204. [Google Scholar] [CrossRef]
- Habib, N.; Choudhry, S. HPLC Quantification of thymoquinone extracted from Nigella sativa L. (Ranunculaceae) seeds and antibacterial activity of its extracts against Bacillus species. eCAM 2021, 2021, 6645680. [Google Scholar] [CrossRef]
- Barashkova, A.S.; Sadykova, V.S.; Salo, V.A.; Zavriev, S.K.; Rogozhin, E.A. Nigellothionins from black cumin (Nigella sativa L.) seeds demonstrate strong antifungal and cytotoxic activity. Antibiotics 2021, 10, 166. [Google Scholar] [CrossRef]
- Keyhanmanesh, R.; Gholamnezhad, Z.; Boskabady, M.H. The relaxant effect of Nigella sativa on smooth muscles, its possible mechanisms and clinical applications. Iran. J. Basic Med. Sci. 2014, 17, 939–949. [Google Scholar] [PubMed]
- Kadam, D.; Lele, S.S. Extraction, characterization and bioactive properties of Nigella sativa seedcake. J. Food Sci. Technol. 2017, 54, 3936–3947. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Šudomová, M. Comment on: Effects of Nigella Sativa on type-2 diabetes mellitus: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 1630. [Google Scholar] [CrossRef]
- El Rabey, H.A.; Al-Seeni, M.N.; Bakhashwain, A.S. The antidiabetic activity of Nigella sativa and Propolis on streptozotocin-induced diabetes and diabetic nephropathy in male rats. eCAM 2017, 2017, 5439645. [Google Scholar] [PubMed]
- Dalli, M.; Daoudi, N.E.; Azizi, S.E.; Benouda, H.; Bnouham, M.; Gseyra, N. Chemical composition analysis using HPLC-UV/GC-MS and inhibitory activity of different Nigella sativa fractions on pancreatic α-amylase and intestinal glucose absorption. Biomed. Res. Int. 2021, 2021, 9979419. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Kim, J.W.; Kim, J.-K.; Chun, Y.-S.; Choi, J.-S.; Ku, S.-K. Efficacy confirmation test of black cumin (Nigella sativa L.) seeds extract using a high-fat diet mouse model. Metabolites 2023, 13, 501. [Google Scholar] [CrossRef]
- Choi, J.S.; Cheon, E.J.; Kim, T.U.; Moon, W.S.; Kim, J.W.; Kim, M.R. Genotoxicity of rice bran oil extracted by supercritical CO2 extraction. Biol. Pharm. Bull. 2014, 37, 1963–1970. [Google Scholar] [CrossRef]
- Rubin, C.S.; Hirsch, A.; Fung, C.; Rosen, O.M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J. Biol. Chem. 1978, 253, 7570–7578. [Google Scholar] [CrossRef]
- Kamei, Y.; Kawada, T.; Mizukami, J.; Sugimoto, E. The prevention of adipose differentiation of 3T3-L1 cells caused by retinoic acid is elicited through retinoic acid receptor alpha. Life Sci. 1994, 55, PL307–PL312. [Google Scholar] [CrossRef]
- Chuang, T.Y.; Cheng, A.J.; Chen, I.T.; Lan, T.Y.; Huang, I.H.; Shiau, C.W.; Hsu, C.L.; Liu, Y.W.; Chang, Z.F.; Tseng, P.H.; et al. Suppression of LPS-induced inflammatory responses by the hydroxyl groups of dexamethasone. Oncotarget 2017, 8, 49735–49748. [Google Scholar] [CrossRef]
- Cho, I.J.; Kim, S.G. A novel mitogen-activated protein kinase phosphatase-1 and glucocorticoid receptor (GR) interacting protein-1-dependent combinatorial mechanism of gene transrepression by GR. Mol. Endocrinol. 2009, 23, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, J.E.; Jung, E.H.; Jung, J.Y.; Jung, D.H.; Ku, S.-K.; Cho, I.J.; Kim, S.C. Hemistepsin A ameliorates acute inflammation in macrophages via inhibition of nuclear factor-κB and activation of nuclear factor erythroid 2-related factor 2. Food Chem Toxicol. 2018, 111, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Suryawan, A.; Hu, C.Y. Effect of serum on differentiation of porcine adipose stromal-vascular cells in primary culture. Comp. Biochem. Physiol. Mol. 1993, 105, 485–492. [Google Scholar] [CrossRef]
- Levene, A. Pathological factors influencing excision of tumours in the head and neck. Clin. Otolaryngol. Allied Sci. 1981, 6, 145–151. [Google Scholar] [CrossRef]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta 2015, 1851, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Phillai, S. Cellular and Molecular Immunology, 7th ed.; Saunders: Philadelphia, PA, USA, 2012; pp. 41–88. [Google Scholar]
- Zhang, G.; Ghosh, S. Toll-like receptor-mediated NF-kappaB activation: A phylogenetically conserved paradigm in innate immunity. J. Clin. Invest. 2001, 107, 13–19. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428. [Google Scholar] [CrossRef]
- Pais, R.; Charlotte, F.; Fedchuk, L.; Bedossa, P.; Lebray, P.; Poynard, T.; Ratziu, V. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J. Hepatol. 2013, 59, 550–556. [Google Scholar] [CrossRef]
- Tan, Y.; Kim, J.; Cheng, J.; Ong, M.; Lao, W.G.; Jin, X.L.; Lin, Y.G.; Xiao, L.; Zhu, X.Q.; Qu, X.Q. Green tea polyphenols ameliorate non-alcoholic fatty liver disease through upregulating AMPK activation in high fat fed Zucker fatty rats. World J. Gastroenterol. 2017, 23, 3805–3814. [Google Scholar] [CrossRef]
- Samuel, V.T.; Liu, Z.X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of hepatic insulin resistance in nonalcoholic fatty liver disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.B.; Petersen, K.F.; Shulman, G.I. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 2007, 87, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Rotman, Y.; Sanyal, A.J. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut 2017, 66, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qu, Z.; Fu, L.; Dong, P.; Zhang, X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2009, 74, C469–C474. [Google Scholar] [CrossRef]
- Hays, N.P.; Galassetti, P.R.; Coker, R.H. Prevention and treatment of type 2 diabetes: Current role of lifestyle, natural product, and pharmacological interventions. Pharmacol. Ther. 2008, 118, 181–191. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour. Technol. 2008, 99, 2981–2988. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.S.; Seol, D.J.; Cho, I.J.; Ku, S.-K.; Choi, J.-S.; Lee, H.J. Anti-obesity and fatty liver-preventing activities of Lonicera caerulea in high-fat diet-fed mice. Int. J. Mol. Med. 2018, 42, 3047–3064. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Clancy, R.M.; Amin, A.R.; Abramson, S.B. The role of nitric oxide in inflammation and immunity. Arthritis. Rheum. 1998, 41, 1141–1151. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 6, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef]
- Kats, A.; NorgAard, M.; Wondimu, Z.; Koro, C.; Concha Quezada, H.; Andersson, G.; Yucel-Lindberg, T. Aminothiazoles inhibit RANKL-and LPS-mediated osteoclastogenesis and PGE 2 production in RAW 264.7 cells. J. Cell. Mol. Med. 2016, 20, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.V.; McManus, A.T.; Chambers, J.P. Production of tumor necrosis factor-alpha, interleukin 1-beta, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides 2003, 37, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006, 97, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Cerami, A. The biology of cachectin/TNF-α primary mediator of the host response. Annu. Rev. Immunol. 1989, 7, 625–655. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 26, 313–326. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kB by toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Spiegelman, V.S.; Stavropoulos, P.; Latres, E.; Pagano, M.; Ronai, Z.; Siaga, T.J.; Fuchs, S.Y. Induction of beta-tranducin repeat-containing protein by JNK signaling and its role in the activation of NF-κB. J. Biol. Chem. 2001, 276, 27152–27158. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef]
- Green, H.; Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell 1974, 3, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ailhaud, G. Early adipocyte differentiation. Biochem. Soc. Trans. 1996, 24, 400–402. [Google Scholar] [CrossRef]
- Cryer, A. Tissue lipoprotein lipase activity and its action in lipoprotein metabolism. Int. J. Biochem. 1981, 13, 525–541. [Google Scholar] [CrossRef]
- Brun, R.P.; Kim, J.B.; Hu, E.; Altiok, S.; Spiegelman, B.M. Adipocyte differentiation: A transcriptional regulatory cascade. Curr. Opin. Cell Biol. 1996, 8, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Spiegelman, B.M. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996, 10, 1096–1107. [Google Scholar] [CrossRef]
- Bernlohr, D.A.; Angus, C.W.; Lane, M.D.; Bolanowski, M.A.; Kelly, T.J., Jr. Expression of specific mRNAs during adipose differentiation: Identification of an mRNA encoding a homologue of myelin P2 protein. Proc. Natl. Acad. Sci. USA 1984, 81, 5468–5472. [Google Scholar] [CrossRef]
- Antras-Ferry, J.; Mahéo, K.; Morel, F.; Guillouzo, A.; Cillard, P.; Cillard, J. Dexamethasone differently modulates TNF-α- and IL-1β-induced transcription of the hepatic Mn-superoxide dismutase gene. FEBS Lett. 1997, 403, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; Lewis-Molock, Y.; White, C.W. Thiol modulation of TNF alpha and IL-1 induced MnSOD gene expression and activation of NF-kappa B. Mol. Cell. Biochem. 1995, 148, 45–57. [Google Scholar] [CrossRef]
- Beato, M. Gene regulation by steroid hormones. Cell 1989, 56, 335–344. [Google Scholar] [CrossRef]
- Caldenhoven, E.; Liden, J.; Wissink, S.; Van de Stolpe, A.; Raaijmakers, J.; Koenderman, L.; Okret, S.; Gustafsson, J.A.; Van der Saag, P.T. Negative cross-talk between RelA and the glucocorticoid receptor: A possible mechanism for the antiinflammatory action of glucocorticoids. Mol. Endocrinol. 1995, 9, 401–412. [Google Scholar]
- Necela, B.M.; Cidlowski, J.A. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proc. Am. Thor. Soc. 2004, 1, 239–246. [Google Scholar] [CrossRef]
- Mihailidou, I.; Pelekanou, A.; Pistiki, A.; Spyridaki, A.; Tzepi, I.M.; Damoraki, G.; Giamarellos-Bourboulis, E.J. Dexamethasone down-regulates expression of triggering receptor expressed on myeloid cells-1: Evidence for a TNFα-related effect. Front. Public Health 2013, 1, 50. [Google Scholar] [CrossRef] [PubMed]
- Tun, S.; Spainhower, C.J.; Cottrill, C.L.; Lakhani, H.V.; Pillai, S.S.; Dilip, A.; Chaudhry, H.; Shapiro, J.I.; Sodhi, K. Therapeutic efficacy of antioxidants in ameliorating obesity phenotype and associated comorbidities. Front. Pharmacol. 2020, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, H.; Haugen, F.; Zadelaar, S.; Kleemann, R.; Kooistra, T.; Drevon, C.A.; Blomhoff, R. Diet-induced obesity increases NF-κB signaling in reporter mice. Genes Nutr. 2009, 4, 215–222. [Google Scholar] [CrossRef]
- Burgess, A.; Li, M.; Vanella, L.; Kim, D.H.; Rezzani, R.; Rodella, L.; Sodhi, K.; Canestraro, M.; Martasek, P.; Peterson, S.J.; et al. Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice. Hypertension 2010, 56, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Wood Dos Santos, T.; Cristina Pereira, Q.; Teixeira, L.; Gambero, A.; Villena, J.A.; Lima Ribeiro, M. Effects of polyphenols on thermogenesis and mitochondrial biogenesis. Int. J. Mol. Sci. 2018, 19, 2757. [Google Scholar] [CrossRef]
- Calzadilla, P.; Sapochnik, D.; Cosentino, S.; Diz, V.; Dicelio, L.; Calvo, J.C.; Guerra, L.N. N-acetylcysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2011, 12, 6936–6951. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Peterson, S.J.; Sodhi, K.; Vanella, L.; Barbagallo, I.; Rodella, L.F.; Schwartzman, M.L.; Abraham, N.G.; Kappas, A. Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet. Hypertension 2012, 60, 467–475. [Google Scholar] [CrossRef]
- Khitan, Z.; Harsh, M.; Sodhi, K.; Shapiro, J.I.; Abraham, N.G. HO-1 upregulation attenuates adipocyte dysfunction, obesity, and isoprostane levels in mice fed high fructose diets. J. Nutr. Metab. 2014, 2014, 980547. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and its human metabolites-effects on metabolic health and obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, K.M.I.; Kim, J.-K.; Chun, Y.-S.; Choi, J.-S.; Ku, S.-K. In Vitro Assessment of Anti-Adipogenic and Anti-Inflammatory Properties of Black Cumin (Nigella sativa L.) Seeds Extract on 3T3-L1 Adipocytes and Raw264.7 Macrophages. Medicina 2023, 59, 2028. https://doi.org/10.3390/medicina59112028
Bashir KMI, Kim J-K, Chun Y-S, Choi J-S, Ku S-K. In Vitro Assessment of Anti-Adipogenic and Anti-Inflammatory Properties of Black Cumin (Nigella sativa L.) Seeds Extract on 3T3-L1 Adipocytes and Raw264.7 Macrophages. Medicina. 2023; 59(11):2028. https://doi.org/10.3390/medicina59112028
Chicago/Turabian StyleBashir, Khawaja Muhammad Imran, Jong-Kyu Kim, Yoon-Seok Chun, Jae-Suk Choi, and Sae-Kwang Ku. 2023. "In Vitro Assessment of Anti-Adipogenic and Anti-Inflammatory Properties of Black Cumin (Nigella sativa L.) Seeds Extract on 3T3-L1 Adipocytes and Raw264.7 Macrophages" Medicina 59, no. 11: 2028. https://doi.org/10.3390/medicina59112028
APA StyleBashir, K. M. I., Kim, J.-K., Chun, Y.-S., Choi, J.-S., & Ku, S.-K. (2023). In Vitro Assessment of Anti-Adipogenic and Anti-Inflammatory Properties of Black Cumin (Nigella sativa L.) Seeds Extract on 3T3-L1 Adipocytes and Raw264.7 Macrophages. Medicina, 59(11), 2028. https://doi.org/10.3390/medicina59112028





