Factors Associated with Increased Risk of Urosepsis during Pregnancy and Treatment Outcomes, in a Urology Clinic
Abstract
1. Introduction
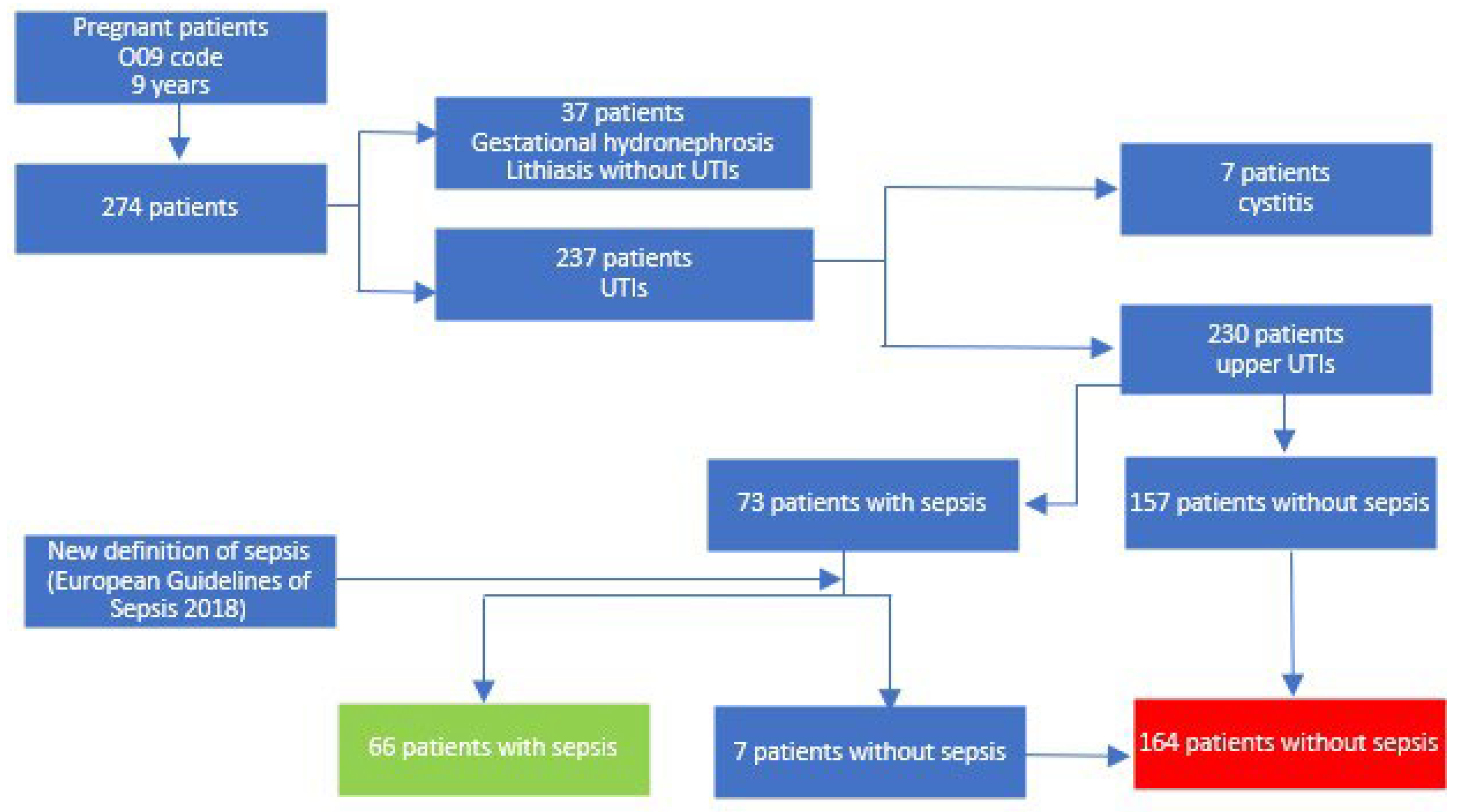
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oud, L. Pregnancy-Associated Severe Sepsis: Contemporary State and Future Challenges. Infect. Dis. Ther. 2014, 3, 175–189. [Google Scholar] [CrossRef][Green Version]
- Cantwell, R.; Clutton-Brock, T.; Cooper, G.; Dawson, A.; Drife, J.; Garrod, D.; Harper, A.; Hulbert, D.; Lucas, S.; McClure, J.; et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG 2011, 118 (Suppl. S1), 1–203. [Google Scholar] [PubMed]
- Fernandez-Perez, E.R.; Salman, S.; Pendem, S.; Farmer, J.C. Sepsis during pregnancy. Crit. Care Med. 2005, 33, S286–S292. [Google Scholar] [CrossRef] [PubMed]
- Cordioli, R.L.; Cordioli, E.; Negrini, R.; Silva, E. Sepsis and pregnancy. Do we know to treat this situation? Rev. Bras. Ter. Intensive 2013, 25, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Lin, R.; Zhang, W.; Huang, S.; Luo, Y.; Wang, D. Epidemiology and clinical features of maternal sepsis: A retrospective study of whole pregnancy period. Medicine 2022, 101, e30599. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ren, L.-W.; Li, X.Y.; Sun, W.; Chen, Y.H.; Chen, J.S.; Chen, D.J. Evaluation of the etiology and risk factors for maternal sepsis: A single center study in Guangzhou, China. World J. Clin. Cases 2021, 9, 7704–7716. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, H.; Ming, X.; Chen, X.; Zhou, W. Analysis of risk factors, pathogenic bacteria of maternal sepsis in term pregnant women with positive blood culture during hospitalisation. Medicine 2021, 100, e24847. [Google Scholar] [CrossRef]
- van Nieuwkoop, C.; Bonten, T.N.; van’t Wout, J.W.; Kuijper, E.J.; Groeneveld, G.H.; Becker, M.J.; Koster, T.; Wattel-Louis, G.H.; Delfos, N.M.; Ablij, H.C.; et al. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: A prospective observational study. Crit. Care 2010, 14, R206. [Google Scholar] [CrossRef]
- Matuszkiewicz-Rowinska, J.; Malyszko, J.; Wieliczko, M. Urinary tract infections in pregnancy: Old and new unresolved diagnostic and therapeutic problems. Arch. Med. Sci. 2015, 11, 67–77. [Google Scholar] [CrossRef]
- Ko, G.J.; Ahn, S.Y.; Kim, J.E.; Cho, E.J.; Lee, K.M.; Kim, H.Y.; Kwon, Y.J.; Oh, M.J.; Han, S.W.; Cho, G.J. Clinical Predictors Implicated in the Incidence of Acute Pyelonephritis during the Antepartum Period: A Population-Based Cohort Study. Kidney Blood Press. Res. 2020, 45, 297–306. [Google Scholar] [CrossRef]
- Jolley, J.A.; Wing, D.A. Pyelonephritis in pregnancy. Drugs 2010, 70, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.Y.; Rocheleau, C.M.; Howley, M.M.; Chiu, S.R.; Arnold, K.E.; Ailes, E.C. Characteristics of Women with Urinary Tract Infection in Pregnancy. J. Women Health 2021, 30, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Gilstrap, L.C.; Ramin, S.M. Urinary Tract Infections During Pregnancy. Obstet. Gynecol. Clin. N. Am. 2001, 28, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Thapa, L. Acute pyelonephritis in pregnancy: A retrospective study. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 313–315. [Google Scholar] [CrossRef]
- Blauvelt, C.A.; Nguyen, K.C.; Cassidy, A.G.; Gaw, S.L. Perinatal Outcomes Among Patients With Sepsis During Pregnancy. JAMA Netw. Open. 2021, 4, e2124109. [Google Scholar] [CrossRef]
- Ngai, H.Y.; Salih, H.Q.; Albeer, A.; Aghaways, I.; Buchholz, N. Double-J ureteric stenting in pregnancy: A single-centre experience from Iraq. Arab. J. Urol. 2013, 11, 148–151. [Google Scholar] [CrossRef]
- Çeçen, K.; Ülker, K. The comparison of double J stent insertion and conservative treatment alone in severe pure gestational hydronephrosis: A case controlled clinical study. Sci. World J. 2014, 2014, 989173. [Google Scholar] [CrossRef]
- Can, U.; Tuncer, M.; Narter, F.; Sabunen, K.; Sarica, K. Ureteral Stent Use in Pregnant Women with Persistent Flank Pain: Our Clinical Experience. South Clin. Ist. Euras. 2018, 29, 285–289. [Google Scholar]
- Jin, X.; Liu, B.; Xiong, Y.; Wang, Y.; Tu, W.; Shao, Y.; Zhang, L.; Wang, D. Outcomes of ureteroscopy and internal ureteral stent for pregnancy with urolithiasis: A systematic review and meta-analysis. BMC Urol. 2022, 22, 150. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Bowyer, L.; Robinson, H.L.; Barrett, H.; Crozier, T.M.; Giles, M.; Idel, I.; Lowe, S.; Lust, K.; Marnoch, C.A.; Morton, M.R.; et al. SOMANZ guidelines for the investigation and management sepsis in pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 2017, 57, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.; Köves, B.; Kranz, J.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; et al. Urinary tract infections. In EAU Guidelines on Urolgical Infections; EAU Guidelines Office: Arnhem, The Netherlands, 2023; pp. 28–31. [Google Scholar]
- Onen, A. Grading of Hydronephrosis: An Ongoing Challenge. Front. Pediatr. 2020, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Huitfeldt, A. Is caviar a risk factor for being a millionaire? BMJ 2016, 355, i6536. [Google Scholar] [CrossRef] [PubMed]
- Committee on Clinical Consensus—Obstetrics. Urinary Tract Infections in Pregnant Individuals. Obstet. Gynecol. 2023, 142, 435–445. [Google Scholar] [CrossRef] [PubMed]
- (RCOG) RCoOaG. Bacterial Sepsis in Pregnancy. 2012. Available online: https://wwwrcogorguk/media/ea1p1r4h/gtg_64apdf (accessed on 25 September 2023).
- Miftode, I.L.; Nastase, E.V.; Miftode, R.; Miftode, E.G.; Iancu, L.S.; Luncă, C.; Păduraru, D.A.; Costache, I.; Stafie, C.; Dorneanu, O. Insights into multidrug resistant K. pneumoniae urinary tract infections: From susceptibility to mortality. Exp. Ther. Med. 2021, 22, 1086. [Google Scholar] [CrossRef] [PubMed]
- Miftode, I.-L.; Leca, D.; Miftode, R.-S.; Roşu, F.; Plesca, C.; Loghin, I.; Timpau, A.S.S.; Mitu, I.; Mititiuc, I.; Dorneanu, O.; et al. The Clash of the Titans: COVID-19, Carbapenem-Resistant Enterobacterales, and First mcr-1-Mediated Colistin Resistance in Humans in Romania. Antibiotics 2023, 12, 324. [Google Scholar] [CrossRef]
- Miftode, I.L.; Pasare, M.A.; Miftode, R.S.; Nastase, E.; Plesca, C.E.; Lunca, C.; Miftode, E.G.; Timpau, A.S.; Iancu, L.S.; Dorneanu, O.S. What Doesn’t Kill Them Makes Them Stronger: The Impact of the Resistance Patterns of Uri-nary Enterobacterales Isolates in Patients from a Tertiary Hospital in Eastern Europe. Antibiotics 2022, 11, 548. [Google Scholar] [CrossRef]
- Rasmussen, P.E.; Nielsen, F.R. Hydronephrosis during pregnancy: A literature survey. Eur. J. Obstet. Gynecol. Reprod. Biol. 1988, 27, 249–259. [Google Scholar] [CrossRef]
- Miftode, R.S.; Costache, I.I.; Cianga, P.; Petris, A.O.; Cianga, C.M.; Maranduca, M.A.; Miftode, I.L.; Constantinescu, D.; Timpau, A.S.; Crisan, A.; et al. The Influence of Socioeconomic Status on the Prognosis and Profile of Patients Admitted for Acute Heart Failure during COVID-19 Pandemic: Overestimated Aspects or a Multifaceted Hydra of Cardiovascular Risk Factors? Healthcare 2021, 9, 1700. [Google Scholar] [CrossRef]
- Bernstein, I.M.; Ziegler, W.; Badger, G.J. Plasma volume expansion in early pregnancy. Obstet. Gynecol. 2001, 97, 669–672. [Google Scholar]
- Wing, D.A.; Fassett, M.J.; Getahun, D. Acute pyelonephritis in pregnancy: An 18-year retrospective analysis. Am. J. Obstet. Gynecol. 2014, 210, 219.e1–219.e6. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Housey, M.; Bauer, S.T.; Behrmann, S.; Chau, A.; Clancy, C.; Clark, E.A.S.; Einav, S.; Langen, E.; Leffert, L.; et al. Risk Factors, Etiologies, and Screening Tools for Sepsis in Pregnant Women: A Multicenter Case-Control Study. Anesth. Analg. 2019, 129, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Radu, V.D.; Costache, R.C.; Onofrei, P.; Miftode, E.; Linga, I.; Ouatu, R.M.; Boiculese, L.; Bobeica, R.L.; Tanasa Vasilache, I.; Mititiuc, I.L. Multidrug-Resistant (MDR) Urinary Tract Infections Associated with Gut Microbiota in CoV and Non-CoV Patients in a Urological Clinic during the Pandemic: A Single Center Experience. Antibiotics 2023, 12, 973. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.M.; Schutte, J.M.; Zwart, J.J.; Schuitemaker, N.W.; Steegers, E.A.; van Roosmalen, J. Maternal mortality and severe morbidity from sepsis in the Netherlands. Acta Obstet. Gynecol. Scand. 2009, 88, 647–653. [Google Scholar] [CrossRef]
- Radu, V.D.; Vasilache, I.A.; Costache, R.C.; Scripcariu, I.S.; Nemescu, D.; Carauleanu, A.; Nechifor, V.; Groza, V.; Onofrei, P.; Boiculese, L.; et al. Pregnancy Outcomes in a Cohort of Patients Who Underwent Double-J Ureteric Stenting-A Single Center Experience. Medicina 2022, 58, 619. [Google Scholar] [CrossRef] [PubMed]
- de Cueto, M.; Aliaga, L.; Alós, J.I.; Canut, A.; Los-Arcos, I.; Martínez, J.A.; Mensa, J.; Pintado, V.; Rodriguez-Pardo, D.; Yuste, J.R.; et al. Executive summary of the diagnosis and treatment of urinary tract infection: Guidelines of the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC). Enferm. Infecc. Microbiol. Clin. 2017, 35, 314–320. [Google Scholar] [CrossRef]
| Infection Site | Sepsis (n = 66) | Non-Sepsis (n = 164) | p Values for chi2 Test | |||
| Right | 47 (71.2%) | 124 (75.6%) | 0.491 | |||
| Left | 14 (21.2%) | 26 (15.9%) | 0.333 | |||
| Bilateral | 5 (7.6%) | 14 (8.5%) | 0.811 | |||
| Age (mean, standard deviation) | 24.97 ± 6.51 | 25.18 ± 5.49 | 0.801 (t) | |||
| Trimester of pregnancy (Nr, %) | I | 5 (7.6%) | 16 (9.8%) | 0.604 | ||
| II | 35 (53.0%) | 88 (53.7%) | 0.931 | |||
| III | 26 (39.4%) | 60 (36.6%) | 0.691 | |||
| Place of origin (Nr, %) | Rural | 38 (57.6%) | 90 (54.9%) | 0.709 | ||
| Urban | 28 (42.4%) | 74 (45.1%) | 0.709 | |||
| Parity (Nr, %) | Nulliparas | 45 (68.2%) | 111 (67.7%) | 0.942 | ||
| Parity ≥ 1 | 21 (31.8%) | 53 (32.3%) | 0.942 | |||
| Comorbidities (Nr, %) | Anemia | Mild | 36 (54.5%) | 73 (44.5%) | 0.169 | |
| Moderate | 10 (15.2%) | 9 (5.5%) | 0.016 | |||
| Total | 46 (69.7%) | 82 (50.0%) | 0.006 | |||
| Diabetes mellitus | 1 (1.5%) | 1 (0.6%) | 0.525 | |||
| Gestational hydronephrosis (Nr, %) | Sepsis (n = 66) | Non-Sepsis (n = 164) | p Values for chi2 Test | |
| 1st grade | 8 (12.12%) | 54 (32.93%) | 0.001 | |
| 2nd–3rd grade | 46 (69.70%) | 52 (31.71%) | 0.001 | |
| Total | 54 (81.82%) | 106 (64.63%) | 0.011 | |
| Hydronephrosis secondary to reno-ureteral lithiasis (Nr, %) | 1st grade | 2 (3.03%) | 16 (9.76) | 0.086 |
| 2nd–3rd grade | 8 (12.12%) | 6 (3.65%) | 0.015 | |
| Total | 10 (15.15%) | 22 (13.41%) | 0.730 | |
| Total hydronephrosis cases (Nr, %) | 1st grade | 10 (15.15%) | 65 (39.63%) | 0.001 |
| 2nd–3rd grade | 54 (81.82%) | 86 (52.44%) | 0.001 | |
| Total | 64 (96.96%) | 151 (92.07%) | 0.113 | |
| Presence of other urological disease (Nr, %) | 1 (1.52%) | 1 (0.61%) | 0.504 | |
| History of UTIs (Nr, %) | 13 (19.70%) | 22 (13.41%) | 0.231 | |
| History of endourologic maneuvers during pregnancy (Nr, %) | 7 (10.6%) | 7 (4.3%) | 0.070 | |
| Fever | Sepsis (n = 66) | Non-Sepsis (n = 164) | p Value for Student’s t-test | |
| Mean, ±SD | 38.60 ± 0.64 | 37.74 ± 0.69 | 0.001 | |
| T > 38 °C | 59 (89.4%) | 69 (42.1%) | 0.001 (*) | |
| qSOFA | Mental | 57 (86.4%) | 11 (6.7%) | 0.001 (*) |
| Respiratory | 62 (93.3%) | 18 (11.0%) | 0.001 (*) | |
| BP < 90 mmHg | 23 (34.8%) | 5 (3.0%) | 0.001 (*) | |
| Leukocytosis (mean, ±SD) | 18,191 ± 6414 | 14,350 ± 3860 | 0.001 | |
| CRP (mean, ±SD) | 142.70 ± 83.50 | 72.76 ± 66.37 | 0.001 | |
| Creatinine (mean, ±SD) | 0.77 ± 0.81 | 0.59 ± 0.22 | 0.012 | |
| Septic shock on admission | 12 (18.2%) | 0 (0%) | - | |
| Sepsis (n = 66) | Non-Sepsis (n = 164) | p Values for chi2 Test | |
|---|---|---|---|
| Positive urine cultures | 51 (72.3%) | 103 (62.8%) | 0.023 |
| E. coli | 36 (54.5%) | 77 (47.0%) | 0.297 |
| Klebsiella spp. | 9 (13.6%) | 10 (6.1%) | 0.072 |
| Proteus mirabilis | 1 (1.5%) | 3 (1.8%) | 0.867 |
| Pseudomonas aeruginosa | 0 (0.0%) | 2 (1.2%) | 0.244 |
| Serratia marcescens | 1 (1.5%) | 0 (0.0%) | 0.113 |
| Staphylococcus aureus | 1 (1.5%) | 0 (0.0%) | 0.361 |
| Candida spp. | 0 (0.0%) | 2 (1.2%) | 0.244 |
| Enterococcus spp. | 3 (4.5%) | 9 (5.5%) | 0.768 |
| Sepsis (n = 66) | Non-Sepsis (n = 164) | p Values for chi2 Test | ||
|---|---|---|---|---|
| Double-J insertion | Right | 36 (54.5%) | 36 (22.0%) | 0.001 |
| Left | 11 (16.7%) | 9 (5.5%) | 0.010 | |
| Bilateral | 10 (15.2%) | 11 (6.7%) | 0.043 | |
| Total | 57 (86.4%) | 56 (34.1%) | 0.001 | |
| Percutaneous nephrostomy | Right | 1 (1.5%) | 3 (1.8%) | 0.867 |
| Left | 0 (0.0%) | 0 (0.0%) | - | |
| Bilateral | 0 (0.0%) | 0 (0.0%) | - | |
| Total | 1 (1.5%) | 3 (1.8%) | 0.867 | |
| Total urologic maneuvers | 58 (87.9%) | 59 (36.0%) | 0.001 | |
| Sepsis (n = 66) | Non-Sepsis (n = 164) | p Values for chi2 Test | ||
|---|---|---|---|---|
| Days of hospitalization | Total (mean, SD) | 5.82 ± 1.69 | 4.48 ± 1.46 | 0.001 (t) |
| >7 days | 10 (15.2%) | 4 (2.4%) | 0.001 | |
| Fetal distress (transfer to maternity hospital) | 15 (22.7%) | 2 (1.2%) | 0.001 | |
| Days of hospitalization in the ICU | Nr. of patients (%) | 23 (34.85%) | 5 (3.05%) | 0.001 |
| Nr. of days (mean) on patients in the ICU | 2.52 ± 1 | 1 ± 1 | 0.748 (t) | |
| Surgical complications | Double-J catheter misplacement | 4 (6.1%) | 1 (0.6%) | 0.016 |
| Reflux pyelonephritis | 5 (7.6%) | 6 (3.7%) | 0.227 | |
| Calcification of the double-J catheter after 8 weeks | 2 (3.0%) | 10 (6.1%) | 0.320 | |
| Septic shock after or during the urologic maneuver | 1 (1.5%) | 2 (1.2%) | 0.860 | |
| Failed ureteral catheterization | 1 (1.5%) | 2 (1.2%) | 0.864 | |
| Sepsis after discharge with indwelling double-J catheter | 2 (3.0%) | 0 (0.0%) | 0.025 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, V.D.; Costache, R.C.; Onofrei, P.; Antohi, L.; Bobeica, R.L.; Linga, I.; Tanase-Vasilache, I.; Ristescu, A.I.; Murgu, A.-M.; Miftode, I.-L.; et al. Factors Associated with Increased Risk of Urosepsis during Pregnancy and Treatment Outcomes, in a Urology Clinic. Medicina 2023, 59, 1972. https://doi.org/10.3390/medicina59111972
Radu VD, Costache RC, Onofrei P, Antohi L, Bobeica RL, Linga I, Tanase-Vasilache I, Ristescu AI, Murgu A-M, Miftode I-L, et al. Factors Associated with Increased Risk of Urosepsis during Pregnancy and Treatment Outcomes, in a Urology Clinic. Medicina. 2023; 59(11):1972. https://doi.org/10.3390/medicina59111972
Chicago/Turabian StyleRadu, Viorel Dragos, Radu Cristian Costache, Pavel Onofrei, Liviu Antohi, Razvan Lucian Bobeica, Iacov Linga, Ingrid Tanase-Vasilache, Anca Irina Ristescu, Alina-Mariela Murgu, Ionela-Larisa Miftode, and et al. 2023. "Factors Associated with Increased Risk of Urosepsis during Pregnancy and Treatment Outcomes, in a Urology Clinic" Medicina 59, no. 11: 1972. https://doi.org/10.3390/medicina59111972
APA StyleRadu, V. D., Costache, R. C., Onofrei, P., Antohi, L., Bobeica, R. L., Linga, I., Tanase-Vasilache, I., Ristescu, A. I., Murgu, A.-M., Miftode, I.-L., & Stoica, B. A. (2023). Factors Associated with Increased Risk of Urosepsis during Pregnancy and Treatment Outcomes, in a Urology Clinic. Medicina, 59(11), 1972. https://doi.org/10.3390/medicina59111972








