Patterns of Common Dermatological Conditions among Children and Adolescents in Pakistan
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esposito, S.; Fumagalli, M.; Principi, N. Immunogenicity, safety and tolerability of vaccinations in premature infants. Expert Rev. Vaccines 2012, 11, 1199–1209. [Google Scholar] [CrossRef]
- Ghirano, I.A.; Sheikh, S.; Arain, A.A. Skin diseases: Prevalence in pediatric patients in Hyderabad: Sindh, Pakistan. Prof. Med. J. 2017, 24, 1031–1035. [Google Scholar] [CrossRef]
- Laube, S. Skin infections and ageing. Ageing Res. Rev. 2004, 3, 69–89. [Google Scholar] [CrossRef]
- Carlsten, C.; Dimich-Ward, H.; Ferguson, A.; Watson, W.; Rousseau, R.; DyBuncio, A.; Becker, A.; Chan-Yeung, M. Atopic dermatitis in a high-risk cohort: Natural history, associated allergic outcomes, and risk factors. Ann. Allergy Asthma Immunol. 2013, 110, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, M.; Fa, Z.; Pan, W.; Liao, W.; Gao, X.-H.; Huo, W.; Yang, Y.; Chen, H.-D.; Holahan, H. Skin diseases caused by factors from the Environment. In Practical Immunodermatology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 145–198. [Google Scholar]
- Chung, J.; Simpson, E.L. The socioeconomics of atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Memon, K.N.; Soomro, R.A.; Ansari, M.S. Pattern of skin diseases in patients visiting a tertiary care health facility at Hyderabad, Pakistan. J. Ayub Med. Coll. Abbottabad 2011, 23, 37–39. [Google Scholar]
- Banerji, A. Scabies. Paediatr. Child Health 2015, 20, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S.; Rathi, H.; Lakhani, H.; Hansotia, M. Awareness about scabies among general medical practitioners (GPs) of Karachi, Pakistan. J. Pak. Med. Assoc. 2001, 51, 370–372. [Google Scholar]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.; Rawat, S. Emerging fungal infections among children: A review on its clinical manifestations, diagnosis, and prevention. J. Pharm. Bioallied Sci. 2010, 2, 314. [Google Scholar] [CrossRef]
- Hoare, C.; Li Wan Po, A.; Williams, H. Systematic review of treatments for atopic eczema. Health Technol. Assess. 2001, 4, 1–191. [Google Scholar] [CrossRef]
- Sandhu, J.K.; Salame, N.; Ehsani-Chimeh, N.; Armstrong, A.W. Economic burden of cutaneous infections in children and adults with atopic dermatitis. Pediatr. Dermatol. 2019, 36, 303–310. [Google Scholar] [CrossRef]
- Coe, A. Eczema—An Itchy Problem. Don’t Forget the Bubbles. 2016. Available online: https://dontforgetthebubbles.com (accessed on 5 January 2021).
- Myers, J.M.B.; Hershey, G.K.K. Eczema in early life: Genetics, the skin barrier, and lessons learned from birth cohort studies. J. Pediatr. 2010, 157, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Nuzhat, Y.; Mohammad Riaz, K. Spectrum of common childhood skin disease: A single centre experience. J. Pak. Med. Assoc. 2005, 55, 15. [Google Scholar]
- Thrusfield, M. What sample size should be selected. In Veterinary Epidemiology; Blackwell Publishing: Hoboken, NJ, USA, 2007; p. 232. [Google Scholar]
- Kanbe, T.; Suzuki, Y.; Kamiya, A.; Mochizuki, T.; Fujihiro, M.; Kikuchi, A. PCR-based identification of common dermatophyte species using primer sets specific for the DNA topoisomerase II genes. J. Dermatol. Sci. 2003, 32, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Held, B.; Moriarty, B.; Richardson, T. Microsoft Excel Functions and Formulas with Excel 2019/Office 365; Mercury Learning and Information: Herndon, VA, USA, 2019. [Google Scholar]
- Mavrevski, R.; Traykov, M.; Trenchev, I.; Trencheva, M. Approaches to modeling of biological experimental data with GraphPad Prism software. WSEAS Trans. Syst. Control 2018, 13, 242–247. [Google Scholar]
- Zeba, N.; Shaikh, D.M.; Memon, K.N.; Khoharo, H.K. Scabies in relation to hygiene and other factors in patients visiting Liaquat University Hospital, Sindh, Pakistan. Age Years 2014, 9, 10–19. [Google Scholar]
- Kandi, V. Laboratory diagnosis of scabies using a simple saline mount: A clinical microbiologist’s report. Cureus 2017, 9, e1102. [Google Scholar] [CrossRef]
- Nenoff, P.; Krüger, C.; Ginter-Hanselmayer, G.; Tietz, H.J. Mycology–an update. Part 1: Dermatomycoses: Causative agents, epidemiology and pathogenesis. JDDG J. Dtsch. Dermatol. Ges. 2014, 12, 188–210. [Google Scholar] [CrossRef]
- Zhi, H.-L.; Xia, X.-J.; Shen, H.; Lv, W.-W.; Zhong, Y.; Sang, B.; Li, Q.-P.; Liu, Z.-H. Trichoscopy for early diagnosis and follow-up of pet-related neonatal tinea capitis. Mycopathologia 2023, 188, 571–575. [Google Scholar] [CrossRef]
- Ahmadi, B.; Mirhendi, H.; Shidfar, M.; Nouripour-Sisakht, S.; Jalalizand, N.; Geramishoar, M.; Shokoohi, G. A comparative study on morphological versus molecular identification of dermatophyte isolates. J. Mycol. Méd. 2015, 25, 29–35. [Google Scholar] [CrossRef]
- Thadchanamoorthy, V.; Dayasiri, K. Diagnosis and management of scabies in children. Sri Lanka J. Child Health 2020, 49, 383–389. [Google Scholar] [CrossRef]
- Rizvi, A.; Rossi, L. Scabies prevalence and risk factors in Pakistan: A hospital based survey. Biomed. J. 2018, 2, 5. [Google Scholar]
- Raza, N.; Qadir, S.; Agha, H. Risk factors for scabies among male soldiers in Pakistan: Case-control study. EMHJ-East. Mediterr. Health J. 2009, 15, 1105–1110. [Google Scholar] [CrossRef]
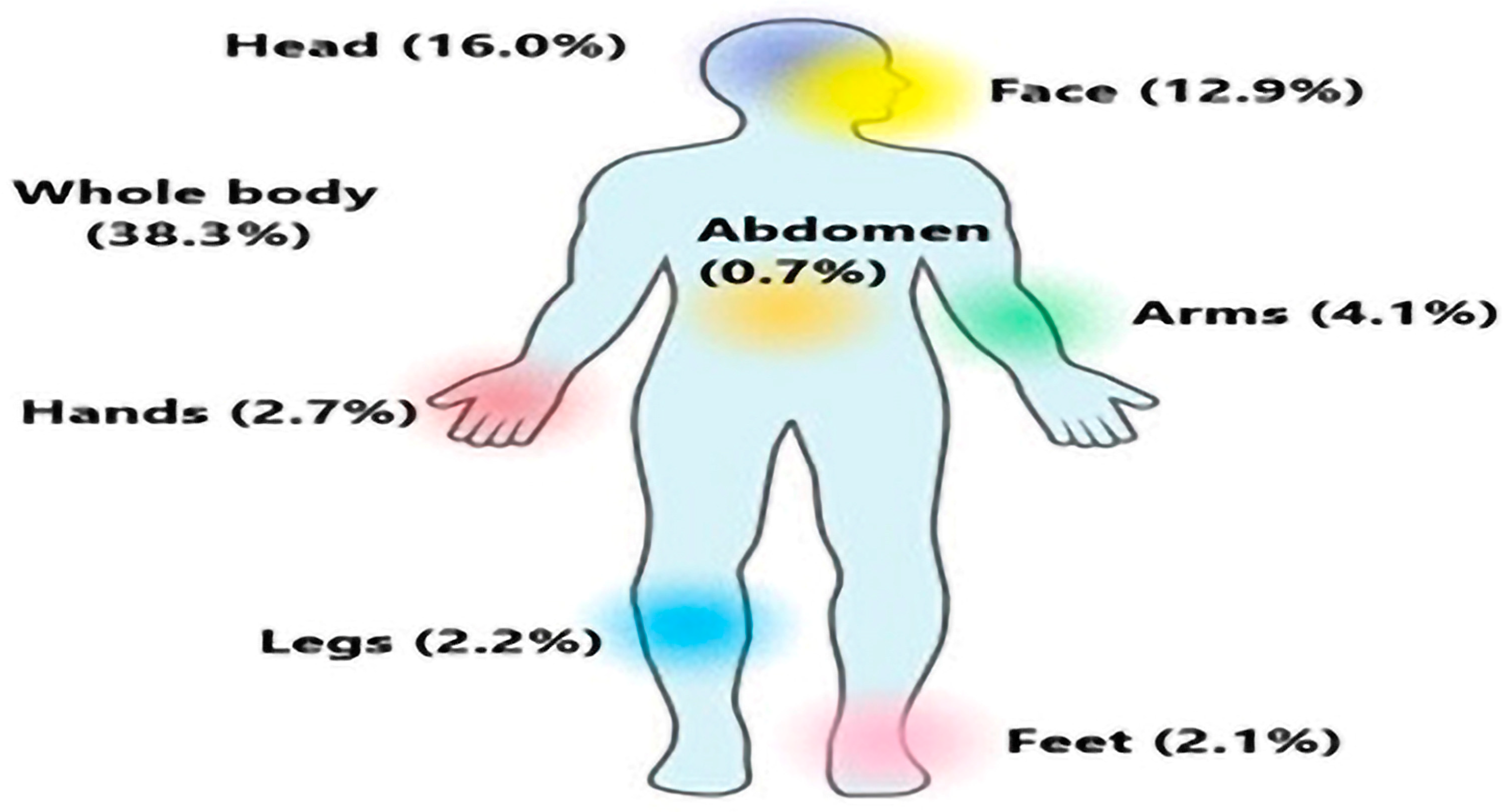
- Khatoon, N.; Khan, A.; Azmi, M.A.; Khan, A.; Shaukat, S.S. Most common body parts infected with scabies in children and its control. Pak. J. Pharm. Sci. 2016, 29, 1715–1717. [Google Scholar] [PubMed]
- Yasmin, S. Epidemiological study of scabies in district Haripur, Pakistan. Arthropods 2016, 5, 151. [Google Scholar]
- Hussain Bux, A.K.; Abdul Sattar, C.; Aijaz Hussain, M. Scabies in community of Jamshoro hills. Med. Forum Mon. 2012, 23, 67–70. [Google Scholar]
- Jackson, A.; Heukelbach, J.; Feldmeier, H. Transmission of scabies in a rural community. Braz. J. Infect. Dis. 2007, 11, 386–387. [Google Scholar] [CrossRef]
- Currie, B.J.; Carapetis, J.R. Skin infections and infestations in Aboriginal communities in northern Australia. Australas. J. Dermatol. 2000, 41, 139–143. [Google Scholar] [CrossRef]
- Stanton, B.; Khanam, S.; Nazrul, H.; Nurani, S.; Khair, T. Scabies in urban Bangladesh. J. Trop. Med. Hyg. 1987, 90, 219–226. [Google Scholar]
- Martin, P.; Koplin, J.; Eckert, J.; Lowe, A.; Ponsonby, A.L.; Osborne, N.; Gurrin, L.; Robinson, M.; Hill, D.; Tang, M. The prevalence and socio-demographic risk factors of clinical eczema in infancy: A population-based observational study. Clin. Exp. Allergy 2013, 43, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Browne, E.; Driessen, M.M.; Ross, R.; Roach, M.; Carver, S. Environmental suitability of bare-nosed wombat burrows for Sarcoptes scabiei. Int. J. Parasitol. Parasites Wildl. 2021, 16, 37–47. [Google Scholar] [CrossRef]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Bashir, Y.; Hassan, I.; Zeerak, S.; Bhat, M.A.; Jeelani, S.; Bhat, Y.J.; Rather, S.P.; Bashir, S. Profile of Dermatological Disorders Among Workers Involved in Fruit Growing Industry of Kashmir Valley in North India. Indian Dermatol. Online J. 2022, 13, 334. [Google Scholar] [PubMed]
- Odhiambo, J.A.; Williams, H.C.; Clayton, T.O.; Robertson, C.F.; Asher, M.I.; Group, I.P.T.S. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J. Allergy Clin. Immunol. 2009, 124, 1251–1258.e23. [Google Scholar] [CrossRef]
- Aman, S.; Nadeem, M.; Mahmood, K.; Ghafoor, M.B. Pattern of skin diseases among patients attending a tertiary care hospital in Lahore, Pakistan. J. Taibah Univ. Med. Sci. 2017, 12, 392–396. [Google Scholar] [CrossRef]
- Maryum, H.; Alam, M.Z.; Ahmed, I. Pattern of skin diseases in a tertiary care private hospital, Karachi. J. Pak. Assoc. Dermatol. 2014, 24, 292–297. [Google Scholar]
- Jabeen, K.; Farooqi, J.; Mirza, S.; Denning, D.; Zafar, A. Serious fungal infections in Pakistan. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 949–956. [Google Scholar] [CrossRef]
- Hussain, I.; Aman, S.; Haroon, T.; Jahangir, M.; Nagi, A. Tinea capitis in Lahore, Pakistan. Int. J. Dermatol. 1994, 33, 255–257. [Google Scholar] [CrossRef]
- Usman, B.; Rehman, A.; Naz, I.; Anees, M. Prevalence and antifungal drug resistance of dermatophytes in the clinical samples from Pakistan. Acta Microbiol. Immunol. Hung. 2021, 68, 291–296. [Google Scholar] [CrossRef]
- Kashif, S.; Uddin, F.; Nasir, F.; Zafar, S.; Jabeen, S.; Kumar, S. Prevalence of dermatophytes in superficial skin infections in a tertiary care hospital. J. Pak. Assoc. Dermatol. 2021, 31, 484–488. [Google Scholar]
- Ansari, F.; Siddiqui, S.A. Prevalence of dermatophytic infections in Karachi, Pakistan during the year 2003–2004. Pak. J. Bot. 2006, 38, 833. [Google Scholar]
- Hussain, A.; Zakki, S.A.; Qureshi, R. Epidemiological Study of Dermatophytosis in Okara, Pakistan. RADS J. Pharm. Pharm. Sci. 2016, 4, 184–187. [Google Scholar]
- Bongomin, F.; Olum, R.; Nsenga, L.; Namusobya, M.; Russell, L.; de Sousa, E.; Osaigbovo, I.I.; Kwizera, R.; Baluku, J.B. Estimation of the burden of tinea capitis among children in Africa. Mycoses 2021, 64, 349–363. [Google Scholar] [CrossRef]
- Jehangir, F.; Vohra, E.A. Frequency of Tinea Capitis in Children 5–15 Years of Age Presenting to Primary Health Care Centre in Karachi, Pakistan. Infect. Dis. J. Pak. 2013, 22, 592–610. [Google Scholar]
- Hameed, K.; Ch, F.R.; Nawaz, M.A.; Naqvi, S.M.S.; Gräser, Y.; Kupsch, C.; Pasquetti, M.; Rossi, L.; Min, A.R.M.; Tizzani, P. Trichophyton verrucosum infection in livestock in the Chitral district of Pakistan. J. Infect. Dev. Ctries. 2017, 11, 326–333. [Google Scholar] [CrossRef][Green Version]
- Connole, M.; Yamaguchi, H.; Elad, D.; Hasegawa, A.; Segal, E.; Torres-Rodriguez, J. Natural pathogens of laboratory animals and their effects on research. Med. Mycol. 2000, 38, 59–65. [Google Scholar] [CrossRef][Green Version]
- Rabinowitz, P.M.; Gordon, Z.; Odofin, L. Pet-related infections. Am. Fam. Physician 2007, 76, 1314–1322. [Google Scholar]
- Chomel, B.B. Diseases transmitted by less common house pets. In Infections of Leisure; Wiley: Hoboken, NJ, USA, 2016; pp. 171–199. [Google Scholar]
- Oehler, R.L.; Velez, A.P.; Mizrachi, M.; Lamarche, J.; Gompf, S. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect. Dis. 2009, 9, 439–447. [Google Scholar] [CrossRef]
- Jenerowicz, D.; Silny, W.; Danczak-Pazdrowska, A.; Polanska, A.; Osmola-Mankowska, A.; Olek-Hrab, K. Environmental factors and allergic diseases. Ann. Agric. Environ. Med. 2012, 19, 475–481. [Google Scholar]
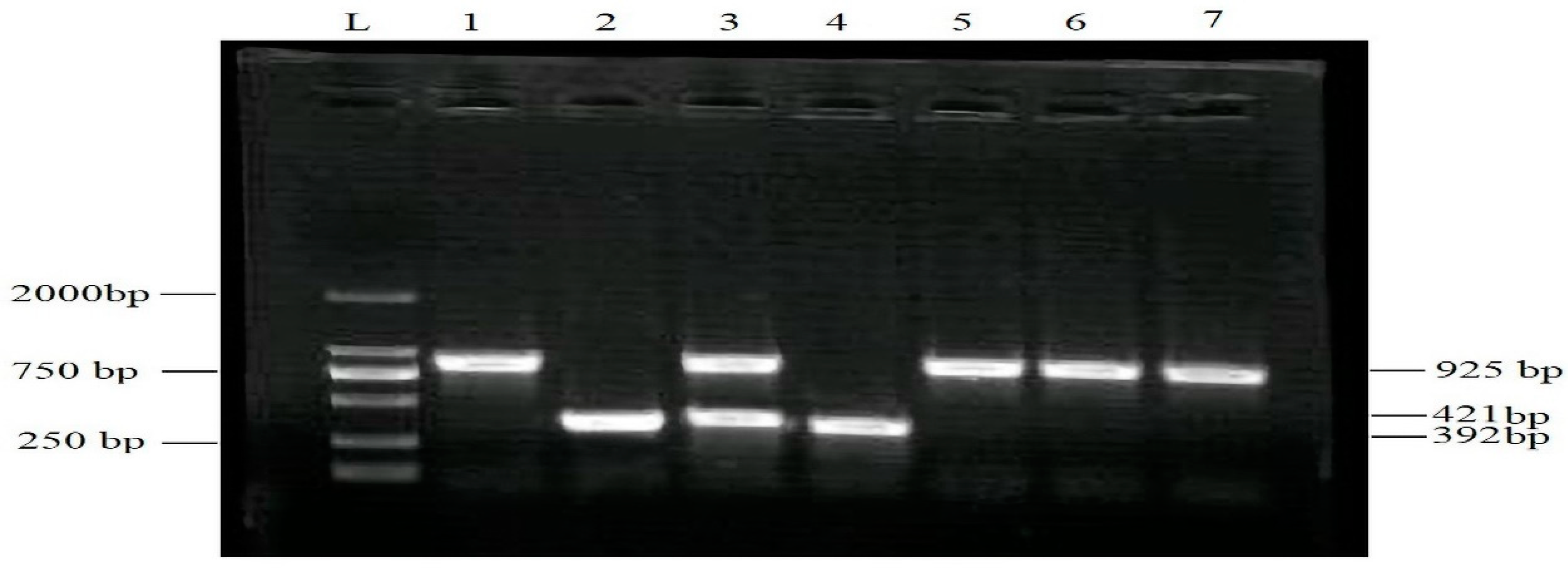
| Target Species | Forward Primer | Amplicon Size |
|---|---|---|
| T. rubrum | dMF2/86: RCGAGGAGAGGACCCRACHTCTGAC dMR2/138: TTCCTTAGTACCRGCYTTG | 925 bp |
| T. violaceum | TVCF2/34: GATCCACAAGGTATGTATTAGTTA TVCR2/76: GGTGCCAGCCATGTCGTAGAC | 421 bp |
| T. mentagrophytes | TRBF2/253: GCCTGTTGTTCCGCTCATTCTT TRBR2/346: CGGCTAGGAGGGCGTGGTAGAA | 392 bp |
| Microsporum spp. | TMTF2/38: GCATGATTTAGAAGTGTAATGCTG MCNR2/138: TTCCTTGGTACCAGCTTTG | 522 bp |
| Disorders | Age Stratification | ||||
|---|---|---|---|---|---|
| Infants and Toddlers | Children | Adolescents | Total Percentage | p-Value | |
| Scabies | 5.55% (0.28–25.75) | 33.33% (16.27–56.25) | 16.66% (5.83–39.22) | 45.55% (41.66–49.49) | <0.0001 |
| Eczema | 17.64% (6.19–41.02) | 35.29% (17.3–58.69) | 17.64% (6.19–41.02) | 18.48% (15.62–21.74) | |
| Dermatitis | 21.42% (7.57–47.58) | 35.71% (16.34–61.23) | 0% (0–21.53) | 9.73% (7.63–12.32) | |
| Tinea capitis | 0% (0–24.24) | 66.66% (39.06–86.18) | 16.66% (2.96–44.8) | 5.35% (3.84–7.42) | |
| Tinea corporis | 0% (0–43.44) | 100% (56.55–100) | 0% (0–43.44) | 2.76% (1.73–4.37) | |
| Impetigo | 0% (0–48.98) | 50% (8.88–91.11) | 50% (8.88–91.11) | 2.6% (1.61–4.18) | |
| Folliculitis | 0% (0–56.14) | 33.33% (1.7–88.15) | 33.33% (1.7–88.15) | 2.44% (1.48–3.98) | |
| Others | 0% (0–56.14) | 100% (43.85–100) | 0% (0–56.14) | 13.13% (10.69–16.03) | |
| Disorders | Dog | Cats | Birds | Livestock | Total | p-Value |
|---|---|---|---|---|---|---|
| Scabies | 9.63% (4.96–17.88) | 1.2% (0.06–6.51) | 7.22% (3.35–14.88) | 3.61% (0.98–10.09) | 22.22% (6.96–17.29) | 0.2402 |
| Tinea capitis | 6.02% (2.6–13.33) | 3.61% (0.98–10.09) | 7.22% (3.35–14.88) | 3.61% (0.98–10.09) | 20.84% (6.42–16.48) | |
| Dermatitis | 7.22% (3.35–14.88) | 3.61% (0.98–10.09) | 6.02% (2.6–13.33) | 0% (0–4.42) | 15.28% (4.32–13.17) | |
| Eczema | 2.4% (0.42–8.36) | 0% (0–4.42) | 9.63% (4.96–17.88) | 2.4% (0.42–8.36) | 13.89% (3.82–12.32) | |
| Tinea inguinal | 0% (0–4.42) | 0% (0–4.42) | 6.02% (2.6–13.33) | 0% (0–4.42) | 5.56% (1.09–6.93) | |
| Tinea corporis | 0% (0–4.42) | 0% (0–4.42) | 2.4% (0.42–8.36) | 2.4% (0.42–8.36) | 5.56% (1.09–6.93) | |
| Urticaria | 1.2% (0.06–6.51) | 0% (0–4.42) | 1.2% (0.06–6.51) | 1.2% (0.06–6.51) | 4.17% (0.57–5.95) | |
| Tinea faciei | 0% (0–4.42) | 0% (0–4.42) | 3.61% (0.98–10.09) | 0% (0–4.42) | 4.17% (0.57–5.95) | |
| Others | 0% (0–4.42) | 0% (0–4.42) | 7.22% (3.35–14.88) | 1.2% (0.06–6.51) | 8.33% (1.93–8.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majeed, A.; Mahmood, S.; Tahir, A.H.; Ahmad, M.; Shabbir, M.A.B.; Ahmad, W.; Iqbal, A.; Mushtaq, R.M.Z.; Aroosa, S.; Ahmed, H.S.; et al. Patterns of Common Dermatological Conditions among Children and Adolescents in Pakistan. Medicina 2023, 59, 1905. https://doi.org/10.3390/medicina59111905
Majeed A, Mahmood S, Tahir AH, Ahmad M, Shabbir MAB, Ahmad W, Iqbal A, Mushtaq RMZ, Aroosa S, Ahmed HS, et al. Patterns of Common Dermatological Conditions among Children and Adolescents in Pakistan. Medicina. 2023; 59(11):1905. https://doi.org/10.3390/medicina59111905
Chicago/Turabian StyleMajeed, Arfa, Sammina Mahmood, Adnan Hassan Tahir, Mehmood Ahmad, Muhammad Abu Bakr Shabbir, Waqas Ahmad, Asif Iqbal, Rana Muhammad Zahid Mushtaq, Sadaf Aroosa, Hafiz Saleet Ahmed, and et al. 2023. "Patterns of Common Dermatological Conditions among Children and Adolescents in Pakistan" Medicina 59, no. 11: 1905. https://doi.org/10.3390/medicina59111905
APA StyleMajeed, A., Mahmood, S., Tahir, A. H., Ahmad, M., Shabbir, M. A. B., Ahmad, W., Iqbal, A., Mushtaq, R. M. Z., Aroosa, S., Ahmed, H. S., Rasool, N., & Ramish, W. (2023). Patterns of Common Dermatological Conditions among Children and Adolescents in Pakistan. Medicina, 59(11), 1905. https://doi.org/10.3390/medicina59111905






