Esculeoside A Decreases Diabetic Cardiomyopathy in Streptozotocin-Treated Rats by Attenuating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis: Impressive Role of Nrf2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Establishment of the T1DM Model
2.3. Preparing ESA
2.4. Experimental Groups
2.5. Assessment of LV Function
2.6. Assessment of Biochemical Parameters in the Plasma and Serum
2.7. Analyses of the Biochemical Markers in the Tissues
2.8. Real-Time Polymerase Chain Reaction (qPCR)
2.9. Histology Studies
2.10. Statistical Analysis
3. Results
3.1. ESA Increases Body Weight, Reduces Heart Weight, and Improves LV Function in STZ-T1DM-Treated Rats by Stimulating Nrf2
3.2. ESA Fails to Improve Glucose and Insulin Levels but Attenuates Dyslipidemia in STZ-T1DM Rats through an Nrf2-Dependent Mechanism
3.3. ESA Reduces Lipid Peroxidation and Inflammatory Cytokine Production and Increases Antioxidant Levels in the LVs STZ-T1DM Rats in an Nrf2-Dependent Mechanism
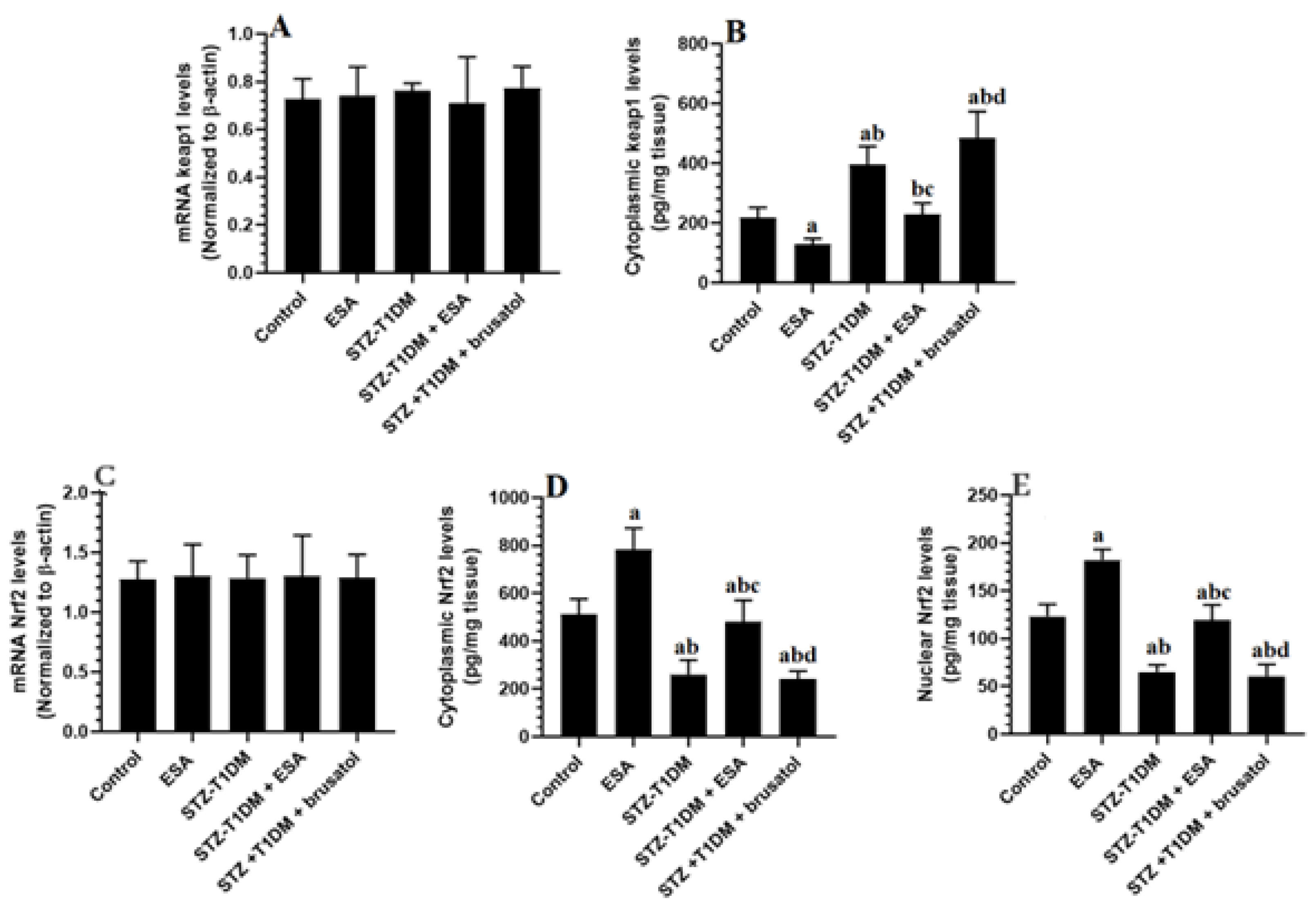
3.4. ESA Reduces Cytoplasmic and Nuclear Levels of Nrf2 by Increasing the Cytoplasmic Levels of kepa-1 in the LVs of STZ-T1DM Rats
3.5. ESA Suppresses the Transcription and the NF-κB Activation by Activating Nrf2
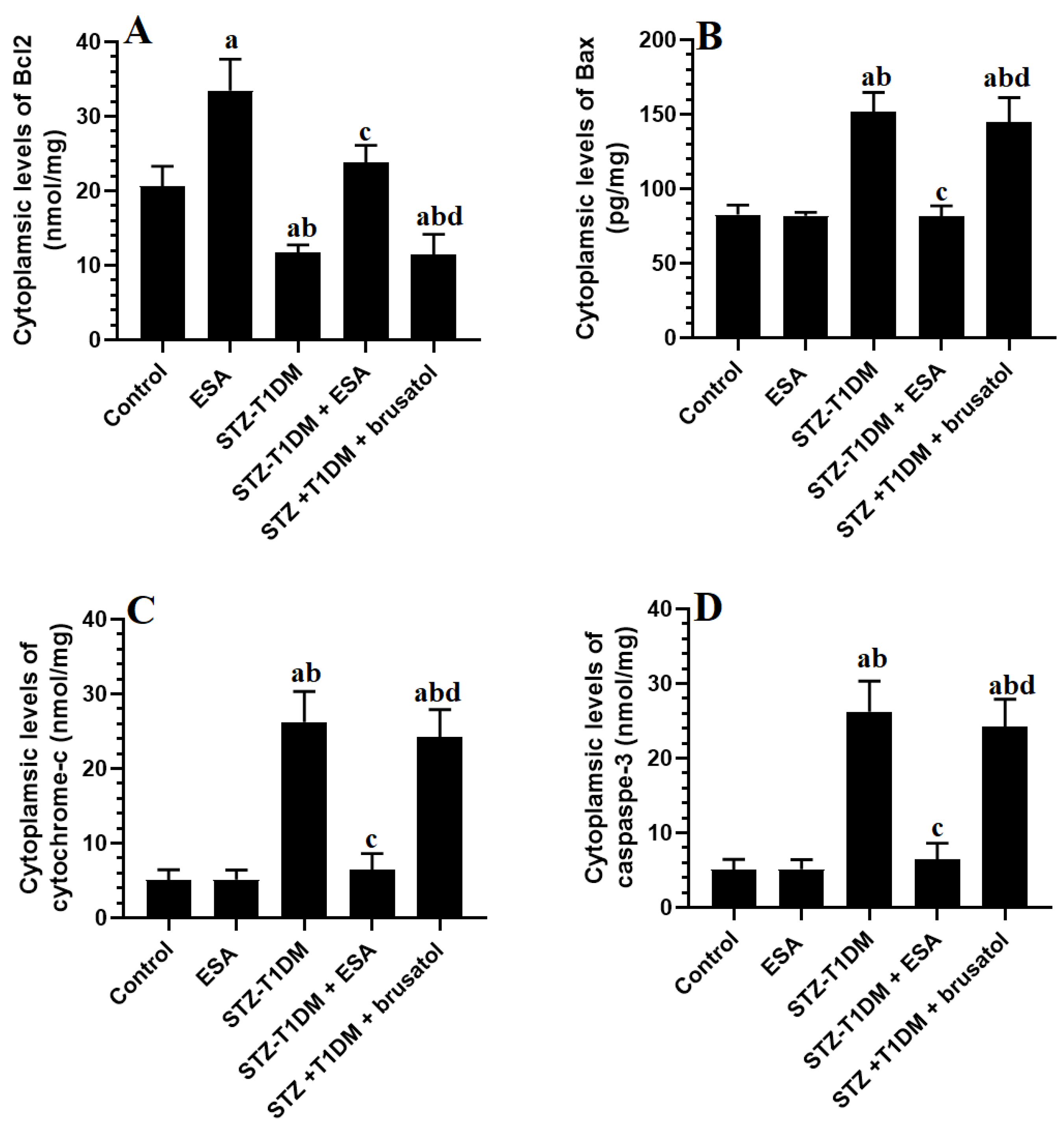
3.6. ESA Inhibits Intrinsic Cell Apoptosis in LVs by Stimulating Nrf2
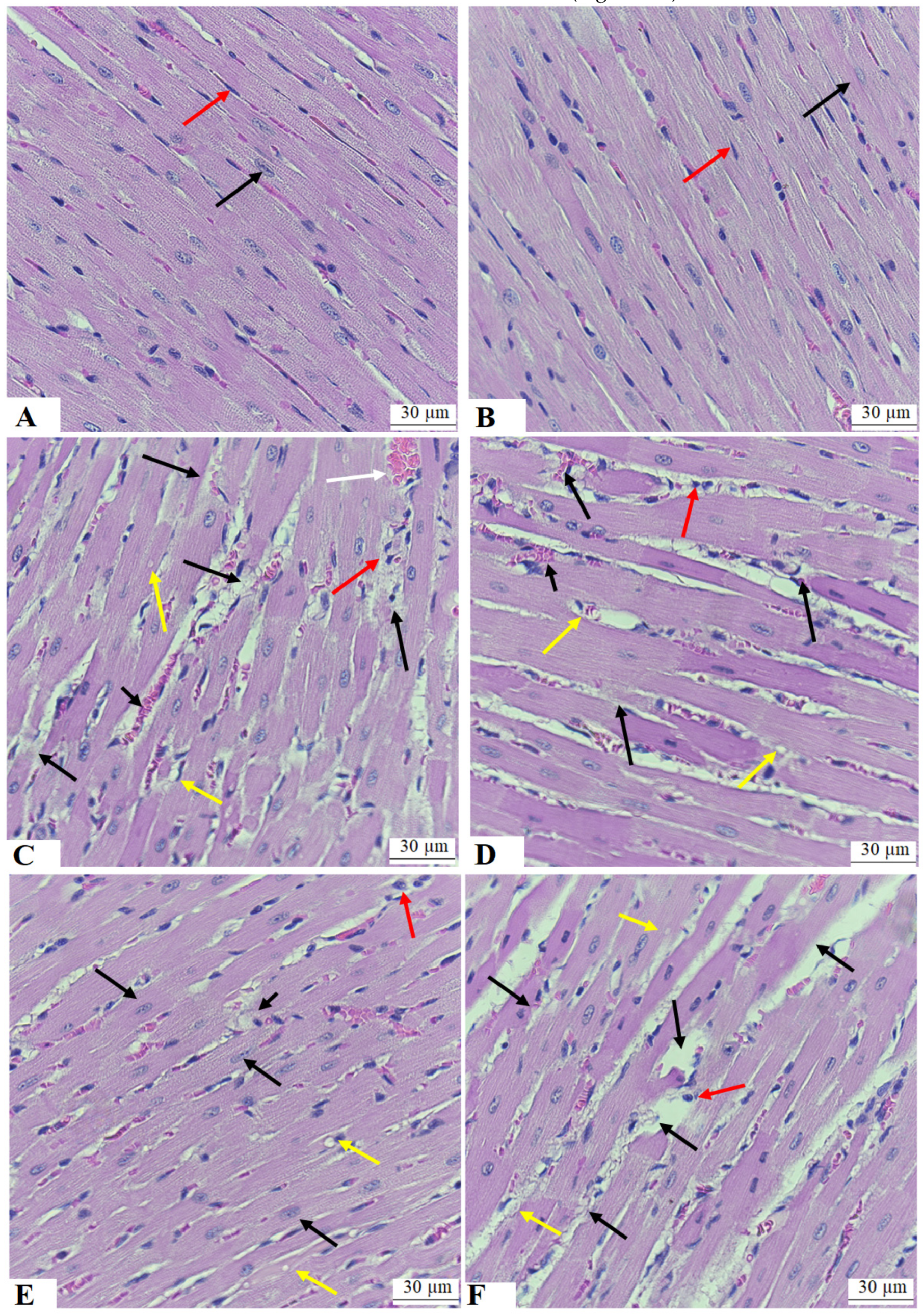
3.7. ESA Improves the Structure of the Cardiomyocytes of the LVs of STZ-T1DM
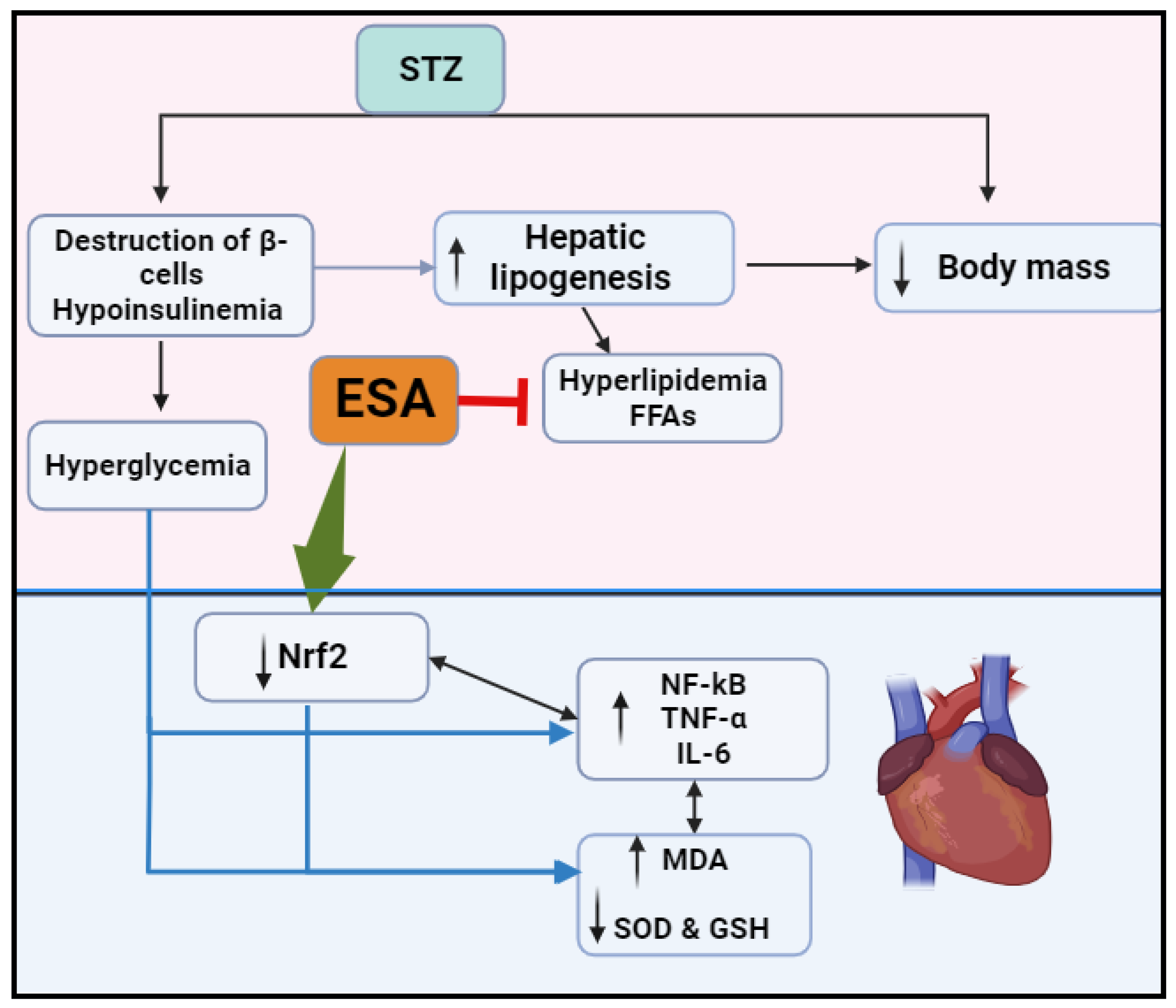
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregory, G.A.; Robinson, T.I.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; Magliano, D.J.; Maniam, J.; Orchard, T.J. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: A modelling study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Abdulaziz Al Dawish, M.; Alwin Robert, A.; Braham, R.; Abdallah Al Hayek, A.; Al Saeed, A.; Ahmed Ahmed, R.; Sulaiman Al Sabaan, F. Diabetes mellitus in Saudi Arabia: A review of the recent literature. Curr. Diabetes Rev. 2016, 12, 359–368. [Google Scholar] [CrossRef]
- Robert, A.A.; Al-Dawish, A.; Mujammami, M.; Dawish, M.A.A. Type 1 diabetes mellitus in Saudi Arabia: A soaring epidemic. Int. J. Pediatr. 2018, 2018, 9408370. [Google Scholar] [CrossRef] [PubMed]
- Dillmann, W.H. Diabetic cardiomyopathy: What is it and can it Be Fixed? Circ. Res. 2019, 124, 1160–1162. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Wang, X.; Chen, Y.; Pang, P.; Yang, Q.; Lin, J.; Deng, S.; Wu, S.; Fan, G. Diabetic cardiomyopathy: Clinical phenotype and practice. Front. Endocrinol. 2022, 13, 1032268. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Cai, L. Diabetic cardiomyopathy and its mechanisms: Role of oxidative stress and damage. J. Diabetes Investig. 2014, 5, 623–634. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef]
- De Geest, B.; Mishra, M. Role of oxidative stress in diabetic cardiomyopathy. Antioxidants 2022, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhao, Y.; Liu, Y.; Liu, Y.; Chen, K.; Lyu, S. Roles and mechanisms of herbal medicine for diabetic cardiomyopathy: Current status and perspective. Oxid. Med. Cell. Longev. 2017, 2017, 8214541. [Google Scholar] [CrossRef] [PubMed]
- Kaludercic, N.; Di Lisa, F. Mitochondrial ROS formation in the pathogenesis of diabetic cardiomyopathy. Front. Cardiovasc. Med. 2020, 7, 12. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Wang, W.; Chen, J.; Zhang, Z.; Zheng, Q.; Liu, Q.; Cai, L. Protection against diabetic cardiomyopathy is achieved using a combination of sulforaphane and zinc in type 1 diabetic OVE26 mice. J. Cell. Mol. Med. 2019, 23, 6319–6330. [Google Scholar] [CrossRef]
- Tan, Y.; Ichikawa, T.; Li, J.; Si, Q.; Yang, H.; Chen, X.; Goldblatt, C.S.; Meyer, C.J.; Li, X.; Cai, L. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress–induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 2011, 60, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, S.; Miao, L.; Cai, L. Prevention of diabetic complications by activation of Nrf2: Diabetic cardiomyopathy and nephropathy. Exp. Diabetes Res. 2012, 2012, 216512. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Murtaza, G.; Nadeem, N.; Shao, X.; Siddiqi, B.G.; Shafique, Z.; Ahmad, S.; Amjad, S.T.; Haroon, S.; Tanoli, M. A questionnaire based survey study for the evaluation of knowledge of Pakistani University teachers regarding their awareness about ibuprofen as an over the counter analgesic. Acta Pol. Pharm. 2014, 71, 337–342. [Google Scholar] [PubMed]
- Deshmukh, P.; Unni, S.; Krishnappa, G.; Padmanabhan, B. The Keap1–Nrf2 pathway: Promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 2017, 9, 41–56. [Google Scholar] [CrossRef]
- He, X.; Kan, H.; Cai, L.; Ma, Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J. Mol. Cell. Cardiol. 2009, 46, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cui, W.; Xin, Y.; Miao, X.; Barati, M.T.; Zhang, C.; Chen, Q.; Tan, Y.; Cui, T.; Zheng, Y. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J. Mol. Cell. Cardiol. 2013, 57, 82–95. [Google Scholar] [CrossRef]
- Luo, J.; Yan, D.; Li, S.; Liu, S.; Zeng, F.; Cheung, C.W.; Liu, H.; Irwin, M.G.; Huang, H.; Xia, Z. Allopurinol reduces oxidative stress and activates Nrf2/p62 to attenuate diabetic cardiomyopathy in rats. J. Cell. Mol. Med. 2020, 24, 1760–1773. [Google Scholar] [CrossRef]
- Ying, Y.; Jin, J.; Ye, L.; Sun, P.; Wang, H.; Wang, X. Phloretin prevents diabetic cardiomyopathy by dissociating Keap1/Nrf2 complex and inhibiting oxidative stress. Front. Endocrinol. 2018, 9, 774. [Google Scholar] [CrossRef]
- Raish, M.; Ahmad, A.; Jardan, Y.A.B.; Shahid, M.; Alkharfy, K.M.; Ahad, A.; Ansari, M.A.; Abdelrahman, I.A.; Al-Jenoobi, F.I. Sinapic acid ameliorates cardiac dysfunction and cardiomyopathy by modulating NF-κB and Nrf2/HO-1 signaling pathways in streptozocin induced diabetic rats. Biomed. Pharmacother. 2022, 145, 112412. [Google Scholar] [CrossRef] [PubMed]
- Dodda, D.; Ciddi, V. Plants used in the management of diabetic complications. Indian J. Pharm. Sci. 2014, 76, 97. [Google Scholar] [PubMed]
- Bilal, M.; Iqbal, M.S.; Shah, S.B.; Rasheed, T.; Iqbal, H. Diabetic complications and insight into antidiabetic potentialities of ethno-medicinal plants: A review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Shabab, S.; Gholamnezhad, Z.; Mahmoudabady, M. Protective effects of medicinal plant against diabetes induced cardiac disorder: A review. J. Ethnopharmacol. 2021, 265, 113328. [Google Scholar] [CrossRef] [PubMed]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, L.; Liu, J.; Lu, F.; Wang, L.; Chen, Y.; Li, D. Hypoglycemic effects of esculeoside A are mediated via activation of AMPK and upregulation of IRS-1. BMC Complement. Altern. Med. 2019, 19, 136. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kiyota, N.; Tsurushima, K.; Yoshitomi, M.; Horlad, H.; Ikeda, T.; Nohara, T.; Takeya, M.; Nagai, R. Tomatidine, a tomato sapogenol, ameliorates hyperlipidemia and atherosclerosis in apoE-deficient mice by inhibiting acyl-CoA: Cholesterol acyl-transferase (ACAT). J. Agric. Food Chem. 2012, 60, 2472–2479. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kiyota, N.; Hori, M.; Matsushita, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Takeya, M.; Ikeda, T.; Nohara, T. Esculeogenin A, a new tomato sapogenol, ameliorates hyperlipidemia and atherosclerosis in ApoE-deficient mice by inhibiting ACAT. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2400–2406. [Google Scholar] [CrossRef]
- Zhou, J.-R.; Kanda, Y.; Tanaka, A.; Manabe, H.; Nohara, T.; Yokomizo, K. Anti-hyaluronidase activity in vitro and amelioration of mouse experimental dermatitis by tomato saponin, Esculeoside A. J. Agric. Food Chem. 2016, 64, 403–408. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Sitasawad, S.L. Multiple antioxidants improve cardiac complications and inhibit cardiac cell death in streptozotocin-induced diabetic rats. PLoS ONE 2013, 8, e67009. [Google Scholar] [CrossRef]
- Zhou, J.-R.; Yamada, R.; Huruiti, E.; Kitahara, N.; Nakamura, H.; Fang, J.; Nohara, T.; Yokomizo, K. Ripe tomato saponin esculeoside A and sapogenol esculeogenin A suppress CD4+ T lymphocyte activation by modulation of Th2/Th1/Treg differentiation. Nutrients 2022, 14, 2021. [Google Scholar] [CrossRef] [PubMed]
- ALTamimi, J.Z.; AlFaris, N.A.; Aljabryn, D.H.; Alagal, R.I.; Alshammari, G.M.; Aldera, H.; Alqahtani, S.; Yahya, M.A. Ellagic acid improved diabetes mellitus-induced testicular damage and sperm abnormalities by activation of Nrf2. Saudi J. Biol. Sci. 2021, 28, 4300–4310. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandeh, H.; Pourbagher-Shahri, A.M.; Hasanpour, M.; Iranshahi, M.; Forouzanfar, F. Effects of Capparis Spinosa extract on the neuropathic pain induced by chronic constriction injury in rats. Metab. Brain Dis. 2022, 37, 2839–2852. [Google Scholar] [CrossRef]
- Thommana, D.D. Optimization of CUBIC Tissue Clearing and Cellular Labeling Methods Applied to the Cerebral Cortex of Rodents; McGill University: Montreal, QC, Canada, 2022. [Google Scholar]
- ALTamimi, J.Z.; BinMowyna, M.N.; AlFaris, N.A.; Alagal, R.I.; El-Kott, A.F.; Al-Farga, A.M. Fisetin protects against streptozotocin-induced diabetic cardiomyopathy in rats by suppressing fatty acid oxidation and inhibiting protein kinase R. Saudi Pharm. J. 2021, 29, 27–42. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, H.; Zhang, J.; Xia, Z.; Chen, W. Streptozotocin-induced diabetic cardiomyopathy in rats: Ameliorative effect of PIPERINE via Bcl2, Bax/Bcl2, and caspase-3 pathways. Biosci. Biotechnol. Biochem. 2020, 84, 2533–2544. [Google Scholar] [CrossRef]
- Marino, F.; Salerno, N.; Scalise, M.; Salerno, L.; Torella, A.; Molinaro, C.; Chiefalo, A.; Filardo, A.; Siracusa, C.; Panuccio, G. Streptozotocin-induced type 1 and 2 diabetes mellitus mouse models show different functional, cellular and molecular patterns of diabetic cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 1132. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Rodrigues, B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H1489–H1506. [Google Scholar] [CrossRef]
- Ke, J.; Pan, J.; Lin, H.; Gu, J. Diabetic cardiomyopathy: A brief summary on lipid toxicity. ESC Heart Fail. 2023, 10, 776–790. [Google Scholar] [CrossRef]
- Schilling, J.D.; Mann, D.L. Diabetic cardiomyopathy: Bench to bedside. Heart Fail. Clin. 2012, 8, 619–631. [Google Scholar] [CrossRef]
- Deng, J.; Yan, F.; Tian, J.; Qiao, A.; Yan, D. Potential clinical biomarkers and perspectives in diabetic cardiomyopathy. Diabetol. Metab. Syndr. 2023, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Bodor, G.S. Biochemical markers of myocardial damage. Ejifcc 2016, 27, 95. [Google Scholar]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.J.; Rajasekaran, N.S.; Abel, E.D.; Bugger, H. Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free Radic. Biol. Med. 2021, 169, 317–342. [Google Scholar] [CrossRef]
- Zarain-Herzberg, A.; García-Rivas, G.; Estrada-Avilés, R. Regulation of SERCA pumps expression in diabetes. Cell Calcium 2014, 56, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Al Kury, L.T. Calcium homeostasis in ventricular myocytes of diabetic cardiomyopathy. J. Diabetes Res. 2020, 2020, 1942086. [Google Scholar] [CrossRef]
- Peng, M.-l.; Fu, Y.; Wu, C.-W.; Zhang, Y.; Ren, H.; Zhou, S.-S. Signaling pathways related to oxidative stress in diabetic cardiomyopathy. Front. Endocrinol. 2022, 13, 907757. [Google Scholar] [CrossRef]
- Sirker, A.; Zhang, M.; Murdoch, C.; Shah, A.M. Involvement of NADPH oxidases in cardiac remodelling and heart failure. Am. J. Nephrol. 2007, 27, 649–660. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Vasudeva, N.; Sharma, S. Acute and chronic animal models for the evaluation of anti-diabetic agents. Cardiovasc. Diabetol. 2012, 11, 9. [Google Scholar] [CrossRef]
- Gupta, S.K.; Dongare, S.; Mathur, R.; Mohanty, I.R.; Srivastava, S.; Mathur, S.; Nag, T.C. Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats. Mol. Cell. Biochem. 2015, 408, 63–72. [Google Scholar] [CrossRef]
- Zou, F.; Wang, L.; Liu, H.; Wang, W.; Hu, L.; Xiong, X.; Wu, L.; Shen, Y.; Yang, R. Sophocarpine suppresses NF-κB-mediated inflammation both in vitro and in vivo and inhibits diabetic cardiomyopathy. Front. Pharmacol. 2019, 10, 1219. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.K.; Zhao, Y.Y.; Shi, M.L.; Zhang, G.; Zhao, Y.J.; Zhang, B.G.; Liang, R.J. Skimmin protects diabetic cardiomyopathy in streptozotocin-induced diabetic rats. Kaohsiung J. Med. Sci. 2021, 37, 136–144. [Google Scholar] [CrossRef]
- Zhang, X.; Ke, P.-X.; Yuan, X.; Zhang, G.-P.; Chen, W.-L.; Zhang, G.-S. Forskolin protected against streptozotocin-induced diabetic cardiomyopathy via inhibition of oxidative stress and cardiac fibrosis in mice. BioMed Res. Int. 2021, 2021, 8881843. [Google Scholar] [CrossRef]
- Abukhalil, M.H.; Althunibat, O.Y.; Aladaileh, S.H.; Al-Amarat, W.; Obeidat, H.M.; Alayn’Al-marddyah, A.; Hussein, O.E.; Alfwuaires, M.A.; Algefare, A.I.; Alanazi, K.M. Galangin attenuates diabetic cardiomyopathy through modulating oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2021, 138, 111410. [Google Scholar] [CrossRef]
- Albasher, G.; Alkahtani, S.; Al-Harbi, L.N. Urolithin A prevents streptozotocin-induced diabetic cardiomyopathy in rats by activating SIRT1. Saudi J. Biol. Sci. 2022, 29, 1210–1220. [Google Scholar] [CrossRef]
- AlTamimi, J.Z.; AlFaris, N.A.; Alshammari, G.M.; Alagal, R.I.; Aljabryn, D.H.; Yahya, M.A. The Protective Effect of 11-Keto-β-Boswellic Acid against Diabetic Cardiomyopathy in Rats Entails Activation of AMPK. Nutrients 2023, 15, 1660. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hua, Y.; Li, X.; Arslan, I.M.; Zhang, W.; Meng, G. Distinct types of cell death and the implication in diabetic cardiomyopathy. Front. Pharmacol. 2020, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Ndebele, K.; Gona, P.; Jin, T.-G.; Benhaga, N.; Chalah, A.; Degli-Esposti, M.; Khosravi-Far, R. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced mitochondrial pathway to apoptosis and caspase activation is potentiated by phospholipid scramblase-3. Apoptosis 2008, 13, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.T.; Dagher, P.; Hile, K.L.; Zhang, H.; Meldrum, D.R.; Rink, R.C.; Meldrum, K.K. Tumor necrosis factor-α induces intrinsic apoptotic signaling during renal obstruction through truncated bid activation. J. Urol. 2008, 180, 2694–2700. [Google Scholar] [CrossRef]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Jaiswal, A.K. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 2012, 287, 9873–9886. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2016, 116, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Ryoo, I.-G.; Ha, H.; Kwak, M.-K. Inhibitory role of the KEAP1-NRF2 pathway in TGFβ1-stimulated renal epithelial transition to fibroblastic cells: A modulatory effect on SMAD signaling. PLoS ONE 2014, 9, e93265. [Google Scholar] [CrossRef]
- Wong, D.P.W.; Ng, M.Y.; Leung, J.Y.; Boh, B.K.; Lim, E.C.; Tan, S.H.; Lim, S.; Seah, W.H.; Hu, C.Z.; Ho, B.C. Regulation of the NRF2 transcription factor by andrographolide and organic extracts from plant endophytes. PLoS ONE 2018, 13, e0204853. [Google Scholar] [CrossRef]
- El Aziz, M.M.A.; Ashour, A.S.; Melad, A.G. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res. 2019, 8, 282–288. [Google Scholar]

| Target | Primers Sequence 5′→3′ | Accession No. | Base Pair Length |
|---|---|---|---|
| Nrf2 | F:AAAATCATTAACCTCCCTGTTGAT R:CGGCGACTTTATTCTTACCTCTC | NM_031789 | 118 |
| NF-κB | F:GTGCAGAAAGAAGACATTGAGGTG R:AGGCTAGGGTCAGCGTATGG | XM_342346.4 | 176 |
| Keap1 | F:CTTCGGGGAGGA GGAGTTCT R:CGTTCAGATCATCGCGGCTG | NM_057152.2 | 122 |
| β-actin | F:CGAGTACAACCTTCTTGCAGC R:CCTTCTGACCCATACCCACC | NM_031144.3 | 209 |
| Parameter | Control | ESA | STZ-T1DM | STZ-T1DM + ESA | DM + ESA + Brusatol |
|---|---|---|---|---|---|
| Final body weight (g) | 523.2 ± 43.2 | 541.1 ± 44.6 | 346.2 ± 28.4 ab | 463.3 ± 22.9 abc | 331.3 ± 34.1 abd |
| Heart weight (g) | 1.68 ± 0.25 | 1.59 ± 0.24 | 2.23 ± 0.21 ab | 1.61 ± 0.11 c | 2.17 ± 0.21 abd |
| LVSP (mmHg) | 117.1 ± 10.2 | 112.8 ± 11.4 | 73.4 ± 6.5 ab | 103.3 ± 9.7 c | 69.8 ± 7.6 abd |
| LVEDP (mmHg) | 2.75 ± 0.36 | 3.11 ± 0.37 | 12.36 ± 1.4 ab | 4.43 ± 0.56 abc | 11.46 ± 1.6 abd |
| Dp/dtmax (×103 mmHg) | 6.74 ± 0.54 | 6.29 ± 0.73 | 3.46 ± 0.43 ab | 5.89 ± 0.73 c | 3.18 ± 0.36 abd |
| Dp/dtmin (×103 mmHg) | 5.43 ± 0.63 | 5.88 ± 0.49 | 2.73 ± 0.22 ab | 5.17 ± 0.63 c | 2.39 ± 0.26 abd |
| TpI (pg/mL) | 24.5 ± 2.7 | 22.5 ± 2.1 | 127.2 ± 11.5 ab | 27.8 ± 3.9 bc | 136.3 ± 15.2 abd |
| CK-MB (IU/L) | 16.4 ± 2.6 | 15.8 ± 2.4 | 85.4 ± 7.9 ab | 18.8 ± 2.9 c | 91.2 ± 10.4 abd |
| LDH (IU/mL) | 229.2 ± 29.8 | 231.7 ± 18.3 | 387.3 ± 42.3 ab | 247.2 ± 33.1 c | 415.6 ± 52.4 abd |
| Parameter | Control | ESA | STZ-DM | STZ-DM + ESA | STZ-DM + ESA + Brusatol |
|---|---|---|---|---|---|
| FPG (mg/dL) | 113.5 ± 10.4 | 108.9 ± 10.2 | 328.4 ± 44.7 ab | 318.4 ± 28.5 ab | 341.2 ± 38.1 ab |
| FPI (µIU/mL) | 3.23 ± 0.42 | 3.59 ± 0.33 | 1.63 ± 0.19 ab | 1.52 ± 0.27 ab | 1.48 ± 0.20 ab |
| TGs (mg/dL) | 74.4 ± 8.1 | 61.2 ± 8.4 a | 167.1 ± 15.9 ab | 92.3 ± 8.4 abc | 181.2 ± 22.3 abd |
| CHOL (mg/dL) | 84.6 ± 7.4 | 68.5 ± 7.6 a | 182.3 ± 15.2 ab | 81.2 ± 8.3 bc | 167.3 ± 17.4 abd |
| LDL-c (mg/dL) | 36.7 ± 3.1 | 24.5 ± 2.5 a | 87.9 ± 7.7 ab | 35.9 ± 4.2 bc | 92.3 ± 10.3 abd |
| Parameter | Control | ESA | STZ-DM | STZ-DM + ESA | STZ-DM + ESA + Brusatol |
|---|---|---|---|---|---|
| MDA (nmol/mg tissue) | 0.53 ± 0.08 | 0.63 ± 0.072 | 1.72 ± 0.21 ab | 0.61 ± 0.09 c | 1.68 ± 0.15 ab |
| GSH (nmol/mg tissue) | 63.4 ± 7.3 | 78.4 ± 6.4 a | 36.6 ± 4.1 ab | 65.2 ± 6.4 bc | 31.1 ± 2.9 ab |
| HO-1 (pg/mg tissue) | 20.4 ± 3.1 | 32.8 ± 3.3 a | 11.3 ± 2.2 ab | 26.5 ± 2.8 bc | 9.4 ± 1.3 abd |
| SOD1 (pg/mg tissue) | 28.9 ± 2.7 | 41.8 ± 3.7 a | 12.9 ± 1.5 ab | 26.8 ± 2.2 bc | 10.6 ± 1.1 abd |
| TNFα (pg/mg tissue) | 0.89 ± 0.06 | 1.1 ± 0.13 | 4.93 ± 0.82 ab | 1.27 ± 0.16 ac | 5.16 ± 0.08 abd |
| ICAM-1 | 54.6 ± 6.9 | 59.8 ± 5.2 | 134.5 ± 12.9 ab | 57.4 ± 6.6 c | 126.3 ± 14.5 abd |
| IL-6 (pg/mg tissue) | 2.89 ± 0.31 | 3.25 ± 0.58 | 13.22 ± 1.82 ab | 4.22 ± 0.72 abc | 11.6 ± 1.42 abd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ALTamimi, J.Z.; AlFaris, N.A.; Alshammari, G.M.; Alagal, R.I.; Aljabryn, D.H.; Yahya, M.A. Esculeoside A Decreases Diabetic Cardiomyopathy in Streptozotocin-Treated Rats by Attenuating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis: Impressive Role of Nrf2. Medicina 2023, 59, 1830. https://doi.org/10.3390/medicina59101830
ALTamimi JZ, AlFaris NA, Alshammari GM, Alagal RI, Aljabryn DH, Yahya MA. Esculeoside A Decreases Diabetic Cardiomyopathy in Streptozotocin-Treated Rats by Attenuating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis: Impressive Role of Nrf2. Medicina. 2023; 59(10):1830. https://doi.org/10.3390/medicina59101830
Chicago/Turabian StyleALTamimi, Jozaa Z., Nora A. AlFaris, Ghedeir M. Alshammari, Reham I. Alagal, Dalal H. Aljabryn, and Mohammed Abdo Yahya. 2023. "Esculeoside A Decreases Diabetic Cardiomyopathy in Streptozotocin-Treated Rats by Attenuating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis: Impressive Role of Nrf2" Medicina 59, no. 10: 1830. https://doi.org/10.3390/medicina59101830
APA StyleALTamimi, J. Z., AlFaris, N. A., Alshammari, G. M., Alagal, R. I., Aljabryn, D. H., & Yahya, M. A. (2023). Esculeoside A Decreases Diabetic Cardiomyopathy in Streptozotocin-Treated Rats by Attenuating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis: Impressive Role of Nrf2. Medicina, 59(10), 1830. https://doi.org/10.3390/medicina59101830






