The Protective Potential of Petroselinum crispum (Mill.) Fuss. on Paracetamol-Induced Hepatio-Renal Toxicity and Antiproteinuric Effect: A Biochemical, Hematological, and Histopathological Study
Abstract
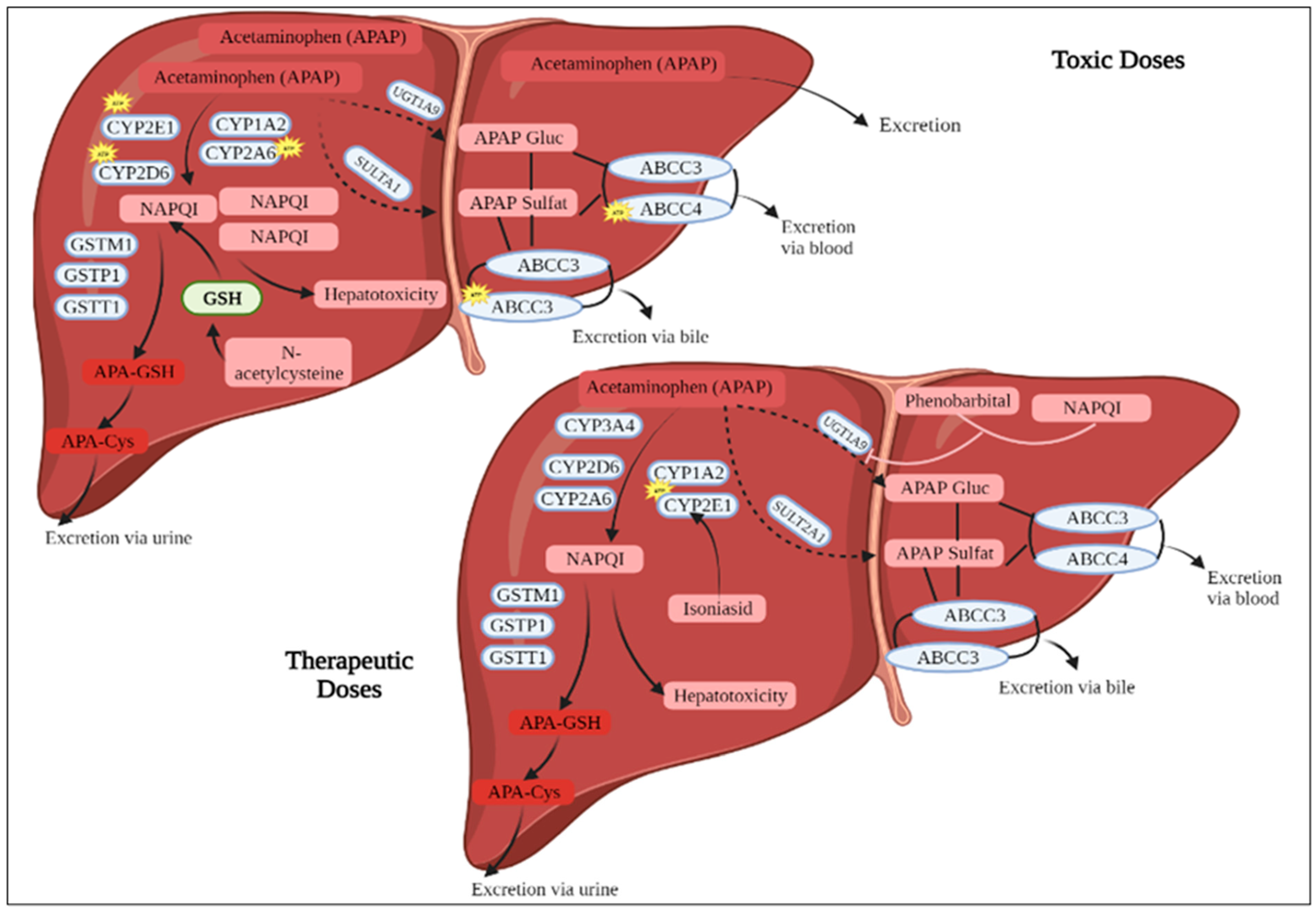
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Plant Extract
2.3. In Vivo Hepatorenal Protective Activity
2.3.1. Test Animals
2.3.2. Experimental Design
2.3.3. Urine Collection and Analysis
2.3.4. Blood Sampling
2.4. Statistical Analysis
3. Results
3.1. Effect of PC-Ext and Paracetamol on Kidneys and Liver Function Tests
3.2. Effect of Paracetamol and PC-Ext on Hematological Parameters
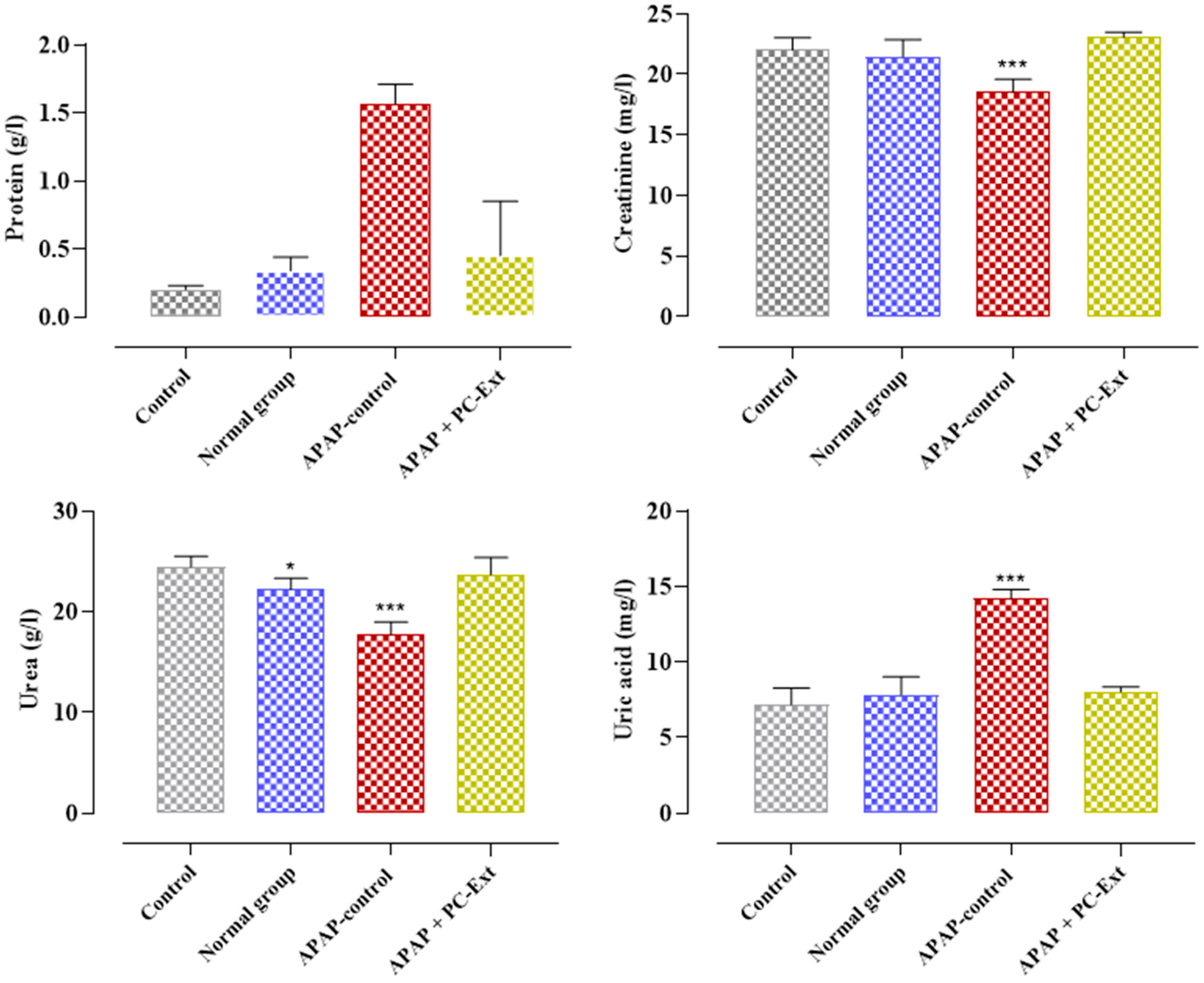
3.3. Influence of Paracetamol and Parsley on Proteinuria
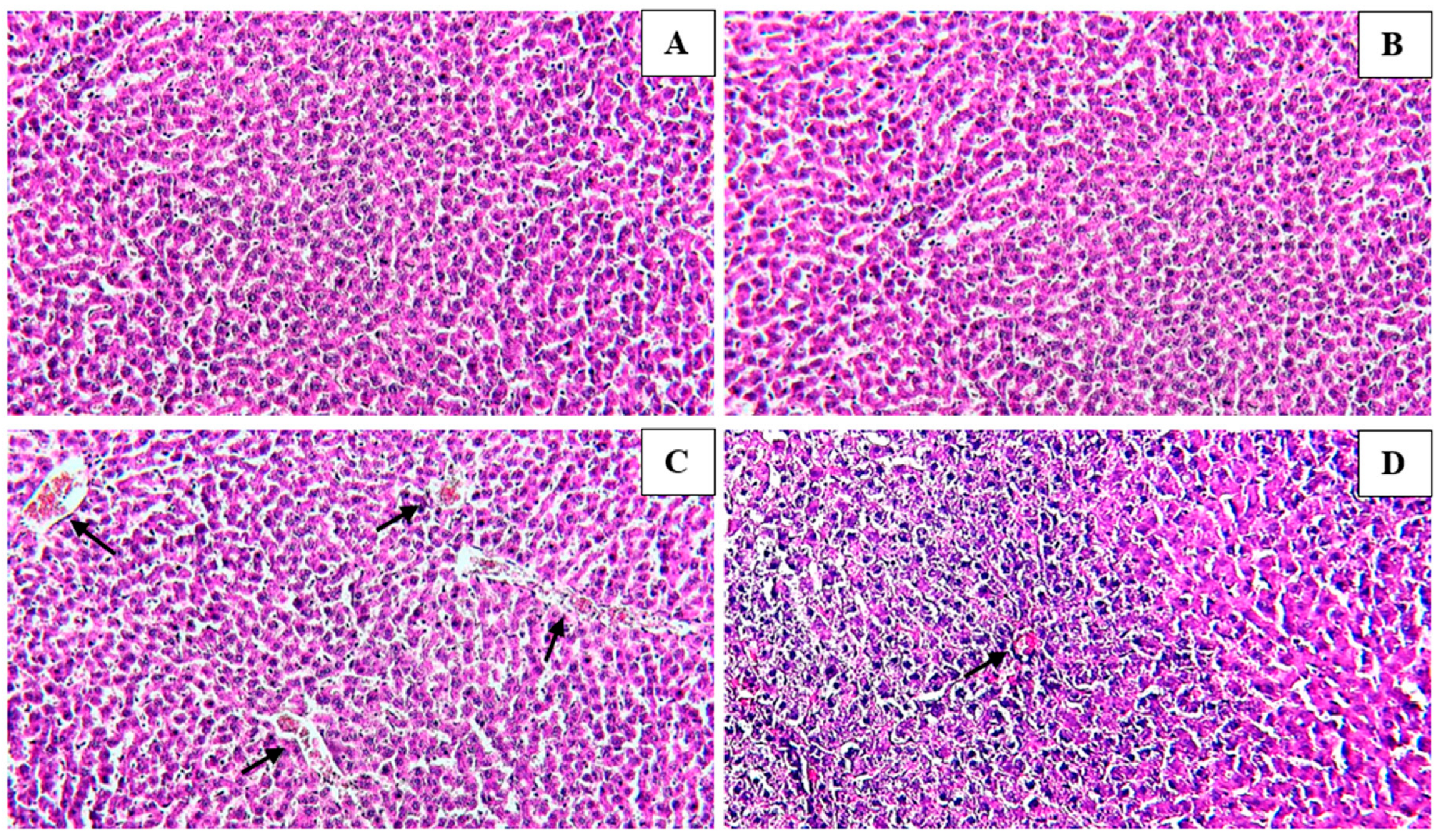
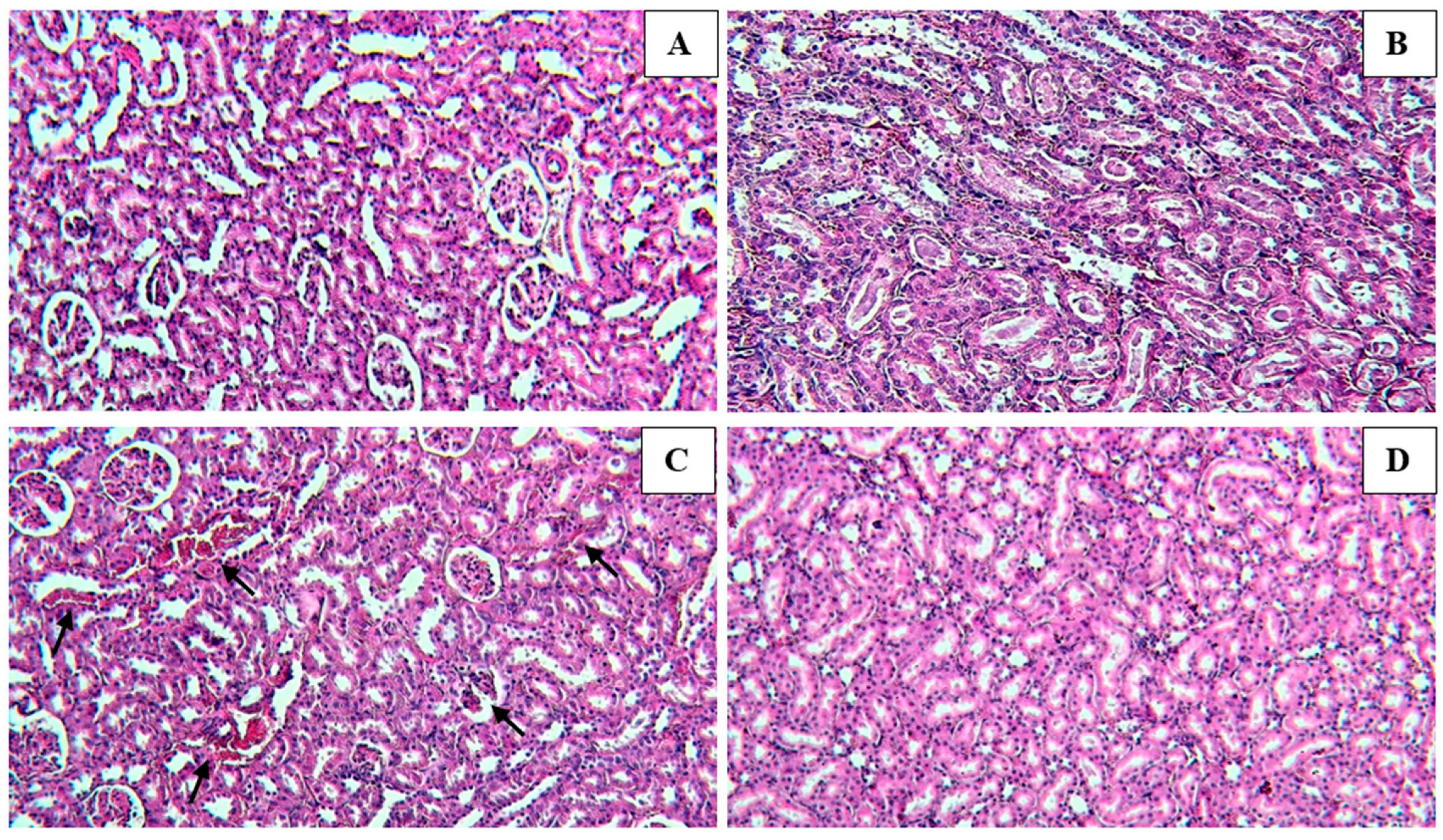
3.4. Histological Findings
4. Discussion
5. Conclusions
6. Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Bayomy, M.; Sakr, S. Biochemical and histological studies on the possible protective impact of the herb basil (Ocimum basilicum) on adriamycin induced toxicity in rats. I. Influence on the liver. J. Biosci. Appl. Res. 2016, 2, 634–640. [Google Scholar] [CrossRef]
- Abdellatief, S.A.; Galal, A.A.; Farouk, S.M.; Abdel-Daim, M.M. Ameliorative effect of parsley oil on cisplatin-induced hepato-cardiotoxicity: A biochemical, histopathological, and immunohistochemical study. Biomed. Pharmacother. 2017, 86, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Alhalmi, A.; Beg, S.; Kohli, K.; Waris, M.; Singh, T. Nanotechnology Based Approach for Hepatocellular Carcinoma Targeting. Curr. Drug Targets 2021, 22, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Bessems, J.G.M.; Vermeulen, N.P.E. Paracetamol (Acetaminophen)-Induced Toxicity: Molecular and Biochemical Mechanisms, Analogues and Protective Approaches. Crit. Rev. Toxicol. 2001, 31, 55–138. [Google Scholar] [CrossRef]
- El Menyiy, N.; Al-Waili, N.; El Ghouizi, A.; Al-Waili, W.; Lyoussi, B. Evaluation of antiproteinuric and hepato-renal protective activities of propolis in paracetamol toxicity in rats. Nutr. Res. Pract. 2018, 12, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Ammar, N.M.; Al-Okbi, S.Y.; Mohamed, D.A. Study of The Anti-Inflammatory Activity of Some Medicinal Edible Plants Growing in Egypt. Med. J. Islam. Acad. Sci. 1997, 10, 113–122. [Google Scholar]
- Garba, S.; Sambo, N.; Bala, U. The Effect Of The Aqueous Extract Of Kohautia Grandiflora On Paracetamol Induced Liver Damage In Albino Rats. Niger. J. Physiol. Sci. 2009, 24, 17–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, R.; Tawa, G.; Wallqvist, A. Locally Weighted Learning Methods for Predicting Dose-Dependent Toxicity with Application to the Human Maximum Recommended Daily Dose. Chem. Res. Toxicol. 2012, 25, 2216–2226. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Misra, N. Hepatoprotective Activity of Aqueous Ethanolic Extract of Chamomile capitula in Paracetamol Intoxicated Albino Rats. Am. J. Pharmacol. Toxicol. 2006, 1, 17–20. [Google Scholar] [CrossRef]
- Oyedeji, K.O.; Bolarinwa, A.F.; Ojeniran, S.S. Effect of Paracetamol (Acetaminophen) on Haematological and Reproductive Parameters in Male Albino Rats. IOSR J. Pharm. Biol. Sci. 2013, 4, 65–70. [Google Scholar] [CrossRef]
- Matić, M.; Milosevic, M.; Paunovic, M.; Ognjanovic, B.; Stajn, A.; Saicic, Z. Paracetamol-induced changes of haematobiochemical and oxidative stress parameters in rat blood: Protective role of vitamin C and β-glucan. Kragujev. J. Sci. 2016, 38, 135–146. [Google Scholar] [CrossRef]
- Megarbane, B.; Deye, N.; Baud, F. Foie toxique: Mécanismes lésionnels et thérapeutiques pharmacologiques spécifiques. Réanimation 2007, 16, 632–642. [Google Scholar] [CrossRef]
- Sabir, S.; Rocha, J. Water-extractable phytochemicals from Phyllanthus niruri exhibit distinct in vitro antioxidant and in vivo hepatoprotective activity against paracetamol-induced liver damage in mice. Food Chem. 2008, 111, 845–851. [Google Scholar] [CrossRef]
- Sheen, C.; Dillon, J.; Bateman, D.; Simpson, K.; Macdonald, T. Paracetamol toxicity: Epidemiology, prevention and costs to the health-care system. Qjm Int. J. Med. 2002, 95, 609–619. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, H.; Kumar, U.; Mayachari, A.; Sangli, G.; Singh, S. Protective effects of Glycyrrhiza glabra supplementation against methotrexate-induced hepato-renal damage in rats: An experimental approach. J. Ethnopharmacol. 2020, 263, 113209. [Google Scholar] [CrossRef] [PubMed]
- Gawai, A.A.; Chalakh, S. In Vivo Study on Hepatoprotective Potential of Nagdantyadi Ghrita in Paracetamol Induced Hepa-totoxicity in Wistar Rats. World J. Pharm. Res. 2022, 11, 918–923. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Sobeh, M.; El-Maadawy, W.H.; Mohammed, H.S.; Khalil, H.; Botros, S.; Wink, M. Chemical Profiling of Polyphenolics in Eucalyptus globulus and Evaluation of Its Hepato–Renal Protective Potential against Cyclophosphamide Induced Toxicity in Mice. Antioxidants 2019, 8, 415. [Google Scholar] [CrossRef]
- Mirza, N. Paracetamol-Induced Hepatotoxicity. In Hepatotoxicity; Chapter 3; Streba, C.-T., Rogoveanu, I., Vere, C.C., Eds.; IntechOpen: Rijeka, Croatia, 2022; ISBN 978-1-80355-781-6. [Google Scholar]
- Byna, J.; Rajaram, S.; Reddy, K.S.; Derangula, S.S.R.; Nathiya, S.; Sankari, S. Attenuation of paracetamol induced hepatotoxicity by Albizia odoratissima in rats. Int. J. Health Sci. 2022, 6, 1676–1685. [Google Scholar] [CrossRef]
- Mateus, A.O.; Bezerra, M.C.O.; Nicoletti, S.d.A.; Miranda, G.C.N.; De Freitas, C.T.A.; Soares, R.M.; Façanha, S.d.O.; Dos Santos, E.I.; Da Costa, R.I.; Nunes, M.P.O.; et al. Dengue and its association with hepatic biomarkers: Antiinflammatory action and utilization of paracetamol as a hepatotoxicity factor. Braz. J. Case Rep. 2022, 3, 10–15. [Google Scholar] [CrossRef]
- Nouioura, G.; Tourabi, M.; El Ghouizi, A.; Kara, M.; Assouguem, A.; Saleh, A.; Al Kamaly, O.; El Ouadrhiri, F.; Lyoussi, B.; Derwich, E.H. Optimization of a New Antioxidant Formulation Using a Simplex Lattice Mixture Design of Apium graveolens L., Coriandrum sativum L., and Petroselinum crispum M. Grown in Northern Morocco. Plants 2023, 12, 1175. [Google Scholar] [CrossRef]
- Ugan, R.A.; Cadirci, E.; Un, H.; Cinar, I.; Gurbuz, M.A. Fisetin Attenuates Paracetamol-Induced Hepatotoxicity by Regulating CYP2E1 Enzyme. An. Acad. Bras. Ciências 2023, 95, e20201408. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.A.; Shreif, N.H.; Gharib, O.A. The Protective Effect of Coriandium sativum Extract on Hepato-renal Toxicity Induced in Irradiated Rats. Eur. J. Med. Plants 2014, 4, 196–205. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Milić, N.B. Perspectives of the Apiaceae Hepatoprotective Effects—A Review. Nat. Prod. Commun. 2017, 12, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Waheeba, E.A.; Ali, A.E.-S.; Ihssan, M.O. Antioxidant Activities of Parsley (Petroselinum crispum) on the Induced Biochemical and Histopathological Changes of Potassium Bromate-Fed Rats. Pak. J. Nutr. 2020, 19, 80–85. [Google Scholar] [CrossRef]
- Emad, A.M.; Ali, S.F.; Abdel-Rahman, E.A.; Meselhy, M.R.; Farag, M.A.; Ali, S.S.; Abdel-Sattar, E.A. Anti-inflammatory and antioxidant effects of Apium graveolens L. extracts mitigate against fatal acetaminophen-induced acute liver toxicity. J. Food Biochem. 2020, 44, e13399. [Google Scholar] [CrossRef]
- Khalil, N.S.A.; Abou-Elhamd, A.S.; Wasfy, S.I.A.; El Mileegy, I.M.H.; Hamed, M.Y.; Ageely, H.M. Antidiabetic and Antioxidant Impacts of Desert Date (Balanites aegyptiaca) and Parsley (Petroselinum sativum) Aqueous Extracts: Lessons from Experimental Rats. J. Diabetes Res. 2016, 2016, 8408326. [Google Scholar] [CrossRef]
- Al-Howiriny, T.A.; Al-Sohaibani, M.O.; El-Tahir, K.H.; Rafatullah, S. Preliminary Evaluation of the Anti-Inflammatory and Anti-Hepatotoxic Activities of “Parsley” Petroselinum crispum in Rats. J. Nat. Remedies 2003, 3, 54–62. [Google Scholar]
- Akıncı, A.; Eşrefoğlu, M.; Taşlıdere, E.; Ateş, B. Petroselinum crispum is Effective in Reducing Stress-Induced Gastric Oxidative Damage. Balk. Med. J. 2017, 34, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Bolkent, S.; Yanardag, R.; Ozsoy-Sacan, O.; Karabulut-Bulan, O. Effects of parsley (Petroselinum crispum) on the liver of diabetic rats: A morphological and biochemical study. Phytother. Res. 2004, 18, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.L.; Rajarajeswaran, J.; Fung, S.; Kanthimathi, M.S. Petroselinum crispum has antioxidant properties, protects against DNA damage and inhibits proliferation and migration of cancer cells. J. Sci. Food Agric. 2015, 95, 2763–2771. [Google Scholar] [CrossRef]
- Jacques, K.K.J.; Nadia, B.A.; Roger, K.K.; Seraphin, D.Y.; Martin, S.Y.; Annick, T. Acute and Subacute Toxic Aqueous Extract of the Leaves of Petroselinum crispum Mill. in Male and Female Wistar Rats. Eur. Sci. J. ESJ 2021, 17, 178–195. [Google Scholar] [CrossRef]
- El Zubeir, I.E.M.; Ishag, H.I.J. Processing and Some Phsico-Chemical Properties of White Cheese Made from Camel Milk Using Capparis decidua Fruits Extract As A Coagulant. Nutr. Food Process. 2022, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Graudins, A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin. Toxicol. 2017, 55, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A.C. Celery and Parsley as Foods in the Greco-Roman Period. Class. Philol. 1949, 44, 91–99. [Google Scholar] [CrossRef]
- Popiolek, I.; Hydzik, P.; Jagielski, P.; Zrodlowska, M.; Mystek, K.; Porebski, G. Risk Factors for Hepatotoxicity Due to Paracetamol Overdose in Adults. Medicina 2021, 57, 752. [Google Scholar] [CrossRef] [PubMed]
- Nouioura, G.; Tourabi, M.; Tahraoui, A.; El-yagoubi, K.; Maache, S.; Elfatemi, H.; Lyoussi, B.; Derwich, E.H. Assessment of the acute and subacute toxicity of the aqueous extract of Moroccan Ferula communis fruit in a mouse model. Saudi Pharm. J. 2023, 31, 101701. [Google Scholar] [CrossRef]
- Coelho, A.M.; Queiroz, I.F.; Perucci, L.O.; de Souza, M.O.; Lima, W.G.; Talvani, A.; Costa, D.C. Piperine as Therapeutic Agent in Paracetamol-Induced Hepatotoxicity in Mice. Pharmaceutics 2022, 14, 1800. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Appiah, T.; Boakye, Y.D.; Apenteng, J.A. Chapter 25—Petroselinum Crispum: A Review. In Medicinal Spices and Vegetables from Africa; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Badr, G.M.; Algefare, A.I.; Alfwuaires, M.A. Antioxidant Potential of Parsley Leaf (Petroselinum crispum) Essential Oil on Hypothyroidism and Testicular Injury in Mice Intoxicated by Carbon Tetrachloride. BioMed Res. Int. 2021, 2021, 9989174. [Google Scholar] [CrossRef] [PubMed]
- Thiviya, P.; Gunawardena, N.; Gamage, A.; Madhujith, T.; Merah, O. Apiaceae Family as a Valuable Source of Biocidal Components and their Potential Uses in Agriculture. Horticulturae 2022, 8, 614. [Google Scholar] [CrossRef]
- Ashry, M.; Atia, I.; Morsy, F.A.; Elmashad, W. Protective efficiency of parsley (Petroselinum crispum) against oxidative stress, DNA damage and nephrotoxicity induced with anti- tuberculosis drugs. Int. J. Cancer Biomed. Res. 2021, 5, 27–36. [Google Scholar] [CrossRef]
- Ertaş, B.; Turan, F.; Özbeyli, D.; Yanardağ, R.; Saçan, O.; Şener, G. Protective Effects of Petroselinum crispum (Parsley) Extract Against Methotrexate-Induced Hepatotoxicity. Eur. J. Biol. 2019, 80, 173–178. [Google Scholar] [CrossRef]
- Punoševac, M.; Radović, J.; Leković, A.; Kundaković-Vasović, T. A review of botanical characteristics, chemical composition, pharmacological activity and use of parsley. Arh. Za Farm. 2021, 71, 177–196. [Google Scholar] [CrossRef]
- Soliman, A.M.; Rizk, H.A.; Shalaby, M.A.; Elkomy, A.A. Mechanisms of Hepato-Renal Protective Activity of Ocimum basilicum Leaf Extract against Paracetamol Toxicity in Rat Model. Adv. Anim. Vet. Sci. 2020, 8, 385–391. [Google Scholar] [CrossRef]
| Blood Variables | Control | Normal Group | APAP-Control | APAP + PC-Ext |
|---|---|---|---|---|
| ALT (U/L) | 89.5 ± 3.54 | 82 ± 2.83 | 143.11 ± 0.34 a*** | 111.5 ± 12.02 a***,b*** |
| AST (U/L) | 223 ± 31.11 | 231 ± 4.35 | 282 ± 7.88 a*** | 232 ± 1.08 b*** |
| ALP (UI/L) | 238.5 ± 3.1 | 245.5 ± 9.09 a*** | 337.5 ± 4.09 a*** | 262.5 ± 7.9 a***,b*** |
| Albumin (g/L) | 43.15 ± 3.75 | 46.7 ± 3.82 | 34.25 ± 0.35 | 44.15 ± 0.45 |
| Creatinine (mg/L) | 4.1 ± 0.13 | 4.45 ± 0.52 | 5.57 ± 0.34 | 4.04 ± 0.25 |
| BUN (g/L) | 0.24 ± 0.06 | 0.36 ± 0.04 | 0.49 ± 0.12 | 0.38 ± 0.00 |
| Uric acid (mg/L) | 18.93 ± 11.78 | 16.1 ± 7.09 | 13.26 ± 0.62 | 17.82 ± 0.40 |
| Protein (g/L) | 66.67 ± 2.35 | 67.32 ± 7.28 | 56.5 ± 3.02 | 65.30 ± 5.38 |
| LDH (U/L) | 107.15 ± 1.48 | 91 ± 5.6 a* | 265.10 ± 4.76 a*** | 130.35 ± 8.13 a***,b*** |
| Triglycerides (g/L) | 0.4 ± 0.14 | 1.01 ± 0.28 | 2.13 ± 2.88 | 1.04 ± 0.25 |
| Parameters | Control | Normal Group | APAP-Control | APAP + PC-Ext | |
|---|---|---|---|---|---|
| White blood cell (mm3) | 8230.00 ± 3.1 | 8143.7 ± 3.01 a*** | 13020 ± 6.11 a*** | 7261.5 ± 2.43 a***,b*** | |
| Red blood cell (M/mm3) | 8.56 ± 0.66 | 8.15 ± 0.77 | 5.02 ± 0.34 | 6.65 ± 0.98 | |
| Hemoglobin (g/dL) | 14.1 ± 0.4 | 14.15 ± 0.92 | 8.98± 1.0 a* | 10.9 ± 1.13 | |
| Hematocrit (%) | 49.3 ± 1.2 | 46.6 ± 4.95 | 36.45 ± 4.22 a*** | 44.85 ± 5.02 b** | |
| VGM (u3) | 57.6 ± 2.06 | 57.2 ± 0.71 | 48.62 ± 1.23 a*** | 55.45 ± 0.64 b* | |
| TCMH (pg) | 16.5 ± 1.1 | 17.4 ± 0.57 | 15.6± 0.2 | 16.4 ± 0.71 | |
| CCMH (g/dL) | 34.6 ± 0.42 | 33.4 ± 1.27 | 28.6 ± 0.41 a* | 30.65 ± 0.92 | |
| Platelets (103/mm3) | 841 ± 4.02 | 818.5 ± 2.15 a*** | 962 ± 10.27 a*** | 722 ± 3.76 a***,b*** | |
| VPM (FL) | 7.6 ± 0.3 | 8 ± 0.42 | 6.02 ± 0.21 | 7.8 ± 0.42 | |
| Differential count (%) | Neutrophils (%) | 24.5 ± 0.2 | 23.7 ± 0.19 | 30.1 ± 2.90 a* | 25 ± 1.27 b* |
| Eosinophils (%) | 0.00 | 0.00 | 0.00 | 0.00 | |
| Basophils (%) | 0.00 | 0.00 | 2.0 ± 0.7 | 1 ± 1.41 | |
| Lymphocytes (%) | 53.5 ± 1.41 | 54 ± 1.04 | 60 ± 2.13 a** | 45 ± 9.90 a***,b*** | |
| Monocytes (%) | 2 ± 0.1 | 3 ± 1.41 | 3.5 ± 0.1 | 3 ± 1.41 | |
| Granulocytes (%) | 0.0 | 0.1 ± 0.00 | 0.7 ± 0.02 | 0.05 ± 0.07 | |
| Interventions mg/kg BWT | Control | Normal Group | APAP-Control | APAP + PC-Ext | |
|---|---|---|---|---|---|
| Urine volume (mL/24 h) | D0 | 6.2 ± 0.41 | 7.25 ± 1.8 | 6 ± 1.3 | 6.10 ± 0.42 |
| D7 | 7.4 ± 0.19 | 10.3 ± 0.1 * | 7.5 ± 0.04 | 7 ± 0.23 | |
| D15 | 6.3 ± 0.5 | 16.5 ± 0.5 *** | 8 ± 1.2 | 10.72 ± 0.7 ** | |
| F/p values | 0.014/0.924 | 0.121/0.773 | 0.067/0.832 | ||
| Urine pH | D0 | 6.53 ± 0.55 | 6.61 ± 0.82 | 6.54 ± 1.04 | 6.67 ± 0.15 |
| D7 | 6.44 ± 0.64 | 6.57 ± 1.2 | 6.75 ± 0.58 | 6.38 ± 0.72 | |
| D15 | 6.45 ± 1.49 | 6.89 ± 0.63 | 7.53 ± 0.35 | 6.04 ± 0.56 | |
| F/p values | 0.622/0.421 | 0.962/0.124 | 0.899/0.205 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouioura, G.; Kettani, T.; Tourabi, M.; Elousrouti, L.T.; Al kamaly, O.; Alshawwa, S.Z.; Shahat, A.A.; Alhalmi, A.; Lyoussi, B.; Derwich, E. The Protective Potential of Petroselinum crispum (Mill.) Fuss. on Paracetamol-Induced Hepatio-Renal Toxicity and Antiproteinuric Effect: A Biochemical, Hematological, and Histopathological Study. Medicina 2023, 59, 1814. https://doi.org/10.3390/medicina59101814
Nouioura G, Kettani T, Tourabi M, Elousrouti LT, Al kamaly O, Alshawwa SZ, Shahat AA, Alhalmi A, Lyoussi B, Derwich E. The Protective Potential of Petroselinum crispum (Mill.) Fuss. on Paracetamol-Induced Hepatio-Renal Toxicity and Antiproteinuric Effect: A Biochemical, Hematological, and Histopathological Study. Medicina. 2023; 59(10):1814. https://doi.org/10.3390/medicina59101814
Chicago/Turabian StyleNouioura, Ghizlane, Tayeb Kettani, Meryem Tourabi, Layla Tahiri Elousrouti, Omkulthom Al kamaly, Samar Zuhair Alshawwa, Abdelaaty A. Shahat, Abdulsalam Alhalmi, Badiaa Lyoussi, and Elhoussine Derwich. 2023. "The Protective Potential of Petroselinum crispum (Mill.) Fuss. on Paracetamol-Induced Hepatio-Renal Toxicity and Antiproteinuric Effect: A Biochemical, Hematological, and Histopathological Study" Medicina 59, no. 10: 1814. https://doi.org/10.3390/medicina59101814
APA StyleNouioura, G., Kettani, T., Tourabi, M., Elousrouti, L. T., Al kamaly, O., Alshawwa, S. Z., Shahat, A. A., Alhalmi, A., Lyoussi, B., & Derwich, E. (2023). The Protective Potential of Petroselinum crispum (Mill.) Fuss. on Paracetamol-Induced Hepatio-Renal Toxicity and Antiproteinuric Effect: A Biochemical, Hematological, and Histopathological Study. Medicina, 59(10), 1814. https://doi.org/10.3390/medicina59101814








