Pilot Study on the Molecular Pathogenesis of Pyeloureteral Junction Obstruction: Underdevelopment or Fibrosis?
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. RNA Extraction and qPCR
2.3. Statistical Analysis
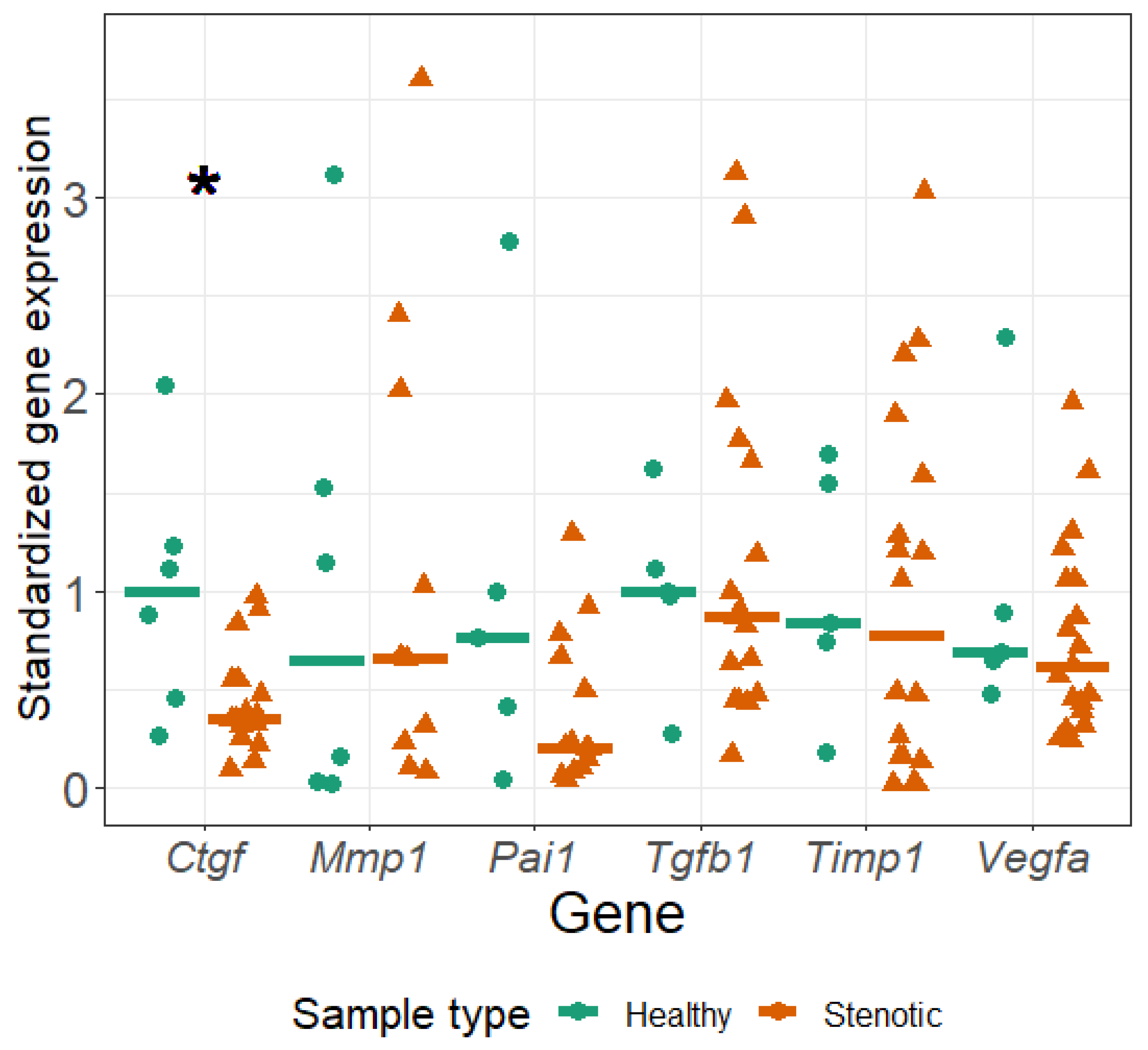
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UB | ureteric bud |
| PUJO | obstructed pyeloureteric junction |
| EGFR | epidermal growth factor receptor |
| AT1R | angiotensin type 1 receptor |
| AT2R | angiotensin type 2 receptor |
| UVJO | ureterovesical junction obstruction |
| ChKD | chronic kidney disease |
| ESRD | end stage renal disease |
| LTBP | latent TGFβ binding proteins |
References
- Zhao, S.; Li, W.; Yu, W.; Rao, T.; Li, H.; Ruan, Y.; Yuan, R.; Li, C.; Ning, J.; Li, S.; et al. Exosomal miR-21 from tubular cells contributes to renal fibrosis by activating fibroblasts via targeting PTEN in obstructed kidneys. Theranostics 2021, 11, 8660–8673. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.Y.; Kim, J.N.; Jun, J.Y.; You, H.J.; Jeon, Y.J.; Park, K.-S.; Yoon, S.P. Repression of apurinic/apyrimidinic endonuclease by p53-dependent apoptosis in hydronephrosis-induced rat kidney. Free Radic. Res. 2011, 45, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.F.; Chu, H.; Peng, B.; Liu, X.; Cao, Y.S. Outcome analysis of early surgery and conservative treatment in neonates and infants with severe hydronephrosis. J. Int. Med. Res. 2021, 49, 3000605211057866. [Google Scholar] [CrossRef] [PubMed]
- Isali, I.; McClellan, P.; Wong, T.R.; Gupta, S.; Woo, L. A systematic review of underlying genetic factors associated with ureteropelvic junction obstruction in stenotic human tissue. J. Pediatr. Urol. 2022, 18, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Senkul, T.; Kucukodaci, Z.; Iseri, C.; Karademir, K.; Erden, D.; Baloglu, H.; Narin, Y. The smooth muscle ratio at the renal pelvis in adults: Does it predict surgical outcome? Urol. Int. 2004, 73, 248–251. [Google Scholar] [CrossRef]
- Kajbafzadeh, A.M.; Payabvash, S.; Salmasi, A.H.; Monajemzadeh, M.; Tavangar, S.M. Smooth muscle cell apoptosis and defective neural development in congenital ureteropelvic junction obstruction. J. Urol. 2006, 176, 718–723, discussion 723. [Google Scholar] [CrossRef]
- Wishahi, M.; Elkholy, A.; Badawy, M.H.; Hafiz, E.; Elganzoury, H.; Nour, H.H.; Ismail, M.; Zayed, A.; Eldahshan, S.K.; Mehena, A.A.; et al. Smooth Muscle Cells and Collagen Fibres Architecture in Equivocal Ureteropelvic Junction Obstruction: Electron Microscopy Study with Clinical Correlation in Adult Egyptians. J. Egypt. Soc. Parasitol. 2020, 50, 453–458. [Google Scholar] [CrossRef]
- Hinz, B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodelling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenes. Tissue Repair 2012, 5 (Suppl. S1), S24. [Google Scholar] [CrossRef]
- Shang, J.; He, Q.; Chen, Y.; Yu, D.; Sun, L.; Cheng, G.; Liu, D.; Xiao, J.; Zhao, Z. miR-15a-5p suppresses inflammation and fibrosis of peritoneal mesothelial cells induced by peritoneal dialysis via targeting VEGFA. J. Cell. Physiol. 2019, 234, 9746–9755. [Google Scholar] [CrossRef]
- Nie, Q.H.; Duan, G.-R.; Luo, X.-D.; Xie, Y.-M.; Luo, H.; Zhou, Y.-X.; Pan, B.-R. Expression of TIMP-1 and TIMP-2 in rats with hepatic fibrosis. World J. Gastroenterol. 2004, 10, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Vaughan, D.E. PAI-1 in tissue fibrosis. J. Cell. Physiol. 2012, 227, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.A.; Bakir, A.S.; Shabana, S.S. Serum TGF-β, serum MMP-1, and HOMA-IR as non-invasive predictors of fibrosis in Egyptian patients with NAFLD. Saudi J. Gastroenterol. 2012, 18, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Ladouceur, M.; Baron, S.; Nivet-Antoine, V.; Maruani, G.; Soulat, G.; Pereira, H.; Blanchard, A.; Boutouyrie, P.; Paul, J.L.; Mousseaux, E. Role of myocardial collagen degradation and fibrosis in right ventricle dysfunction in transposition of the great arteries after atrial switch. Int. J. Cardiol. 2018, 258, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ganger, M.T.; Dietz, G.D.; Ewing, S.J. A common base method for analysis of qPCR data and the application of simple blocking in qPCR experiments. BMC Bioinform. 2017, 18, 534. [Google Scholar] [CrossRef] [PubMed]
- Horbelt, D.; Denkis, A.; Knaus, P. A portrait of Transforming Growth Factor β superfamily signalling: Background matters. Int. J. Biochem. Cell Biol. 2012, 44, 469–474. [Google Scholar] [CrossRef]
- Samarakoon, R.; Overstreet, J.M.; Higgins, P.J. TGF-β signaling in tissue fibrosis: Redox controls, target genes and therapeutic opportunities. Cell. Signal. 2013, 25, 264–268. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, X.; Gao, H.; Ji, S.J.; Wang, C. The expression of epidermal growth factor and transforming growth factor-beta1 in the stenotic tissue of congenital pelvi-ureteric junction obstruction in children. J. Pediatr. Surg. 2003, 38, 1656–1660. [Google Scholar] [CrossRef]
- Koca, O.; Kaya, C.; Ozturk, M.I.; Gunes, M.; Gumrukcu, G.; Karaman, M.I. Analysis of expression of TNF-alpha and TGF-beta3 in intrinsic ureteropelvic junction obstruction. Bratisl. Lek. Listy 2013, 114, 498–502. [Google Scholar] [CrossRef]
- Seremetis, G.M.; Maizels, M. TGF-beta mRNA expression in the renal pelvis after experimental and clinical ureteropelvic junction obstruction. J. Urol. 1996, 156, 261–266. [Google Scholar] [CrossRef]
- Duymelinck, C.; Dauwe, S.E.; De Greef, K.E.; Ysebaert, D.K.; Verpooten, G.A.; De Broe, M.E. TIMP-1 gene expression and PAI-1 antigen after unilateral ureteral obstruction in the adult male rat. Kidney Int. 2000, 58, 1186–1201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaughan, D.E.; Lazos, S.A.; Tong, K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J. Clin. Investig. 1995, 95, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Magyar, Z.; Schönléber, J.; Romics, M.; Hruby, E.; Nagy, B.; Sulya, B.; Beke, A.; Harmath, A.; Jeager, J.; Rigó, J.; et al. Expression of VEGF in neonatal urinary obstruction: Does expression of VEGF predict hydronephrosis? Med. Sci. Monit. 2015, 21, 1319–1323. [Google Scholar]
- Bickelhaupt, S.; Erbel, C.; Timke, C.; Wirkner, U.; Dadrich, M.; Flechsig, P.; Tietz, A.; Pföhler, J.; Gross, W.; Peschke, P.; et al. Effects of CTGF Blockade on Attenuation and Reversal of Radiation-Induced Pulmonary Fibrosis. J. Natl. Cancer Inst. 2017, 109, djw339. [Google Scholar] [CrossRef] [PubMed]
- Valle-Tenney, R.; Rebolledo, D.L.; Lipson, K.E.; Brandan, E. Role of hypoxia in skeletal muscle fibrosis: Synergism between hypoxia and TGF-β signaling upregulates CCN2/CTGF expression specifically in muscle fibers. Matrix Biol. 2020, 87, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Roestenberg, P.; van Nieuwenhoven, F.A.; Joles, J.A.; Trischberger, C.; Martens, P.P.; Oliver, N.; Aten, J.; Höppener, J.W.; Goldschmeding, R. Temporal expression profile and distribution pattern indicate a role of connective tissue growth factor (CTGF/CCN-2) in diabetic nephropathy in mice. Am. J. Physiol. Ren. Physiol. 2006, 290, F1344–F1354. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cretoiu, D. Advances in Experimental Medicine and Biology 913 Telocytes Connecting Cells. Available online: http://www.springer.com/series/5584 (accessed on 8 August 2012).
- Huang, B.L.; Brugger, S.M.; Lyons, K.M. Stage-specific control of connective tissue growth factor (CTGF/CCN2) expression in chondrocytes by Sox9 and β-catenin. J. Biol. Chem. 2010, 285, 27702–27712. [Google Scholar] [CrossRef]
- Airik, R.; Trowe, M.-O.; Foik, A.; Farin, H.F.; Petry, M.; Schuster-Gossler, K.; Schweizer, M.; Scherer, G.; Kist, R.; Kispert, A. Hydroureternephrosis due to loss of Sox9-regulated smooth muscle cell differentiation of the ureteric mesenchyme. Hum. Mol. Genet. 2010, 19, 4918–4929. [Google Scholar] [CrossRef]
- Ampawong, S.; Klincomhum, A.; Likitsuntonwong, W.; Singha, O.; Ketjareon, T.; Panavechkijkul, Y.; Zaw, K.-M.; Kengkoom, K. Expression of aquaporin-1, -2 and -4 in mice with a spontaneous mutation leading to hydronephrosis. J. Comp. Pathol. 2012, 146, 332–337. [Google Scholar] [CrossRef]
- Paces-Fessy, M.; Fabre, M.; Lesaulnier, C.; Cereghini, S. Hnf1b and Pax2 cooperate to control different pathways in kidney and ureter morphogenesis. Hum. Mol. Genet. 2012, 21, 3143–3155. [Google Scholar] [CrossRef]
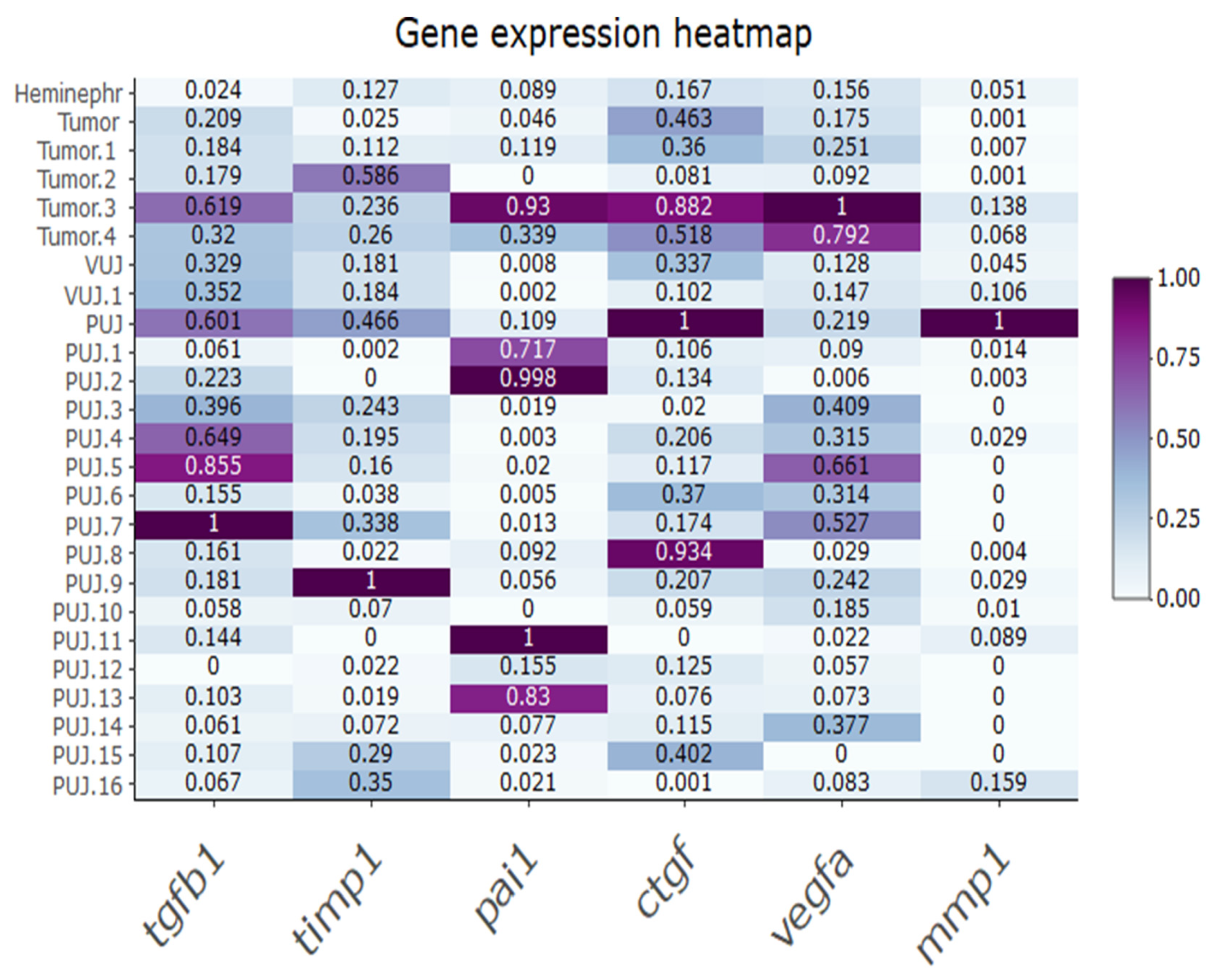
| Sample | Age at Enrollment (Months) | Sex (1-Boy, 0-Girl) | Weight (kg) | Height (cm) |
|---|---|---|---|---|
| Heminephr. | 10.l37 | 1 | n/a | n/a |
| Tumor | 7.47 | 1 | 8.7 | 73 |
| Tumor.1 | 98.13 | 1 | 28.5 | 132 |
| Tumor.2 | 24.87 | 1 | n/a | n/a |
| Tumor.3 | 68.73 | 1 | n/a/ | n/a |
| Tumor.4 | 2.90 | 1 | 5.4 | 64 |
| VUJ | 3.33 | 1 | n/a | n/a |
| VUJ.1 | 29.60 | n/a | n/a | n/a |
| PUJ | 21.43 | 0 | 12.8 | 79 |
| PUJ.1 | 54.83 | 1 | 22 | 114 |
| PUJ.2 | 1.40 | 0 | 4.8 | 55 |
| PUJ.3 | 51.33 | n/a | n/a | n/a |
| PUJ.4 | 3.97 | 1 | 7.24 | 68 |
| PUJ.5 | 111.33 | 0 | 35 | 141 |
| PUJ.6 | 9.53 | 0 | 7.5 | 71 |
| PUJ.7 | 95.43 | 1 | 33.5 | 142 |
| PUJ.8 | 21.50 | 1 | 14.5 | 89 |
| PUJ.9 | 3.77 | 1 | 8.9 | 71 |
| PUJ.10 | 199.73 | n/a | n/a | n/a |
| PUJ.11 | 84.10 | n/a | n/a | n/a |
| PUJ.12 | 11.13 | 1 | 13.5 | 78.5 |
| PUJ.13 | 98.60 | 0 | 24.6 | 127 |
| PUJ.14 | 3.63 | 1 | 7.63 | 64 |
| PUJ.15 | 78.80 | 1 | 27 | 126 |
| PUJ.16 | 2.6 | 0 | 5.3 | 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamasauske, R.Z.; Kazlauskas, V.; Barasa, P.; Krestnikova, N.; Dasevicius, D.; Bilius, V.; Verkauskas, G. Pilot Study on the Molecular Pathogenesis of Pyeloureteral Junction Obstruction: Underdevelopment or Fibrosis? Medicina 2023, 59, 1729. https://doi.org/10.3390/medicina59101729
Tamasauske RZ, Kazlauskas V, Barasa P, Krestnikova N, Dasevicius D, Bilius V, Verkauskas G. Pilot Study on the Molecular Pathogenesis of Pyeloureteral Junction Obstruction: Underdevelopment or Fibrosis? Medicina. 2023; 59(10):1729. https://doi.org/10.3390/medicina59101729
Chicago/Turabian StyleTamasauske, Ramune Zilinskaite, Vytis Kazlauskas, Povilas Barasa, Natalija Krestnikova, Darius Dasevicius, Vytautas Bilius, and Gilvydas Verkauskas. 2023. "Pilot Study on the Molecular Pathogenesis of Pyeloureteral Junction Obstruction: Underdevelopment or Fibrosis?" Medicina 59, no. 10: 1729. https://doi.org/10.3390/medicina59101729
APA StyleTamasauske, R. Z., Kazlauskas, V., Barasa, P., Krestnikova, N., Dasevicius, D., Bilius, V., & Verkauskas, G. (2023). Pilot Study on the Molecular Pathogenesis of Pyeloureteral Junction Obstruction: Underdevelopment or Fibrosis? Medicina, 59(10), 1729. https://doi.org/10.3390/medicina59101729






