Determinants of Increased Effort of Breathing in Non-Intubated Critical COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Effort of Breathing
2.3. Determinants of Effort of Breathing
2.4. Statistical Analysis
2.4.1. Descriptive Analysis
2.4.2. Comparison of Two Groups of Normal and Increased Effort of Breathing
2.4.3. Accuracy Testing
2.4.4. Regression Analysis
3. Results
3.1. Study Population
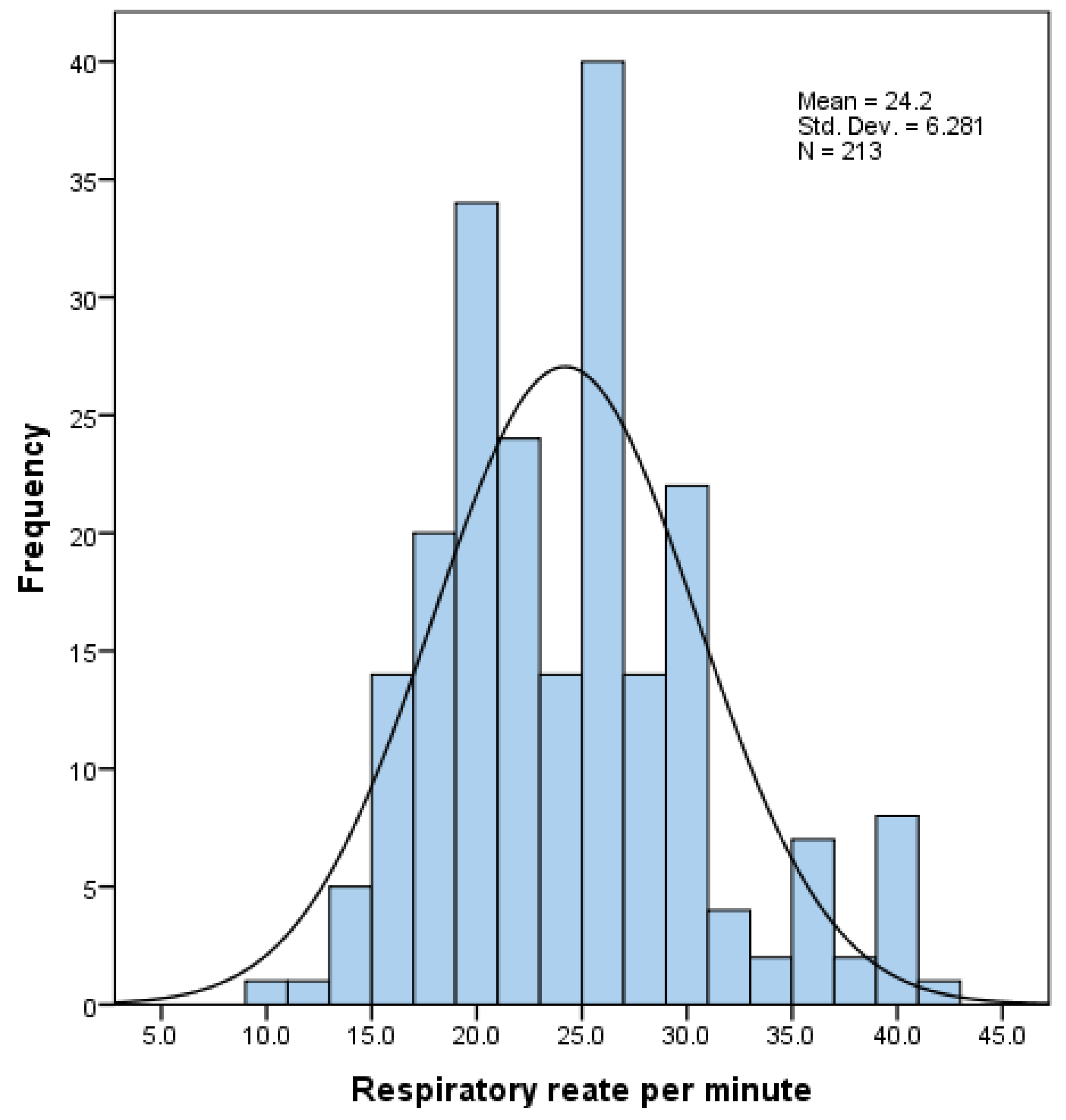
3.2. Effort of Breathing: Incidence and Descriptive Characteristics
3.3. Effort of Breathing: Determinants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weekly Epidemiological Update on COVID-19—7 September 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---7-september-2021 (accessed on 24 March 2022).
- Elsayed, H.H.; Hassaballa, A.S.; Ahmed, T.A.; Gumaa, M.; Sharkawy, H.Y.; Moharram, A.A. Variation in outcome of invasive mechanical ventilation between different countries for patients with severe COVID-19: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252760. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Kane, A.D.; Cook, T.M. Outcomes from intensive care in patients with COVID-19: A systematic review and meta-analysis of observational studies. Anaesthesia 2020, 75, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Anaesthesiology, J.N.M.M.; Gomersall, C.D. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 201, 1299–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaporidi, K.; Akoumianaki, E.; Telias, I.; Goligher, E.C.; Brochard, L.; Georgopoulos, D. Respiratory Drive in Critically Ill Patients. Pathophysiology and Clinical Implications. Am. J. Respir. Crit. Care Med. 2020, 201, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Swenson, K.E.; Ruoss, S.J.; Swenson, E.R. The Pathophysiology and Dangers of Silent Hypoxemia in COVID-19 Lung Injury. Ann. Am. Thorac. Soc. 2021, 18, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Valletta, F.; Franceschi, F. Gemelli Against COVID 2019. Barotrauma during non-invasive ventilation for acute respiratory distress syndrome caused by COVID-19: A balance between risks and benefits. Br. J. Hosp. Med. 2021, 82, 1–9. [Google Scholar]
- Brouqui, P.; Amrane, S.; Million, M.; Cortaredona, S.; Parola, P.; Lagier, J.-C.; Raoult, D. Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int. J. Infect. Dis. 2021, 102, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.J.; Laghi, F.; Jubran, A. Why COVID-19 Silent Hypoxemia Is Baffling to Physicians. Am. J. Respir. Crit. Care Med. 2020, 202, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G.; Bayliss, D.A. Neural Control of Breathing and CO2 Homeostasis. Neuron 2015, 87, 946–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottestad, W.; Seim, M.; Mæhlen, J.O. COVID-19 with silent hypoxemia. Tidsskr. Den Nor. Legeforening 2020, 140. [Google Scholar] [CrossRef]
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012, 38, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Feiner, J.R.; Weiskopf, R.B. Evaluating Pulmonary Function: An Assessment of PaO2/FIO2. Crit. Care Med. 2017, 45, e40–e48. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.A.; Hashmi, M.; Qayyum, A.; Pervaiz, R.; Saleem, M.; Munir, M.F.; Saif, M.M.U. Comparison of Efficacy of Dexamethasone and Methylprednisolone in Improving PaO2/FiO2 Ratio Among COVID-19 Patients. Cureus 2020, 12, e10918. [Google Scholar] [CrossRef] [PubMed]
- Holten, A.R.; Nore, K.G.; Tveiten, C.E.V.W.K.; Olasveengen, T.M.; Tonby, K. Predicting severe COVID-19 in the Emergency Department. Resusc. Plus 2020, 4, 100042. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef] [PubMed]
| Group 1 | High PaCO2 | Low PaO2 |
| Group 2 | High PaCO2 | Normal PaO2 |
| Group 3 | Normal PaCO2 | Low PaO2 |
| Group 4 | Normal PaCO2 | Normal PaO2 |
| Variable | n = 213, (%), Mean ± SD | Median (IQR) |
|---|---|---|
| Demographics | ||
| Gender: | ||
| Female | 83 (39.0) | |
| Male | 130 (61.0) | |
| Age (y) | 61.34 ± 13.26 | |
| Age groups (% within group) | ||
| <50 y | 36 (16.9) | |
| 50–59 y | 64 (30.0) | |
| 60–69 y | 53 (24.9) | |
| 70–79 y | 41 (19.3) | |
| >80 y | 19 (8.9) | |
| Co-morbidities | ||
| Obesity | 68 (31.9) | |
| Hypertension | 162 (76.1) | |
| Chronic cardiac disease | 98 (46.0) | |
| CKD | 72 (33.8) | |
| Immunosuppression | 28 (13.0) | |
| Diabetes | 67 (31.5) | |
| COPD | 21 (9.9) | |
| Asthma | 14 (6.6) | |
| Mortality risk scores | ||
| APACHE II | 12 (8–16) | |
| ISARIC-4C | 10 (7–13) | |
| Clinical course | ||
| MV | 93 (43.7) | |
| Length of stay | 18 (13–28) | |
| Mortality | 89 (42.8) | |
| Variable | Normal Effort of Breathing n = 75 (35.2%) | Increased Effort of Breathing n = 138 (64.8%) | p Value |
|---|---|---|---|
| Demographics | |||
| Gender: | 0.078 | ||
| Female | 23 (30.7) | 60 (72.3) | |
| Male | 52 (69.3) | 78 (56.5) | |
| Age | 62.43 ± 14.13 | 60.75 ± 12.79 | 0.395 |
| Co-morbidities | |||
| Obesity | 23 (30.7) | 45 (32.6) | 0.878 |
| Hypertension | 59 (78.7) | 103 (74.6) | 0.615 |
| Chronic cardiac disease | 37 (49.3) | 61 (44.2) | 0.476 |
| CKD | 27 (36.0) | 45 (32.6) | 0.651 |
| Immunosuppression | 7 (9.3) | 21 (15.2) | 0.290 |
| Diabetes | 26 (34.7) | 41 (29.7) | 0.537 |
| COPD | 9 (12.0) | 12 (8.7) | 0.475 |
| Asthma | 4 (5.3) | 10 (7.2) | 0.774 |
| Median (IQR) | Median (IQR) | ||
| Disease course | |||
| Time to ICU | 8.5 (4.75–12) | 8 (4–14.5) | 0.183 |
| Clinical signs upon admission | |||
| Fever | 36.6 (36.6–37.2) | 36.9 (36.6–37.5) | 0.219 |
| MAP | 88.0 (80.0–98.3) | 96.7 (84.8–105.) | 0.010 |
| Heart rate | 80 (70–92) | 87.5 (76–101) | 0.011 |
| SpO2 | 94 (90–97) | 88 (80–92) | <0.001 |
| PaO2/FiO2 | 186.9 (120.0–250.0) | 95.9 (65.5–152.8) | <0.001 |
| Arterial blood gas analysis | |||
| PaO2 | 81.5 (62.9–107.2) | 62.9 (51.8–86.4) | <0.001 |
| PaCO2 | 35.1 (31.9–38.9) | 32.8 (28.9–36.6) | 0.043 |
| HCO3 | 22.3 (20.6–25.3) | 22.4 (19.1–24.5) | 0.297 |
| pH | 7.44 (7.40–7.49) | 7.45 (7.40–7.48) | 0.361 |
| Laboratory findings | |||
| Urea | 7.5 (5.5–10.2) | 8.0 (6.2–11.6) | 0.269 |
| Creatinine | 78 (61.7–106) | 85 (62.5–103.5) | 0.793 |
| PCT | 0.15 (0.08–0.54) | 0.25 (0.13–0.81) | 0.171 |
| CRP | 106.5 (60–187) | 128.4 (91–215) | 0.041 |
| D-dimer | 720 (410–1170) | 805 (430–1647) | 0.585 |
| IL-6 | 27.2 (11.5–79.8) | 40.7 (16.6–89.2) | 0.158 |
| Feritin | 768 (425–2299) | 1131 (581–2114) | 0.180 |
| Lymph. count | 0.7 (0.6–0.95) | 0.65 (0.5–0.9) | 0.171 |
| WBC | 7.46 (5.10–10.49) | 8.68 (6.47–12.54) | 0.018 |
| N (%) | Mean ± SD | CI 95% | p Value | |
|---|---|---|---|---|
| Group 1 | 2 (0.9) | 25.7 ± 8.0 | 22.7–28.7 | <0.001 |
| Group 2 | 12 (5.6) | 22.2 ± 6.0 | 20.7–23.8 | |
| Group 3 | 71 (33.3) | 27.5 ± 5.0 | 26.0–29.1 | |
| Group 4 | 128 (60.1) | 24.20 ± 6.3 | 22.0–24.5 |
| Univariate Regression | Multivariate Regression | |||||
|---|---|---|---|---|---|---|
| Variable | Exp(B) | 95% CI | p Value | Exp(B) | 95% CI | p Value |
| Clinical signs | ||||||
| MAP | 1.024 | 1.005–1.044 | 0.015 | n.s. | n.s. | 0.085 |
| Heart rate | n.s. | n.s. | 0.062 | n.i. | n.i. | n.i. |
| SpO2 | 0.894 | 0.850–0.940 | <0.001 | 0.922 | 0.874–0.973 | 0.033 |
| PaO2/FiO2 | 0.992 | 0.989–0.996 | <0.001 | 0.996 | 0.922–1.000 | 0.003 |
| Arterial blood gas | ||||||
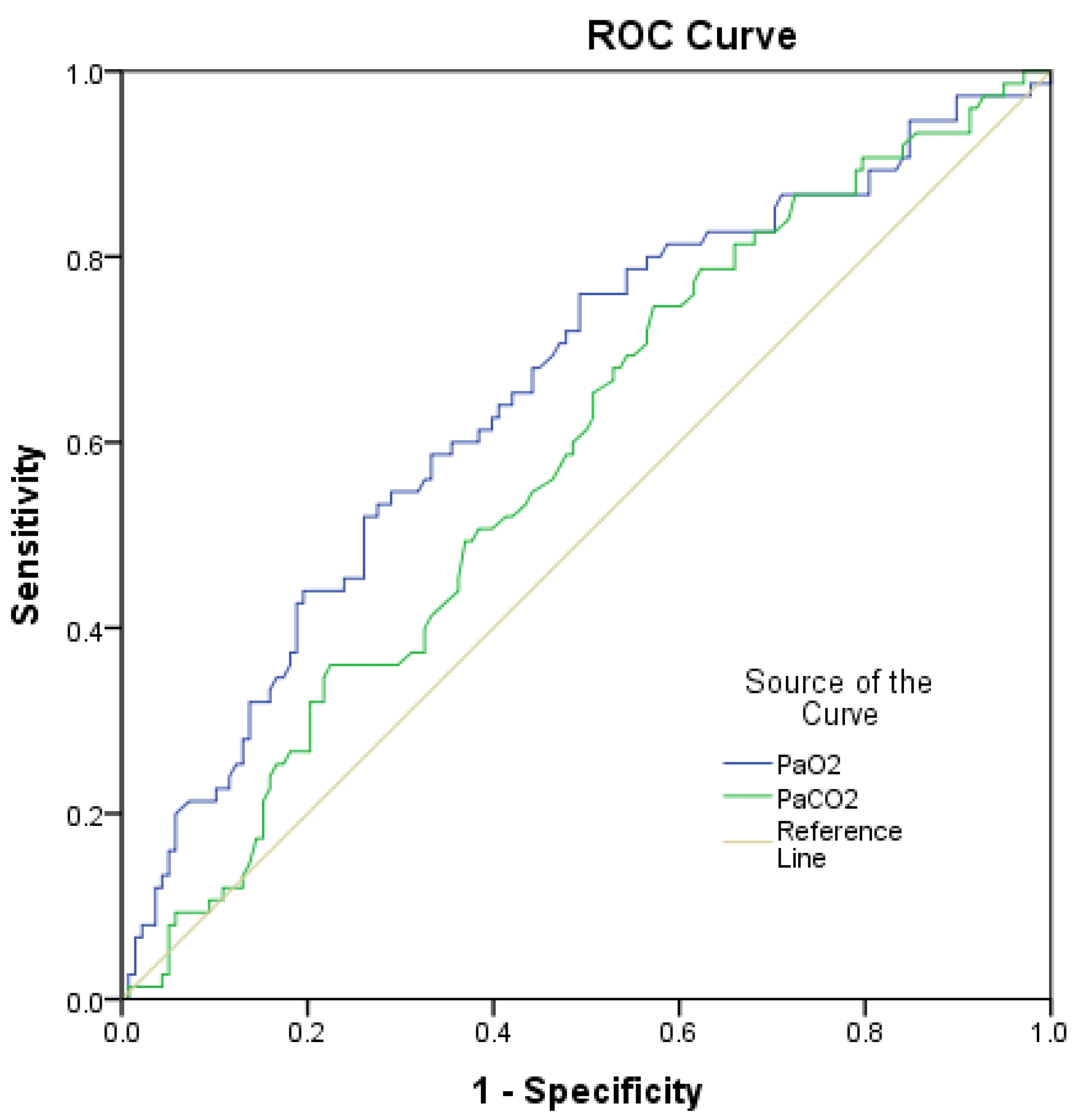
| PaO2 | 0.989 | 0.982–0.996 | 0.003 | 0.989 | 0.982–0.996 | 0.003 |
| PaCO2 | n.s. | n.s. | 0.218 | n.i. | n.i. | n.i. |
| Laboratory | ||||||
| CRP | n.s. | n.s. | 0.341 | n.i. | n.i. | n.i. |
| WBC | n.s. | n.s. | 0.132 | n.i. | n.i. | n.i. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicka, V.; Januskeviciute, E.; Krauklyte, J.; Aleknaviciene, A.; Ringaitiene, D.; Jancoriene, L.; Sipylaite, J. Determinants of Increased Effort of Breathing in Non-Intubated Critical COVID-19 Patients. Medicina 2022, 58, 1133. https://doi.org/10.3390/medicina58081133
Vicka V, Januskeviciute E, Krauklyte J, Aleknaviciene A, Ringaitiene D, Jancoriene L, Sipylaite J. Determinants of Increased Effort of Breathing in Non-Intubated Critical COVID-19 Patients. Medicina. 2022; 58(8):1133. https://doi.org/10.3390/medicina58081133
Chicago/Turabian StyleVicka, Vaidas, Elija Januskeviciute, Justina Krauklyte, Aiste Aleknaviciene, Donata Ringaitiene, Ligita Jancoriene, and Jurate Sipylaite. 2022. "Determinants of Increased Effort of Breathing in Non-Intubated Critical COVID-19 Patients" Medicina 58, no. 8: 1133. https://doi.org/10.3390/medicina58081133
APA StyleVicka, V., Januskeviciute, E., Krauklyte, J., Aleknaviciene, A., Ringaitiene, D., Jancoriene, L., & Sipylaite, J. (2022). Determinants of Increased Effort of Breathing in Non-Intubated Critical COVID-19 Patients. Medicina, 58(8), 1133. https://doi.org/10.3390/medicina58081133







