Natural Course of Early Detected Acute Peripancreatic Fluid Collection in Moderately Severe or Severe Acute Pancreatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Severity Assessment
2.3.1. Based on Clinical Symptoms, Signs and Laboratory Test
2.3.2. Based on Clinical Scoring System
2.3.3. Based on Initial and Follow-Up CT
2.4. Treatment of AP and Its Complications
2.5. Statistical Analysis
3. Results
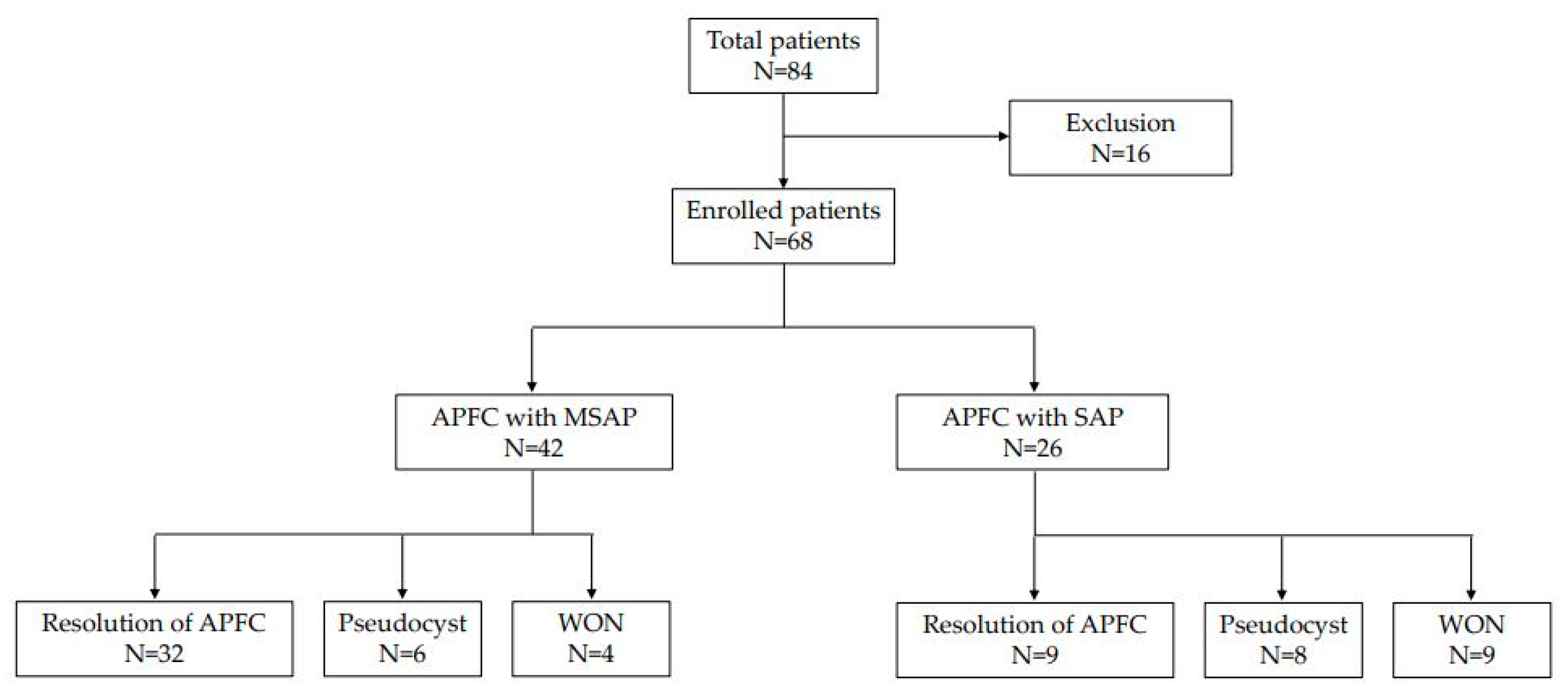
3.1. Natural Course of APFC
3.2. Baseline Characteristics of the Patients and AP
3.3. Laboratory Findings of Initial Status
3.4. Clinical Score and Organ Failure
3.5. Nutritional Support
3.6. Initial Pancreatitis Finding and Appearance of Fluid Collection in CT
3.7. Risk Factors for Late Complications after APFC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, K.P.; O’Connor, O.J.; Maher, M.M. Updated imaging nomenclature for acute pancreatitis. Am. J. Roentgenol. 2014, 203, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Brun, A.; Agarwal, N.; Pitchumoni, C.S. Fluid collections in and around the pancreas in acute pancreatitis. J. Clin. Gastroenterol. 2011, 45, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.L., 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch. Surg. 1993, 128, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, E.J. CT diagnosis and staging of acute pancreatitis. Radiol. Clin. N. Am. 1989, 27, 19–37. [Google Scholar] [PubMed]
- Wu, B.U.; Johannes, R.S.; Sun, X.; Conwell, D.L.; Banks, P.A. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Gastroenterology 2009, 137, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.U.; Bakker, O.J.; Papachristou, G.I.; Besselink, M.G.; Repas, K.; van Santvoort, H.C.; Muddana, V.; Singh, V.K.; Whitcomb, D.C.; Gooszen, H.G.; et al. Blood urea nitrogen in the early assessment of acute pancreatitis: An international validation study. Arch. Intern. Med. 2011, 171, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Heath, D.I.; Imrie, C.W. Prediction of outcome in acute pancreatitis: A comparative study of APACHE II, clinical assessment and multiple factor scoring systems. Br. J. Surg. 1990, 77, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Keak, C.; Almeida, N. Predicting Severity in Acute Pancreatitis: A Never Ending Quest. GE-Port. J. Gastroenterol. 2019, 26, 232–234. [Google Scholar]
- Wu, B.U.; Johannes, R.S.; Sun, X.; Tabak, Y.; Conwell, D.L.; Banks, P.A. The early prediction of mortality in acute pancreatitis: A large population-based study. Gut 2008, 57, 1698–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.K.; Wu, B.U.; Bollen, T.L.; Repas, K.; Maurer, R.; Johannes, R.S.; Mortele, K.J.; Conwell, D.; Banks, P.A. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am. J. Gastroenterol. 2009, 104, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Imrie, C.W. Classification of acute pancreatitis and the role of prognostic factors in assessing severity of disease. Schweiz. Med. Wochenschr. 1997, 127, 798–804. [Google Scholar] [PubMed]
- Mofidi, R.; Duff, M.D.; Wigmore, S.J.; Madhavan, K.K.; Garden, O.J.; Parks, R.W. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br. J. Surg. 2006, 93, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, D.W.; Boerma, D.; van Santvoort, H.C.; Horvath, K.D.; Werner, J.; Carter, C.R.; Bollen, T.L.; Gooszen, H.G.; Besselink, M.G.; Bakker, O.J. Staged multidisciplicary step-up management for necrotizing pancreatitis. Br. J. Surg. 2014, 101, e65–e79. [Google Scholar] [CrossRef] [PubMed]
- Pezzilli, R.; Zerbi, A.; Carlo, V.D.; Bassi, C.; Fave, G.F.D. Working Group of the Italian Association for the study of the Pancreas on Acute Pancreatitis. Pancreatology 2010, 10, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Freeman, M.L. Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am. J. Gastroenterol. 2006, 101, 2379–2400. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, E.J. Staging of acute pancreatitis. Radiol. Clin. N. Am. 2002, 40, 1199–1209. [Google Scholar] [CrossRef]
- Gianotti, L.; Meier, R.; Lobo, D.N.; Bassi, C.; Dejong, C.H.C.; Ockenga, J.; Irtun, O.; MacFie, J.; ESPEN. ESPEN Guidelines on Parenteral Nutrition: Pancreas. Clin. Nutr. 2009, 28, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Thoeni, R.F. The revised Atlanta classification of acute pancreatitis: Its importance for the radiologist and its effect on treatment. Radiology 2012, 262, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Yokoe, M.; Takada, T.; Mayumi, T.; Yoshida, M.; Isaji, S.; Wada, K.; Itoi, T.; Sata, N.; Gabata, T.; Igarashi, H.; et al. Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J. Hepatobiliary Pancreat. Sci. 2015, 22, 405–432. [Google Scholar] [CrossRef] [PubMed]
| APFC with MSAP (N = 42) | APFC with SAP (N = 26) | p-Value | |
|---|---|---|---|
| Baselinecharacteristics | |||
| Age, years | 52.3 (17.6) | 51.6 (13.2) | 0.314 |
| Sex, male | 28 (66.7) | 18 (69.2) | 0.683 |
| Height, cm | 167.3 (9.6) | 166.2 (8.7) | 0.516 |
| Weight, kg | 68.7 (12.3) | 67.1 (13.7) | 0.411 |
| BMI, kg/cm2 | 25.8 (3.1) | 24.4 (3.9) | 0.105 |
| Pain onset time, hours | 32.1 (42.6) | 33.0 (39.2) | 0.797 |
| Pain score, VAS | 5.1 (2.1) | 5.8 (2.2) | 0.197 |
| Etiology | |||
| Alcohol | 20 (47.6) | 13 (50.0) | 0.619 |
| Gallstone | 11 (26.2) | 6 (23.1) | 0.785 |
| HyperTG | 4 (9.5) | 2 (7.7) | 0.299 |
| Autoimmune | 3 (7.1) | 2 (7.7) | 0.817 |
| Idiopathic | 2 (4.8) | 2 (7.7) | 0.376 |
| Divisum | 2 (4.8) | 1 (3.8) | 0.772 |
| APFC with MSAP (N = 42) | APFC with SAP (N = 26) | p-Value | |
|---|---|---|---|
| Hemoglobin, g/dL | 13.8 (2.4) | 13.2 (2.7) | 0.486 |
| Hematocrit | 41.1 (6.6) | 40.2 (5.1) | 0.817 |
| CRP, at admission, mg/L | 47.8 (65.8) | 59.1 (48.3) | 0.284 |
| CRP, after 48 h, mg/L | 81.9 (77.3) | 168.7 (82.9) | 0.036 |
| BUN, mg/dL | 15.5 (10.9) | 26.8 (11.4) | 0.024 |
| Creatinine, mg/dL | 0.9 (0.6) | 1.1 (0.5) | 0.083 |
| Amylase, U/L | 378.1 (222.6) | 420.9 (312.1) | 0.758 |
| Lipase, U/L | 614.8 (273.2) | 679.6 (477.2) | 0.647 |
| pH | 7.41 (0.05) | 7.40 (0.06) | 0.599 |
| pO2, mmHg | 83.8 (16.1) | 82.1 (17.9) | 0.231 |
| HCO3, mmol/L | 22.2 (3.3) | 21.3 (4.2) | 0.196 |
| APFC with MSAP (N = 42) | APFC with SAP (N = 26) | p-Value | |
|---|---|---|---|
| BISAP score ≥ 3 | 2 (4.8) | 7 (26.9) | 0.019 |
| Modified Marshall score | |||
| MMS_cardiac ≥ 2 | 3 (7.1) | 3 (11.5) | 0.471 |
| MMS_respiratory ≥ 2 | 4 (9.5) | 2 (7.7) | 0.865 |
| MMS_renal ≥ 2 | 4 (9.5) | 3 (11.5) | 0.755 |
| Ranson score ≥ 3 | 5 (11.9) | 6 (23.1) | 0.093 |
| APACHE II ≥ 8 | 6 (14.3) | 4 (15.4) | 0.392 |
| APFC with MSAP (N = 42) | APFC with SAP (N = 26) | p-Value | |
|---|---|---|---|
| Feeding at admission | |||
| NPO | 24 (57.1) | 17 (65.4) | 0.176 |
| Oral feeding | 18 (42.9) | 9 (34.6) | 0.264 |
| Feeding after 24 h | |||
| NPO | 8 (19.0) | 10 (38.5) | 0.132 |
| Oral feeding | 33 (78.6) | 10 (38.5) | 0.029 |
| Nasojejunal feeding | 1 (2.4) | 6 (23.1) | 0.015 |
| Feeding after 48 h | |||
| NPO | 0 (0.0) | 0 (0.0) | - |
| Oral feeding | 41 (97.6) | 18 (69.2) | 0.042 |
| Nasojejunal feeding | 1 (2.4) | 8 (30.8) | 0.006 |
| APFC with MSAP (N = 42) | APFC with SAP (N = 26) | p-Value | |
|---|---|---|---|
| Fluid collections, multiple | 19 (45.2) | 12 (46.2) | 0.729 |
| Fluid collection diameter > 6 cm | 12 (26.1) | 8 (30.8) | 0.136 |
| Additional CT after 1 week | 3 (7.1) | 8 (30.8) | 0.033 |
| Late complications | 10 (23.8) | 17 (65.4) | 0.029 |
| Pseudocyst | 6 (14.3) | 8 (30.8) | 0.119 |
| WON | 4 (9.5) | 9 (34.6) | 0.018 |
| Additional treatment for pseudocyst/WON | |||
| Percutaneous procedure | 1 (10.0) | 2 (7.7) | 0.815 |
| Endoscopic procedure * | 1 (10.0) | 4 (15.4) | 0.766 |
| Surgery | 0 (0.0) | 1 (3.8) | 0.912 |
| Variables | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| APFC with SAP | 4.02 | 2.912–28.754 | 0.015 |
| CRP, after 48 h ≥ 150 mg/L | 3.51 | 1.914–23.988 | 0.033 |
| BUN ≥ 20 mg/dL | 4.11 | 0.907–13.101 | 0.108 |
| BISAP score ≥ 3 | 2.53 | 1.014–12.487 | 0.042 |
| Nasojejunal feeding after 48 h (yes) | 7.43 | 6.366–32.474 | 0.020 |
| Additional CT after 48 h (yes) | 5.36 | 0.816–21.948 | 0.336 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.W.; Kim, H.G.; Cho, C.M.; Jung, M.K.; Heo, J.; Cho, K.B.; Kim, S.B.; Kim, K.H.; Kim, T.N.; Han, J.; et al. Natural Course of Early Detected Acute Peripancreatic Fluid Collection in Moderately Severe or Severe Acute Pancreatitis. Medicina 2022, 58, 1131. https://doi.org/10.3390/medicina58081131
Lee DW, Kim HG, Cho CM, Jung MK, Heo J, Cho KB, Kim SB, Kim KH, Kim TN, Han J, et al. Natural Course of Early Detected Acute Peripancreatic Fluid Collection in Moderately Severe or Severe Acute Pancreatitis. Medicina. 2022; 58(8):1131. https://doi.org/10.3390/medicina58081131
Chicago/Turabian StyleLee, Dong Wook, Ho Gak Kim, Chang Min Cho, Min Kyu Jung, Jun Heo, Kwang Bum Cho, Sung Bum Kim, Kook Hyun Kim, Tae Nyeun Kim, Jimin Han, and et al. 2022. "Natural Course of Early Detected Acute Peripancreatic Fluid Collection in Moderately Severe or Severe Acute Pancreatitis" Medicina 58, no. 8: 1131. https://doi.org/10.3390/medicina58081131
APA StyleLee, D. W., Kim, H. G., Cho, C. M., Jung, M. K., Heo, J., Cho, K. B., Kim, S. B., Kim, K. H., Kim, T. N., Han, J., & Kim, H. (2022). Natural Course of Early Detected Acute Peripancreatic Fluid Collection in Moderately Severe or Severe Acute Pancreatitis. Medicina, 58(8), 1131. https://doi.org/10.3390/medicina58081131






