High-Sensitivity Cardiac Troponin Impact on the Differential Diagnosis of Non-ST Segment Elevation Coronary Syndromes—Is It Helping?
Abstract
:1. Introduction
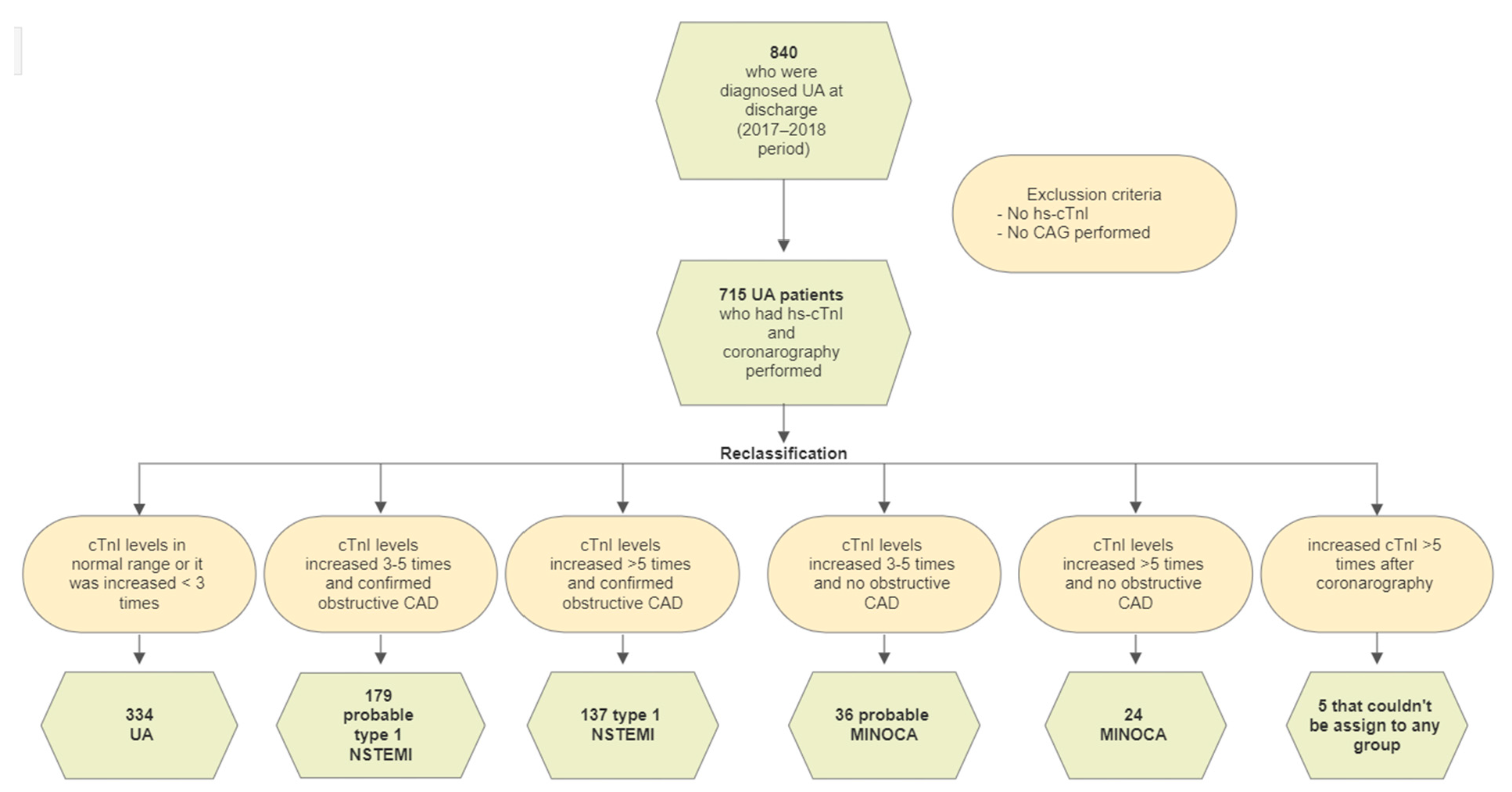
2. Materials and Methods
- Rest angina pectoris that is prolonged (usually >20 min);
- New-onset angina of at least class 3 severity in the Canadian Cardiovascular Society classification;
- Destabilization of previously stable angina.
- Type 1 NSTEMI if followed by obstructive CAD (≥50% diameter stenosis in a major epicardial vessel);
- Myocardial infarction with non-obstructive coronary artery (MINOCA) in cases with no angiographic obstructive coronary artery disease.
3. Results
3.1. Baseline Characteristcs
3.2. Diagnostic Reclassification by a High-Sensitivity Cardiac Troponin I Assay
3.3. Unstable Angina Compared to Reclassified Myocardial Infarction
4. Discussion
4.1. Unstable Angina Versus Myocardial Infarction
4.2. The Difference in Baseline Characteristics
4.3. The Difference in Clinical Characteristics
4.4. The Difference in Instrumental Diagnostics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nichols, M.; Townsend, N.; Luengo-Fernandez, R.; Leal, J.; Gray, A.; Scarborough, P.; Rayner, M. European Cardiovascular Disease Statistics 2012; European Heart Network: Bruxelles, Belgium, 2012. [Google Scholar]
- Movsisyan, N.K.; Vinciguerra, M.; Medina-Inojosa, J.R.; Lopez-Jimenez, F. Cardiovascular Diseases in Central and Eastern Europe: A Call for More Surveillance and Evidence-Based Health Promotion. Ann. Glob. Health 2020, 86, 21. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Zeltser, R. Unstable Angina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Puelacher, C.; Gugala, M.; Adamson, P.D.; Shah, A.; Chapman, A.R.; Anand, A.; Sabti, Z.; Boeddinghaus, J.; Nestelberger, T.; Twerenbold, R.; et al. Incidence and outcomes of unstable angina compared with non-ST-elevation myocardial infarction. Heart 2019, 105, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 42, 1289–1367. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C. Biomarkers and acute coronary syndromes: An update. Eur. Heart J. 2014, 35, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.V.; Anand, A.; Strachan, F.; Ferry, A.V.; Lee, K.K.; Chapman, A.R.; Sandeman, D.; Stables, C.L.; Adamson, P.D.; Andrews, J.P.; et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: A stepped-wedge, cluster-randomised controlled trial. Lancet 2018, 392, 919–928. [Google Scholar] [CrossRef]
- Twerenbold, R.; Jaeger, C.; Rubini Gimenez, M.; Wildi, K.; Reichlin, T.; Nestelberger, T.; Boeddinghaus, J.; Grimm, K.; Puelacher, C.; Moehring, B.; et al. Impact of high-sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. Eur. Heart J. 2016, 37, 3324–3332. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Morrow, D.A. Unstable Angina. Is It Time for a Requiem? Circulation 2013, 127, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Jernberg, T.; Lindhagen, L.; Lindahl, B. High-Sensitivity Cardiac Troponin T Levels Identify Patients with Non–ST-Segment Elevation Acute Coronary Syndrome Who Benefit From Invasive Assessment. JACC Cardiovasc. Interv. 2018, 11, 1665–1667. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Jernberg, T.; Lindahl, B. Unstable Angina in the Era of Cardiac Troponin Assays with Improved Sensitivity-A Clinical Dilemma. Am. J. Med. 2017, 130, 1423–1430.e5. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar]
- Chapman Andrew, R.; Adamson Philip, D.; Shah Anoop, S.V.; Anand, A.; Strachan, F.E.; Ferry, A.V.; Ken Lee, K.; Berry, C.; Findlay, I.; Cruikshank, A.; et al. High-Sensitivity Cardiac Troponin and the Universal Definition of Myocardial Infarction. Circulation 2020, 141, 161–171. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.; Sarkisian, L.; Saaby, L.; Poulsen, T.S.; Gerke, O.; Larsen, T.B.; Diederichsen, A.C.; Jangaard, N.; Diederichsen, S.Z.; Hosbond, S.; et al. Diagnosis of unstable angina pectoris has declined markedly with the advent of more sensitive troponin assays. Am. J. Med. 2015, 128, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, H.W.; Chen, H.; Guo, C.Y. Sex and Age Differences in Patients With Unstable Angina Pectoris: A Single-Center Retrospective Study. Am. J. Med. Sci. 2020, 360, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.K.; Ounpuu, S.; Hawken, S.; Pandey, M.R.; Valentin, V.; Hunt, D.; Diaz, R.; Rashed, W.; Freeman, R.; Jiang, L.; et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: A case-control study. Lancet 2006, 368, 647–658. [Google Scholar] [CrossRef]
- Pechacek, T.F.; Asma, S.; Blair, N.; Eriksen, M.P. Tobacco: Global Burden and Community Solutions. In Evidence-Based Cardiology; Yusuf, S., Cairns, J.A., Camm, A.J., Fallen, E.L., Gersh, B.J., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 103–113. [Google Scholar]
- Elbarouni, B.; Goodman, S.G.; Yan, R.T.; Welsh, R.C.; Kornder, J.M.; DeYoung, J.P.; Wong, G.C.; Rose, B.; Grondin, F.R.; Gallo, R.; et al. Validation of the Global Registry of Acute Coronary Event (GRACE) risk score for in-hospital mortality in patients with acute coronary syndrome in Canada. Am. Heart J. 2009, 158, 392–399. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.D.; Gore, J.; Yarzebski, J.; Spencer, F.; Lessard, D.; Goldberg, R.J. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am. J. Med. 2011, 124, 40–47. [Google Scholar] [CrossRef] [PubMed]
- André, R.; Bongard, V.; Elosua, R.; Kirchberger, I.; Farmakis, D.; Häkkinen, U.; Fusco, D.; Torre, M.; Garel, P.; Araújo, C.; et al. International differences in acute coronary syndrome patients’ baseline characteristics, clinical management and outcomes in Western Europe: The EURHOBOP study. Heart Br. Card Soc. 2014, 100, 1201–1207. [Google Scholar] [CrossRef]
- Mueller, C.; Neumann, F.-J.; Perach, W.; Perruchoud, A.P.; Buettner, H.J. Prognostic value of the admission electrocardiogram in patients with unstable angina/non-ST-segment elevation myocardial infarction treated with very early revascularization. Am. J. Med. 2004, 117, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Golwala, H.; Tripathi, A.; Bin Abdulhak, A.A.; Bavishi, C.; Riaz, H.; Mallipedi, V.; Pandey, A.; Bhatt, D.L. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: A systematic review and meta-analysis. Eur. Heart J. 2017, 38, 3082–3089. [Google Scholar] [CrossRef] [PubMed]

| Initial Diagnosis UA | After Reclassification | ||||
|---|---|---|---|---|---|
| All (n = 715) | Type 1 NSTEMI (n = 137) | UA (n = 334) | p Value | ||
| Baseline characteristics | |||||
| Age (years), mean (SD) | 67.6 (11.02) | 71.4 (9.68) | 65.6 (11.02) | <0.001 | |
| Age by sex (years), median (min-max) | Male | 66 (35–91) a | 70 (44–90) b | 64 (36–91) c | <0.001 |
| Female | 72 (43–90) a | 74 (49–88) b | 68 (46–90) c | 0.004 | |
| Sex, n (%) | Male | 458 (64.1) a | 86 (55.5) | 227 (68.0) | 0.01 |
| Female | 257 (35.9) a | 61 (44.5) | 107 (32.0) | ||
| History of previous MI1, n (%) | 237 (33.1) | 47 (34.3) | 113 (33.8) | 0.92 | |
| History of PCI, n (%) | 259 (36.2) | 57 (41.6) | 127 (38.0) | 0.47 | |
| History of CABG surgery, n (%) | 79 (11.0) | 20 (14.6) | 34 (10.2) | 0.17 | |
| Obstructive CAD, n (%) | 608 (58.0) | 137 (100) | 276 (82.6) | <0.001 | |
| Smoking status, n (%) | Smoking | 111 (15.5) | 13 (9.5) | 54 (16.2) | 0.13 |
| Unknown | 538 (75.2) | 113 (82.5) | 248 (74.3) | ||
| Clinical characteristics | |||||
| Prolonged pain (>20 min.), n (%) | 133 (18.6) | 31 (64.6) | 54 (40.0) | <0.001 | |
| Previous chest pain, n (%) | 529 (74.0) | 76 (57.7) | 276 (85.7) | <0.001 | |
| Exercise-induced chest pain, n (%) | 362 (50.6) | 70 (51.5) | 170 (52.8) | 0.78 | |
| Pain localisation, n (%) | Chest | 615 (86.0) | 115 (83.9) | 309 (92.5) | <0.001 |
| Epigastric region | 12 (1.7) | 1 (0.7) | 8 (2.4) | ||
| Chest and Epigastric region | 10 (1.4) | 1 (0.7) | 2 (0.6) | ||
| No pain at the presentation to emergency room | 58 (8.1) | 18 (13.1) | 1 (0.3) | ||
| Intrascapular | 4 (0.6) | 1 (0.7) | 2 (0.6) | ||
| Left arm | 1 (0.1) | 1 (0.7) | 0 | ||
| Felt typical pain (pressing, dull, tearing), n (%) | 374 (52.3) | 71 (51.8) | 192 (57.5) | 0.08 | |
| Typical irradiation of pain (arm, neck, wide irradiation, mandible, epigastric region and no irradiation), n (%) | 665 (93.0) | 131 (95.6) | 305 (91.3) | 0.17 | |
| Dyspnea, n (%) | 267 (37.3) | 53 (39.0) | 115 (35.3) | 0.45 | |
| Instrumental diagnostics | |||||
| Ischemic changes in ECG (inverted T, ST segment depression and new left bundle branch block) | 364 (50.9) | 75 (54.7) | 148 (44.3) | 0.04 | |
| ECG changes, n (%) | No changes in ECG | 336 (47.0) | 61 (44.5) | 176 (52.7) | 0.2 |
| Negative T wave | 206 (28.8) | 35 (25.5) | 89 (26.6) | ||
| ST depression | 133 (18.6) | 33 (24.1) | 52 (15.6) | ||
| Left bundle branch block | 25 (3.5) | 6 (4.4) | 10 (3.0) | ||
| Right bundle branch block | 6 (0.8) | 1 (0.7) | 4 (1.2) | ||
| Left anterior fascicular block | 5 (0.7) | 1 (0.7) | 0 | ||
| Non specific ECG changes | 4 (0.6) | 0 | 3 (0.9) | ||
| PCI performed, n (%) | 354 (49.5) | 76 (57.7) | 154 (46.1) | 0.02 | |
| Findings of CAG | LMS | 53 (8.2) | 16 (11.7) | 22 (6.6) | 0.07 |
| LAD | 419 (64.7) | 123 (89.8) | 203 (60.8) | <0.001 | |
| LCX | 301 (46.5) | 85 (62.0) | 152 (45.5) | 0.001 | |
| RCA | 291 (45.1) | 83 (60.6) | 145 (43.4) | 0.001 | |
| CABG surgery performed, n (%) | 42 (5.9) | 10 (7.3) | 14 (2.3) | 0.17 | |
| CT coronary angiography performed, n (%) | 20 (2.8) | 1 (0.7) | 10 (3.0) | 0.19 | |
| Exercise tolerance testperformed, n (%) | 19 (2.7) | 2 (1.5) | 14 (4.2) | 0.17 | |
| High sensitivity cardiac troponin-I (hs-cTnI) | |||||
| Hs-cTnI at the presentation to emergency room (ng/L), median (IQR) | 17.6 (76.55) | 184.4 (226.15) | 6.4 (8.10) | <0.001 | |
| Lowest hs-cTnI (ng/L), median (IQR) | 14.1 (46.45) | 114.0 (207.40) | 5.7 (7.28) | <0.001 | |
| Highest hs-cTnI (ng/L), median (IQR) | 32.0 (107.85) | 304.0 (357.60) | 7.1 (8.80) | <0.001 | |
| All Patients | UA | Type 1 NSTEMI | MINOCA | Probable Type 1 NSTEMI | Probable MINOCA | Elevated after Coronary Angiography | |
|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| Hs-cTnI at the presentation, ng/L | 17.6 (902.05) | 6.4 (8.10) | 184.4 (226.15) | 184.7 (165.15) | 47.2 (67.25) | 47.0 (28.45) | 6.9 (7.30) |
| Lowest hs-cTnI, ng/L | 51.7 (125.99) | 5.7 (7.28) | 114.0 (207.40) | 125.6 (147.30) | 37.0 (50.80) | 38.7 (33.08) | 6.9 (3.10) |
| Highest hs-cTnI, ng/L | 115.2 (1112.71) | 7.1 (8.80) | 304.0 (357.60) | 200.3 (317.05) | 61.2 (55.20) | 49.5 (29.15) | 1522.0 (2069.30) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šulskutė, K.; Pilkienė, A.; Meškėnė, E.; Kersnauskaitė, D.; Šerpytis, R.; Petrulionienė, Ž.; Šerpytis, P. High-Sensitivity Cardiac Troponin Impact on the Differential Diagnosis of Non-ST Segment Elevation Coronary Syndromes—Is It Helping? Medicina 2022, 58, 1084. https://doi.org/10.3390/medicina58081084
Šulskutė K, Pilkienė A, Meškėnė E, Kersnauskaitė D, Šerpytis R, Petrulionienė Ž, Šerpytis P. High-Sensitivity Cardiac Troponin Impact on the Differential Diagnosis of Non-ST Segment Elevation Coronary Syndromes—Is It Helping? Medicina. 2022; 58(8):1084. https://doi.org/10.3390/medicina58081084
Chicago/Turabian StyleŠulskutė, Kristina, Aistė Pilkienė, Emilija Meškėnė, Džiugilė Kersnauskaitė, Rokas Šerpytis, Žaneta Petrulionienė, and Pranas Šerpytis. 2022. "High-Sensitivity Cardiac Troponin Impact on the Differential Diagnosis of Non-ST Segment Elevation Coronary Syndromes—Is It Helping?" Medicina 58, no. 8: 1084. https://doi.org/10.3390/medicina58081084
APA StyleŠulskutė, K., Pilkienė, A., Meškėnė, E., Kersnauskaitė, D., Šerpytis, R., Petrulionienė, Ž., & Šerpytis, P. (2022). High-Sensitivity Cardiac Troponin Impact on the Differential Diagnosis of Non-ST Segment Elevation Coronary Syndromes—Is It Helping? Medicina, 58(8), 1084. https://doi.org/10.3390/medicina58081084







