Aqueous Humour Ofloxacin Concentration after Topical Instillation in Patients with Dry Eye Disease
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
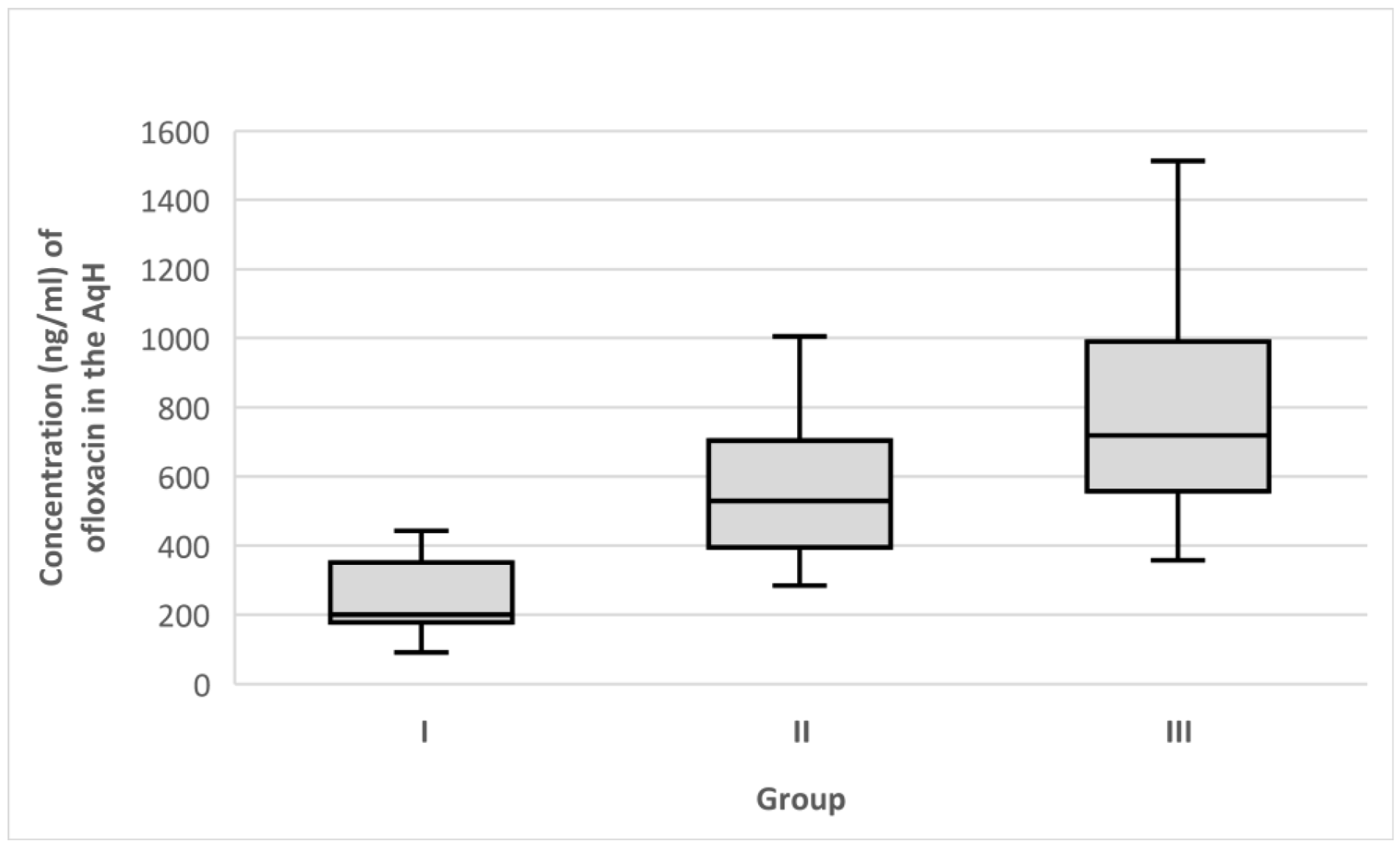
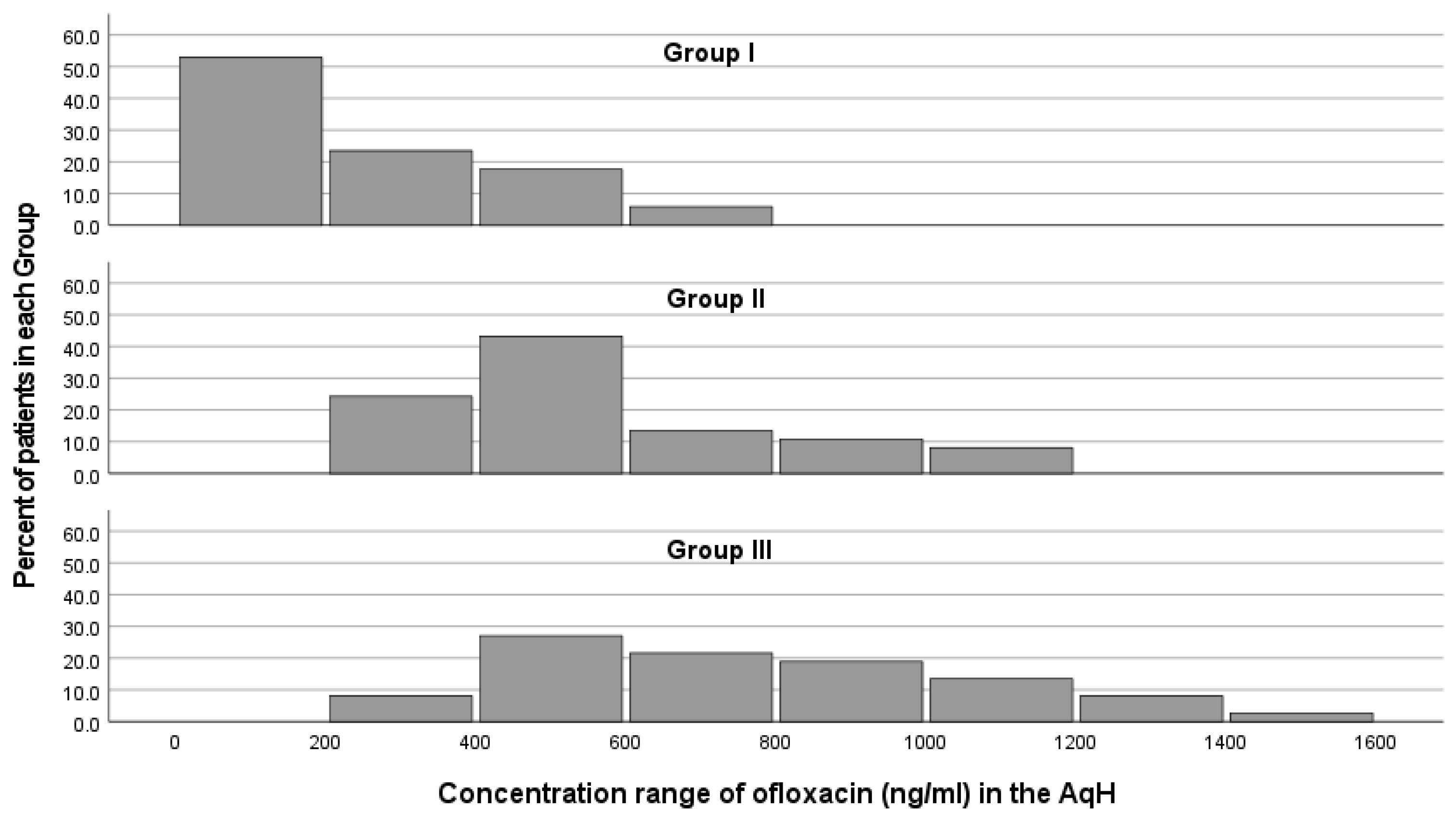
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, J.D.; Craig, J.P.; Akpek, E.K.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Clayton, J.A.; Dogru, M.; Dua, H.S.; Foulks, G.N.; et al. TFOS DEWS II Introduction. Ocul. Surf. 2017, 15, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ganesalingam, K.; Ismail, S.; Sherwin, T.; Craig, J.P. Molecular Evidence for the Role of Inflammation in Dry Eye Disease. Clin. Exp. Optom. 2019, 102, 446–454. [Google Scholar] [CrossRef]
- Periman, L.M.; Perez, V.L.; Saban, D.R.; Lin, M.C.; Neri, P. The Immunological Basis of Dry Eye Disease and Current Topical Treatment Options. J. Ocul. Pharmacol. Ther. 2020, 36, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Pflugfelder, S.C.; Liu, Z.; Baudouin, C.; Kim, H.M.; Messmer, E.M.; Kruse, F.; Liang, L.; Carreno-Galeano, J.T.; Rolando, M.; et al. Defining Dry Eye from a Clinical Perspective. Int. J. Mol. Sci. 2020, 21, 9271. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.L.; Stern, M.E.; Pflugfelder, S.C. Inflammatory Basis for Dry Eye Disease Flares. Exp. Eye Res. 2020, 201, 108294. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.P.; D’Souza, S.; Shetty, R.; Ahuja, P.; Kundu, G.; Khamar, P.; Dadachanji, Z.; Paritekar, P.; Patel, P.; Dickman, M.M.; et al. Altered Ocular Surface Immune Cell Profile in Patients with Dry Eye Disease. Ocul. Surf. 2021, 21, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Akpek, E.K.; Amescua, G.; Farid, M.; Garcia-Ferrer, F.J.; Lin, A.; Rhee, M.K.; Varu, D.M.; Musch, D.C.; Dunn, S.P.; Mah, F.S. Dry Eye Syndrome Preferred Practice Pattern®. Ophthalmology 2019, 126, 286–334. [Google Scholar] [CrossRef]
- Srinivasarao, D.A.; Lohiya, G.; Katti, D.S. Fundamentals, Challenges, and Nanomedicine-based Solutions for Ocular Diseases. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1548. [Google Scholar] [CrossRef]
- Lee, V.H.L. Mechanisms and Facilitation of Corneal Drug Penetration. J. Control. Release 1990, 11, 79–90. [Google Scholar] [CrossRef]
- Suri, R.; Beg, S.; Kohli, K. Target Strategies for Drug Delivery Bypassing Ocular Barriers. J. Drug Deliv. Sci. Technol. 2020, 55, 101389. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A Comprehensive Insight on Ocular Pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Hayakawa, I.; Atarashi, S.; Yokohama, S.; Imamura, M.; Sakano, K.; Furukawa, M. Synthesis and Antibacterial Activities of Optically Active Ofloxacin. Antimicrob. Agents Chemother. 1986, 29, 163–164. [Google Scholar] [CrossRef]
- Gower, E.W.; Lindsley, K.; Tulenko, S.E.; Nanji, A.A.; Leyngold, I.; McDonnell, P.J. Perioperative Antibiotics for Prevention of Acute Endophthalmitis after Cataract Surgery. Cochrane Database Syst. Rev. 2017, 2, CD006364. [Google Scholar] [CrossRef]
- PubChem Compound Summary for CID 4583, Ofloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ofloxacin (accessed on 15 December 2021).
- Abelson, M.B.; Udell, I.J.; Weston, J.H. Normal Human Tear PH by Direct Measurement. Arch. Ophthalmol. (Chic. IL. 1960) 1981, 99, 301. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Medicin. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Kagkelaris, K.A.; Makri, O.E.; Georgakopoulos, C.D.; Panayiotakopoulos, G.D. An Eye for Azithromycin: Review of the Literature. Ther. Adv. Ophthalmol. 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Mah, F.S. Fourth-Generation Fluoroquinolones: New Topical Agents in the War on Ocular Bacterial Infections. Curr. Opin. Ophthalmol. 2004, 15, 316–320. [Google Scholar] [CrossRef]
- Santos, A.; Altamirano-Vallejo, J.C.; Navarro-Partida, J.; la Rosa, A.G.-D.; Hsiao, H.J. Breaking down the Barrier: Topical Liposomes as Nanocarriers for Drug Delivery into the Posterior Segment of the Eyeball. In Role of Novel Drug Delivery Vehicles in Nanobiomedicine, 1st ed.; Tyagi, R.K., Garg, N., Shukla, R., Bisen, P.S., Eds.; IntechOpen: London, UK, 2020; pp. 23–58. ISBN 978-1-78923-986-7. [Google Scholar]
- Baudouin, C.; Aragona, P.; Van Setten, G.; Rolando, M.; Irkeç, M.; Benítez del Castillo, J.; Geerling, G.; Labetoulle, M.; Bonini, S. Diagnosing the Severity of Dry Eye: A Clear and Practical Algorithm. Br. J. Ophthalmol. 2014, 98, 1168–1176. [Google Scholar] [CrossRef]
- Starr, C.E.; Gupta, P.K.; Farid, M.; Beckman, K.A.; Chan, C.C.; Yeu, E.; Gomes, J.A.P.; Ayers, B.D.; Berdahl, J.P.; Holland, E.J.; et al. An Algorithm for the Preoperative Diagnosis and Treatment of Ocular Surface Disorders. J. Cataract Refract. Surg. 2019, 45, 669–684. [Google Scholar] [CrossRef]
- El Mubarak, M.A.; Kagkelaris, K.; Panayiotakopoulos, G.D.; Thomopoulou, V.; Georgakopoulos, C.D.; Sivolapenko, G.B. Quantification of Ofloxacin in Human Aqueous Humour of the Eye by LC-MS/MS. J. Anal. Bioanal. Tech. 2022, 13, 435. [Google Scholar] [CrossRef]
- EMEA Guideline on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 15 December 2021).
- FDA & CDER Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Contains Nonbinding Recommendations. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 15 December 2021).
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A. Dry Eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.L.; Özer Stillman, I.; Pivneva, I.; Guerin, A.; Evans, A.M.; Dana, R. Dry Eye Disease Ranking among Common Reasons for Seeking Eye Care in a Large US Claims Database. Clin. Ophthalmol. 2019, 13, 225–232. [Google Scholar] [CrossRef]
- Tsai, J.H.; Derby, E.; Holland, E.J.; Khatana, A.K. Incidence and Prevalence of Glaucoma in Severe Ocular Surface Disease. Cornea 2006, 25, 530–532. [Google Scholar] [CrossRef]
- Baudouin, C. Side Effects of Antiglaucomatous Drugs on the Ocular Surface. Curr. Opin. Ophthalmol. 1996, 7, 80–86. [Google Scholar] [CrossRef]
- Bouchard, C.S.; King, K.K.; Holmes, J.M. The Kinetics of Anterior Chamber Ofloxacin Penetration. Cornea 1996, 15, 72–75. [Google Scholar] [CrossRef]
- Sadiq, M. Factors Affecting Corneal Penetration of Drugs. Al-Shifa J. Ophthalmol. 2016, 12, 123–124. [Google Scholar]
- Barar, J.; Javadzadeh, A.R.; Omidi, Y. Ocular Novel Drug Delivery: Impacts of Membranes and Barriers. Expert Opin. Drug Deliv. 2008, 5, 567–581. [Google Scholar] [CrossRef]
- Ueda, K.; Ohtori, A.; Tojo, K. Effects of Pathological Conditions on Ocular Pharmacokinetics of Antimicrobial Drugs. Chem. Pharm. Bull. 2010, 58, 1301–1305. [Google Scholar] [CrossRef][Green Version]
- Gatti, G.; Panozzo, G. Effect of Inflammation on Intraocular Penetration of Intravenous Ofloxacin in Albino Rabbits. Antimicrob. Agents Chemother. 1995, 39, 549–552. [Google Scholar] [CrossRef][Green Version]
- Öztürk, F.; Kortunay, S.; Kurt, E.; Inan, Ü.Ü.; Ilker, S.S.; Basci, N.; Bozkurt, A. The Effect of Long-Term Use and Inflammation on the Ocular Penetration of Topical Ofloxacin. Curr. Eye Res. 1999, 19, 461–464. [Google Scholar] [CrossRef]
- Öztürk, F.; Kurt, E.; Inan, Ü.Ü.; Kortunay, S.; Ilker, S.S.; Başci, N.E.; Bozkurt, A. Penetration of Topical and Oral Ofloxacin into the Aqueous and Vitreous Humor of Inflamed Rabbit Eyes. Int. J. Pharm. 2000, 204, 91–95. [Google Scholar] [CrossRef]
- Baudouin, C. The Vicious Circle in Dry Eye Syndrome: A Mechanistic Approach. J. Fr. Ophtalmol. 2007, 30, 239–246. [Google Scholar] [CrossRef]
- Chennakesavalu, M.; Somala, S.R.R.; Dommaraju, S.R.; Peesapati, M.P.; Guo, K.; Rosenblatt, M.I.; Chang, J.H.; Azar, D.T. Corneal Lymphangiogenesis as a Potential Target in Dry Eye Disease-a Systematic Review. Surv. Ophthalmol. 2021, 66, 960–976. [Google Scholar] [CrossRef]
- Seen, S.; Tong, L. Dry Eye Disease and Oxidative Stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef]
- Baudouin, C.; Irkeç, M.; Messmer, E.M.; Benítez-del-Castillo, J.M.; Bonini, S.; Figueiredo, F.C.; Geerling, G.; Labetoulle, M.; Lemp, M.; Rolando, M.; et al. Clinical Impact of Inflammation in Dry Eye Disease: Proceedings of the ODISSEY Group Meeting. Acta Ophthalmol. 2018, 96, 111–119. [Google Scholar] [CrossRef]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry Eye Disease: An Immune-Mediated Ocular Surface Disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef]
- Mather, R.; Karenchak, L.M.; Romanowski, E.G.; Kowalski, R.P. Fourth Generation Fluoroquinolones: New Weapons in the Arsenal of Ophthalmic Antibiotics. Am. J. Ophthalmol. 2002, 133, 463–466. [Google Scholar] [CrossRef]
- Lemley, C.A.; Han, D.P. Endophthalmitis: A Review of Current Evaluation and Management. Retina 2007, 27, 662–680. [Google Scholar] [CrossRef]
- Plotas, P.; Anastasopoulos, C.; Makri, O.E.; Leotsinidis, M.; Georgakopoulos, C.D. A UPLC-MS Method for the Determination of Ofloxacin Concentrations in Aqueous Humor. Anal. Chem. Insights 2014, 9, 27–32. [Google Scholar] [CrossRef]
- Cekic, O.; Batman, C.; Yasar, Ü.; Basci, N.E.; Bozkurt, A.; Kayaalp, S.O. Penetration of Ofloxacin in Human Aqueous and Vitreous Humors Following Oral and Topical Administration. Retina 1998, 18, 521–525. [Google Scholar] [CrossRef]
| Organism | Ofloxacin MIC90 (ng/mL) |
|---|---|
| Streptococcus pneumoniae | 2000 |
| Streptococcus viridans | 2000 |
| Enterococci species | 2000 |
| β-hemolytic Streptococcus | 1500 |
| Staphylococcus aureus | 630 |
| Bacillus species | 380 |
| coagulase-negative Staphylococci | 380 |
| gram-negative bacteria | 190 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kagkelaris, K.; El Mubarak, M.A.; Plotas, P.; Panaretos, D.; Panayiotakopoulos, G.D.; Sivolapenko, G.B.; Georgakopoulos, C.D. Aqueous Humour Ofloxacin Concentration after Topical Instillation in Patients with Dry Eye Disease. Medicina 2022, 58, 1031. https://doi.org/10.3390/medicina58081031
Kagkelaris K, El Mubarak MA, Plotas P, Panaretos D, Panayiotakopoulos GD, Sivolapenko GB, Georgakopoulos CD. Aqueous Humour Ofloxacin Concentration after Topical Instillation in Patients with Dry Eye Disease. Medicina. 2022; 58(8):1031. https://doi.org/10.3390/medicina58081031
Chicago/Turabian StyleKagkelaris, Konstantinos, Mohamed A. El Mubarak, Panagiotis Plotas, Dimitris Panaretos, George D. Panayiotakopoulos, Gregory B. Sivolapenko, and Constantinos D. Georgakopoulos. 2022. "Aqueous Humour Ofloxacin Concentration after Topical Instillation in Patients with Dry Eye Disease" Medicina 58, no. 8: 1031. https://doi.org/10.3390/medicina58081031
APA StyleKagkelaris, K., El Mubarak, M. A., Plotas, P., Panaretos, D., Panayiotakopoulos, G. D., Sivolapenko, G. B., & Georgakopoulos, C. D. (2022). Aqueous Humour Ofloxacin Concentration after Topical Instillation in Patients with Dry Eye Disease. Medicina, 58(8), 1031. https://doi.org/10.3390/medicina58081031







