Evaluation of Remdesivir for Mildly to Moderately Ill Patients with COVID-19: A Single-Arm, Single-Center, Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Patients
2.2. Clinical Characteristics
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
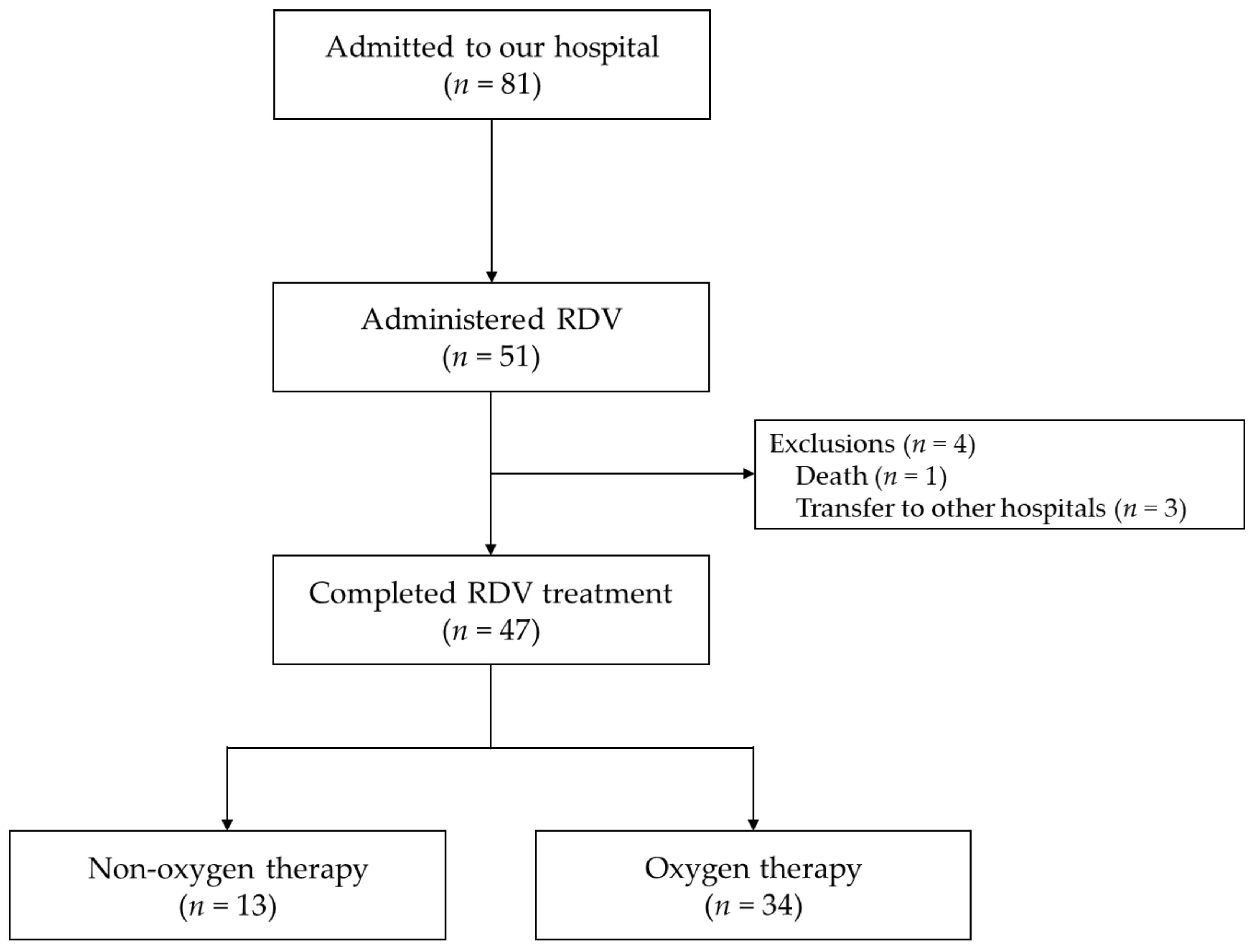
3.1. Clinical Characteristics of Patients Completing RDV Treatment
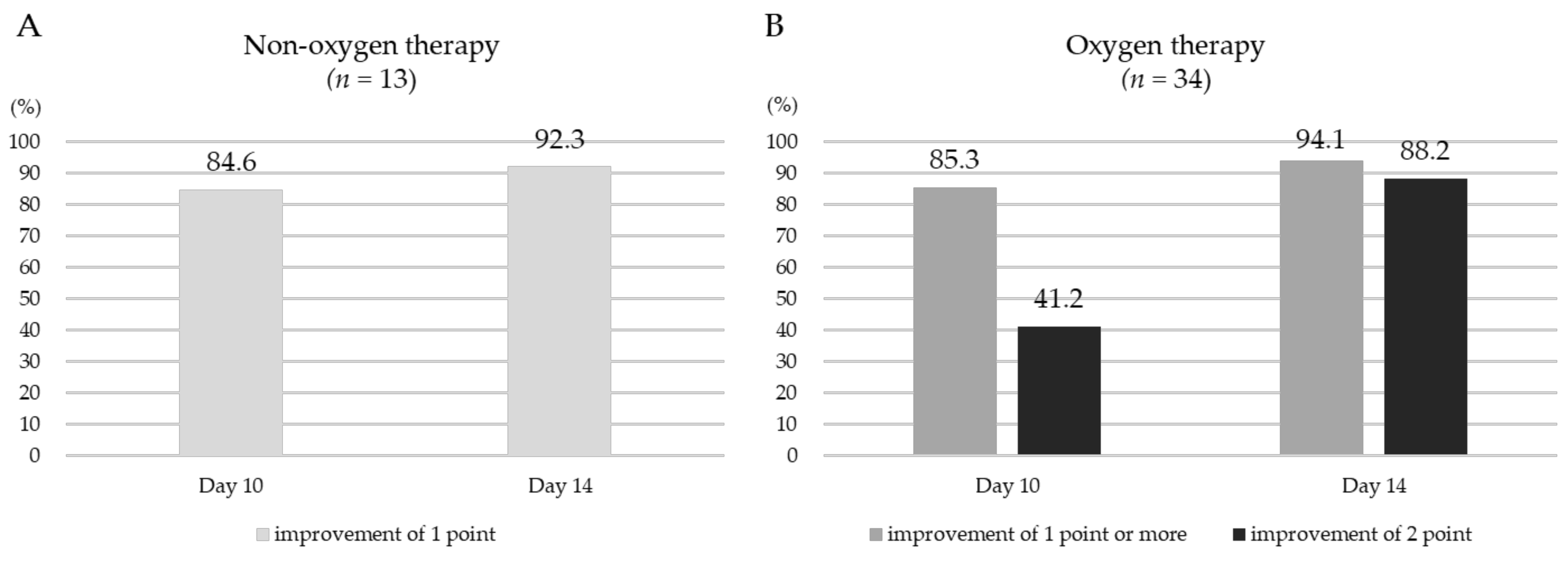
3.2. Outcomes of Patients Completing RDV Treatment
3.3. Adverse Events in Patients Who Completed RDV Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 26 March 2022).
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- WHO Solidarity Trial Consortium; Pan, H.; Peto, R.; Henao-Restrepo, A.M.; Preziosi, M.P.; Sathiyamoorthy, V.; Karim, Q.A.; Alejandria, M.M.; García, C.H.; Kieny, M.P.; et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchesnokov, E.P.; Gordon, C.J.; Woolner, E.; Kocinkova, D.; Perry, J.K.; Feng, J.Y.; Porter, D.P.; Götte, M. Template-dependent inhibition of coronavirus RNA-dependent RNA polymerase by remdesivir reveals a second mechanism of action. J. Biol. Chem. 2020, 295, 16156–16165. [Google Scholar] [CrossRef]
- Eastman, R.T.; Roth, J.S.; Brimacombe, K.R.; Simeonov, A.; Shen, M.; Patnaik, S.; Hall, M.D. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent. Sci. 2020, 6, 672–683. [Google Scholar] [CrossRef]
- Olender, S.A.; Walunas, T.L.; Martinez, E.; Perez, K.K.; Castagna, A.; Wang, S.; Kurbegov, D.; Goyal, P.; Ripamonti, D.; Balani, B.; et al. Remdesivir Versus Standard-of-Care for Severe Coronavirus Disease 2019 Infection: An Analysis of 28-Day Mortality. Open Forum Infect. Dis. 2021, 8, ofab278. [Google Scholar]
- Olender, S.A.; Perez, K.K.; Go, A.S.; Balani, B.; Price-Haywood, E.G.; Shah, N.S.; Wang, S.; Walunas, T.L.; Swaminathan, S.; Slim, J.; et al. Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) versus a Cohort Receiving Standard of Care. Clin. Infect. Dis. 2021, 73, e4166–e4174. [Google Scholar] [CrossRef]
- Mozaffari, E.; Chandak, A.; Zhang, Z.; Liang, S.; Thrun, M.; Gottlieb, R.L.; Kuritzkes, D.R.; Sax, P.E.; Wohl, D.A.; Casciano, R.; et al. Remdesivir treatment in hospitalized patients with COVID-19: A comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort. Clin. Infect. Dis. 2021, ciab875. [Google Scholar] [CrossRef]
- Dal-Ré, R.; Becker, S.L.; Bottieau, E.; Holm, S. Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach. Lancet. Infect. Dis. 2022, 22, e231–e238. [Google Scholar] [CrossRef]
- Piscoya, A.; Ng-Sueng, L.F.; Parra Del Riego, A.; Cerna-Viacava, R.; Pasupuleti, V.; Roman, Y.M.; Thota, P.; White, C.M.; Hernandez, A.V. Efficacy and harms of remdesivir for the treatment of COVID-19: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0243705. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.X.; et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Földi, M.; Farkas, N.; Kiss, S.; Zádori, N.; Váncsa, S.; Szakó, L.; Dembrovszky, F.; Solymár, M.; Bartalis, E.; Szakács, Z.; et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13095. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Morieri, M.L.; Longato, E.; Avogaro, A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J. Endocrinol. Invest. 2020, 43, 867–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, K.; Gončarova, K.; Fedders, D.; Kalbitz, S.; Kellner, N.; Fedders, M.; Lübbert, C. Clinical outcomes of hospitalized COVID-19 patients treated with remdesivir: A retrospective analysis of a large tertiary care center in Germany. Infection 2022, 50, 1–12. [Google Scholar] [CrossRef]
- Wong, C.K.H.; Lau, K.T.K.; Au, I.C.H.; Xiong, X.; Lau, E.H.Y.; Cowling, B.J. Clinical Improvement, Outcomes, Antiviral Activity, and Costs Associated with Early Treatment with Remdesivir for Patients With Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2022, 74, 1450–1458. [Google Scholar] [CrossRef]
- Falcone, M.; Suardi, L.R.; Tiseo, G.; Barbieri, C.; Giusti, L.; Galfo, V.; Forniti, A.; Caroselli, C.; Della Sala, L.; Tempini, S.; et al. Early Use of Remdesivir and Risk of Disease Progression in Hospitalized Patients with Mild to Moderate COVID-19. Clin. Ther. 2022, 44, 364–373. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Hayakawa, K.; Uemura, Y.; Shinozaki, T.; Matsunaga, N.; Terada, M.; Suzuki, S.; Asai, Y.; Kitajima, K.; Saito, S.; et al. Effectiveness of remdesivir in hospitalized nonsevere patients with COVID-19 in Japan: A large observational study using the COVID-19 Registry Japan. Int. J. Infect. Dis. 2022, 118, 119–125. [Google Scholar] [CrossRef]
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Chiba, S.; Halfmann, P.; Nagai, H.; et al. Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant. N. Engl. J. Med. 2022, 386, 995–998. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef] [PubMed]
- Price, C.C.; Altice, F.L.; Shyr, Y.; Koff, A.; Pischel, L.; Goshua, G.; Azar, M.M.; Mcmanus, D.; Chen, S.C.; Gleeson, S.E.; et al. Tocilizumab Treatment for Cytokine Release Syndrome in Hospitalized Patients with Coronavirus Disease 2019: Survival and Clinical Outcomes. Chest 2020, 158, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Liu, Y.; Qiu, L.; Liu, X.; Liu, D.; Li, J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020, 92, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, S.; Gasperetti, A.; Winterton, D.; Vicenzi, M.; Busana, M.; Pedrazzini, G.; Biasco, L.; Tersalvi, G. Echocardiographic assessment of the right ventricle in COVID-19: A systematic review. Int. J. Cardiovasc. Imaging 2021, 37, 3499–3512. [Google Scholar] [CrossRef]
| Characteristics | Non-Oxygen Therapy (n = 13) | Oxygen Therapy (n = 34) | p-Value | |
|---|---|---|---|---|
| Age (years) # | 46 (41–63) | 51 (43–57) | 0.6856 | |
| ≥65 year | 3 (23.1) | 5 (14.7) | 0.6664 a | |
| Gender | Male | 6 (46.2) | 21 (61.8) | 0.3329 |
| Female | 7 (53.9) | 13 (38.2) | ||
| BMI (kg/m2) # | 24.6 (20.6–27.7) | 25.2 (22.1–28.0) | 0.6428 | |
| <25 | 8 (61.5) | 16 (47.0) | 0.8097 | |
| 25–30 | 4 (30.8) | 14 (41.2) | ||
| 30< | 1 (7.7) | 4 (11.8) | ||
| Prior vaccination | 1 (7.7) | 5 (14.7) | >0.9999 a | |
| Current smoking | 3 (23.1) | 12 (35.3) | 0.5033 a | |
| Comorbidities | ||||
| Hypertension | 3 (23.1) | 7 (20.6) | >0.9999 a | |
| DM | 0 (0) | 4 (11.8) | 0.5639 a | |
| Dyslipidemia | 1 (7.7) | 1 (2.9) | 0.4810 a | |
| Cerebrovascular diseases | 0 (0) | 1 (2.9) | >0.9999 a | |
| Asthma | 0 (0) | 3 (8.8) | 0.5502 a | |
| Malignant tumor | 2 (15.4) | 1 (2.9) | 0.1812 a | |
| Laboratory findings # | ||||
| WBC (103/µL) | 3.9 (2.9–4.8) | 5.2 (3.5–5.6) | 0.0373 | |
| RBC (103/µL) | 444 (415–478) | 477 (445–516) | 0.0363 | |
| Hb (g/dL) | 13.4 (11.3–14.5) | 15.0 (13.6–15.7) | 0.0109 | |
| Plt (104/µL) | 16.9 (13.6–23.7) | 17.9 (16.0–22.4) | 0.3949 | |
| TP (g/dL) | 7.2 (6.9–7.7) | 7.4 (7.1–7.7) | 0.3162 | |
| ALB (g/dL) | 3.6 (3.4–3.8) | 3.5 (3.4–3.6) | 0.3786 | |
| AST (U/L) | 32 (27–41) | 41 (31–57) | 0.1006 | |
| ALT (U/L) | 26 (19–35) | 33 (20–57) | 0.2952 | |
| LDH (U/L) | 258 (197–341) | 348 (285–501) | 0.0163 | |
| CK (U/L) | 90 (48–193) | 103 (74–173) | 0.5338 | |
| ALP (U/L) | 63 (50–76) | 69 (58–84) | 0.5841 | |
| γ-GT (U/L) | 26 (16–54) | 65 (25–87) | 0.0825 | |
| BUN (mg/dL) | 10 (9–12) | 12 (10–14) | 0.0707 | |
| Cre (mg/dL) | 0.63 (0.56–0.90) | 0.79 (0.60–0.95) | 0.4049 | |
| eGFR (mL/min) | 82.3 (67.4–92.5) | 76.0 (61.4–91.1) | 0.4904 | |
| CRP (mg/dL) | 4.27 (0.66–8.54) | 5.22 (3.79–7.86) | 0.2344 | |
| Concomitant drug | ||||
| Dexamethasone | 7 (53.9) | 34 (100) | 0.0002 a | |
| Baricitinib | 0 (0) | 7 (20.6) | 0.1660 a | |
| Heparin | 0 (0) | 7 (20.6) | 0.1660 a | |
| Antibiotics | 1 (7.7) | 3 (8.8) | >0.9999 a |
| Patient | Age (Years) | Gender | BMI (kg/m2) | Underlying Diseases | Symptom Onset to RDV (Days) | Duration of RDV Treatment (Days) | Concomitant Drug | RDV Initiation to Clinical Improvement by 2 Points (Days) |
|---|---|---|---|---|---|---|---|---|
| I | 51 | Female | 18.1 | None | 9 | 5 | Antibiotics | 14 |
| II | 56 | Male | 25.5 | DM | 8 | 10 | Baricitinib Heparin Antibiotics | 24 |
| III | 59 | Female | 35.2 | None | 4 | 5 | None | 15 |
| IV | 43 | Male | 25.7 | DM | 6 | 10 | Baricitinib Heparin | 22 |
| Patients | Age (Years) | Gender | BMI (kg/m2) | Underlying Diseases | RDV Treatment (Days) | Concomitant Drug | Oxygen | Adverse Events | Change in Value a |
|---|---|---|---|---|---|---|---|---|---|
| A | 54 | Male | 26.1 | None | 5 | Dexamethasone | No | ALT (U/L) elevation | Day 1: 32 Day 5: 172 |
| B | 45 | Male | 25.1 | None | 5 | Dexamethasone | Yes | ALT (U/L) elevation | Day 1: 38 Day 8: 200 |
| C | 47 | Male | 21.5 | None | 5 | Dexamethasone | Yes | ALT (U/L) elevation | Day 1: 31 Day 6: 193 |
| D | 40 | Female | 20.7 | None | 5 | None | No | Neutropenia (/µL) | Day 1: 3432 Day 3: 364 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, M.; Yanagida, R.; Nakashima, A.; Matsuo, K.; Moriwaki, N.; Uchiyama, M.; Yamada, Y.; Hirata, H.; Kushima, H.; Kinoshita, Y.; et al. Evaluation of Remdesivir for Mildly to Moderately Ill Patients with COVID-19: A Single-Arm, Single-Center, Retrospective Study. Medicina 2022, 58, 1007. https://doi.org/10.3390/medicina58081007
Miyazaki M, Yanagida R, Nakashima A, Matsuo K, Moriwaki N, Uchiyama M, Yamada Y, Hirata H, Kushima H, Kinoshita Y, et al. Evaluation of Remdesivir for Mildly to Moderately Ill Patients with COVID-19: A Single-Arm, Single-Center, Retrospective Study. Medicina. 2022; 58(8):1007. https://doi.org/10.3390/medicina58081007
Chicago/Turabian StyleMiyazaki, Motoyasu, Ryoko Yanagida, Akio Nakashima, Koichi Matsuo, Norihiro Moriwaki, Masanobu Uchiyama, Yota Yamada, Hitomi Hirata, Hisako Kushima, Yoshiaki Kinoshita, and et al. 2022. "Evaluation of Remdesivir for Mildly to Moderately Ill Patients with COVID-19: A Single-Arm, Single-Center, Retrospective Study" Medicina 58, no. 8: 1007. https://doi.org/10.3390/medicina58081007
APA StyleMiyazaki, M., Yanagida, R., Nakashima, A., Matsuo, K., Moriwaki, N., Uchiyama, M., Yamada, Y., Hirata, H., Kushima, H., Kinoshita, Y., Ishii, H., & Imakyure, O. (2022). Evaluation of Remdesivir for Mildly to Moderately Ill Patients with COVID-19: A Single-Arm, Single-Center, Retrospective Study. Medicina, 58(8), 1007. https://doi.org/10.3390/medicina58081007








