Evaluating the Phagocytic Index of Peripheral Leukocytes in Endometriosis by Plasma Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Preparation of Opsonized and FITC-Labeled Zymosan Particles
2.3. Separation of Plasma Fractions, Granulocytes, and Monocytes
2.4. Plasma Incubation
2.5. Cell Number Adjustment and Adhesion
2.6. Phagocytic Activity, Cell Membrane, and Nucleus of Cells
2.7. Statistical Analysis
3. Results
3.1. Clinical Data of Participants
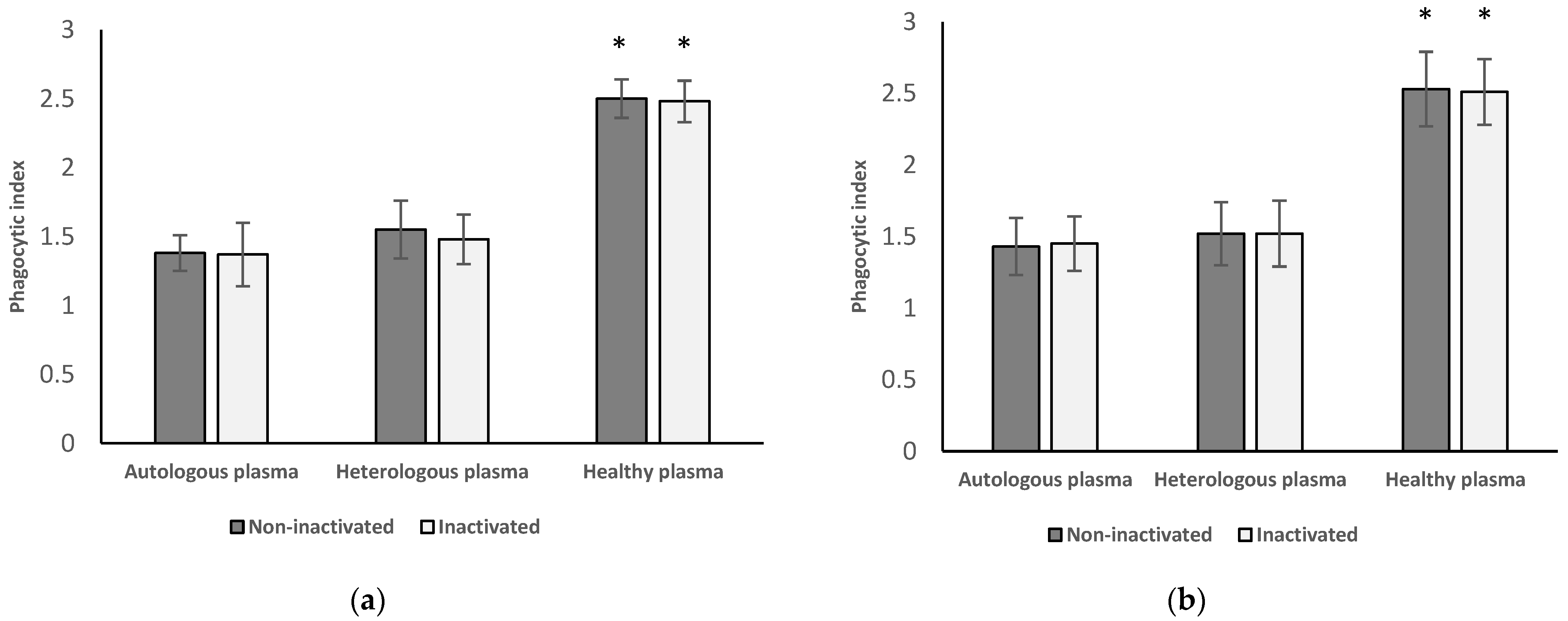
3.2. Phagocytic Index of Neutrophil Granulocytes and Monocytes from Healthy Women
3.2.1. Results of Neutrophil Granulocytes from Healthy Women
3.2.2. Results of Monocytes Isolated from Healthy Women
3.2.3. Results from Inactivated versus Non-Inactivated and Heterologous versus Autologous Plasma of Healthy Women
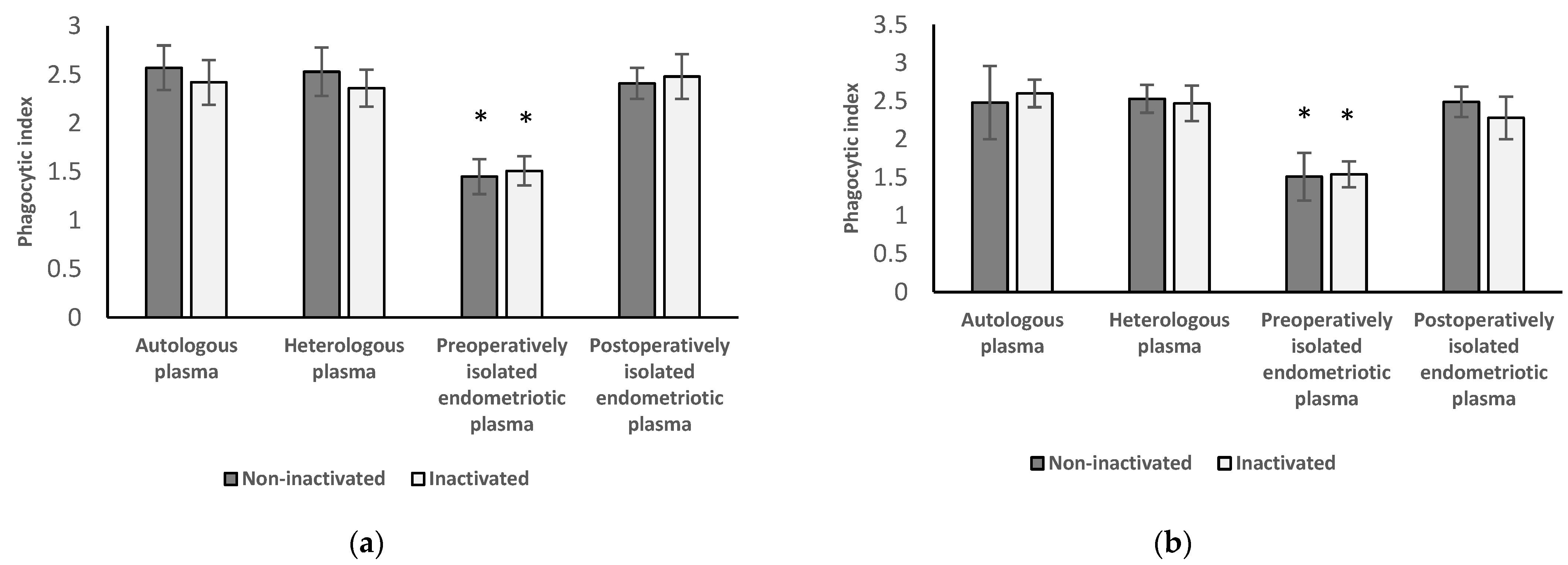
3.3. Phagocytic Index of Neutrophil Granulocytes and Monocytes from Women with Endometriosis before Surgery
3.3.1. Results of Neutrophil Granulocytes Preoperatively Separated from Women with Endometriosis
3.3.2. Results of Monocytes Preoperatively Isolated from Women with Endometriosis
3.3.3. Results from Inactivated versus Non-Inactivated and Heterologous versus Autologous Plasma from Preoperative Women
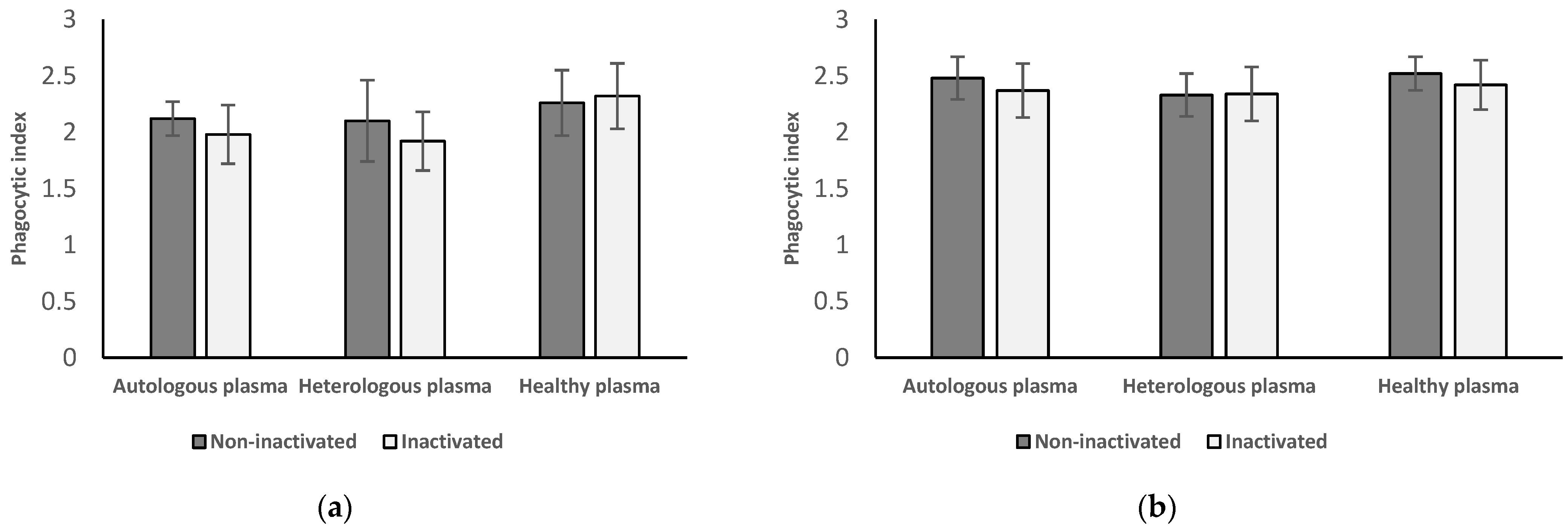
3.4. Phagocytic Index of Neutrophil Granulocytes and Monocytes from Women with Endometriosis after Surgery
3.4.1. Results of Neutrophil Granulocytes Postoperatively Separated from Women with Endometriosis
3.4.2. Results of Monocytes Postoperatively Isolated from Women with Endometriosis
3.4.3. Results from Inactivated vs. Non-Inactivated Plasma and Heterologous vs. Autologous Plasma Regarding Postoperative Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballard, K.D.; Seaman, H.E.; de Vries, C.S.; Wright, J.T. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study-Part 1. BJOG 2008, 115, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2013, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Bokor, A.; Koszorús, E.; Brodszky, V.; D’Hooghe, T.; Rigó, J. The impact of endometriosis on quality of life in Hungary. Orv. Hetil. 2013, 154, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Mehedintu, C.; Plotogea, M.N.; Ionescu, S.; Antonovici, M. Endometriosis still a challenge. J. Med. Life 2014, 7, 349–357. [Google Scholar]
- D’Alterio, M.N.; Saponara, S.; Agus, M.; Laganà, A.S.; Noventa, M.; Loi, E.S.; Feki, A.; Angioni, S. Medical and surgical interventions to improve the quality of life for endometriosis patients: A systematic review. Gynecol. Surg. 2021, 18, 13. [Google Scholar] [CrossRef]
- Berlanda, N.; Alio, W.; Angioni, S.; Bergamini, V.; Bonin, C.; Boracchi, P.; Candiani, M.; Centini, G.; D’Alterio, M.N.; Del Forno, S.; et al. Impact of endometriosis on obstetric outcome after natural conception: A Multicenter Italian study. Arch. Gynecol. Obstet. 2021, 305, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, F.; De Padova, M.; Falafario, M.; D’Alterio, M.N.; Di Spiezio Sardo, A.; Pacheco, L.A.; Carugno, J.T.; Nappi, L. Endometriosis and adverse pregnancy outcome—Minerva. Obstet. Gynecol. 2022, 74, 31–44. [Google Scholar]
- Murgia, F.; Angioni, S.; D’Alterio, M.N.; Pirarba, S.; Noto, A.; Santoru, M.L.; Tronci, L.; Fanos, V.; Atzori, L.; Congiu, F. Metabolic Profile of Patients with Severe Endometriosis: A Prospective Experimental Study. Reprod. Sci. 2020, 28, 728–735. [Google Scholar] [CrossRef]
- Mol, B.W.J.; Bayram, N.; Lijmer, J.G.; Wiegerinck, M.A.H.M.; Bongers, M.Y.; van der Veen, F.; Bossuyt, P.M.M. The performance of CA-125 measurement in the detection of endometriosis: A meta-analysis. Fertil. Steril. 1998, 70, 1101–1108. [Google Scholar] [CrossRef]
- Ottolina, J.; Bartiromo, L.; Dolci, C.; Salmeri, N.; Schimberni, M.; Villanacci, R.; Viganò, P.; Candiani, M. Assessment of coagulation parameters in women affected by endometriosis: Validation study and systematic review of the literature. Diagnostics 2020, 10, 567. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angioni, S.; D’Alterio, M.N.; Coiana, A.; Anni, F.; Gessa, S.; Deiana, D. Genetic characterization of endometriosis patients: Review of the literature and a prospective cohort study on a mediterranean population. Int. J. Mol. Sci. 2020, 21, 1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viganó, D.; Zara, F.; Pinto, S.; Loddo, E.; Casula, L.; Soru, M.B.; D’Ancona, G.; D’Alterio, M.N.; Giuliani, C.; Angioni, S.; et al. How is small bowel permeability in endometriosis patients? A case control pilot study. Gynecol. Endocrinol. 2020, 36, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- D’alterio, M.N.; Giuliani, C.; Scicchitano, F.; Laganà, A.S.; Oltolina, N.M.; Sorrentino, F.; Nappi, L.; Orrù, G.; Angioni, S. Possible role of microbiome in the pathogenesis of endometriosis. Minerva Gynecol. 2021, 73, 193–214. [Google Scholar] [CrossRef]
- Suardika, A.; Astawa Pemayun, T.G. New Insights on the pathogenesis of endometriosis and novel non-surgical therapies. J. Turk. Ger. Gynecol. Assoc. 2018, 19, 158–164. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Control of phagocytosis by microbial pathogens. Front. Immunol. 2017, 8, 1368. [Google Scholar] [CrossRef] [Green Version]
- Lampé, R.; Szűcs, S.; Ádány, R.; Póka, R. Granulocyte superoxide anion production and regulation by plasma factors in normal and preeclamptic pregnancy. J. Reprod. Immunol. 2011, 89, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Lukács, L.; Kovács, A.R.; Pál, L.; Szűcs, S.; Kövér, Á.; Lampé, R. Phagocyte function of peripheral neutrophil granulocytes and monocytes in endometriosis before and after surgery. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101796. [Google Scholar] [CrossRef]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised American Society for Reproductive Medicine Classification of Endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar]
- Hed, J.; Hallden, G.; Johansson, S.G.O.; Larsson, P. The use of fluorescence quenching in flow cytofluorometry to measure the attachment and ingestion phases in phagocytosis in peripheral blood without prior cell separation. J. Immunol. Methods 1987, 101, 119–125. [Google Scholar] [CrossRef]
- Bøyum, A. Isolation of lymphocytes, granulocytes and macrophages. Scand. J. Immunol. 1976, 5, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Vrsalovic, M.; Vrsalovic, M.M.; Presecki, A.V.; Lukac, J. Modulating role of alcohol and acetaldehyde on neutrophil and monocyte functions in vitro. J. Cardiovasc. Pharmacol. 2007, 50, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Králíčková, M.; Vetvicka, V. Immunological aspects of endometriosis: A review. Ann. Transl. Med. 2015, 3, 153. [Google Scholar]
- Na, Y.-J.; Lee, D.-H.; Kim, S.-C.; Joo, J.-K.; Wang, J.-W.; Jin, J.-O.; Kwak, J.-Y.; Lee, K.-S. Effects of peritoneal fluid from endometriosis patients on the release of monocyte-specific chemokines by leukocytes. Arch. Gynecol. Obstet. 2010, 283, 1333–1341. [Google Scholar] [CrossRef]
- Giudice, L.C. Endometriosis. N. Eng. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef]
- Voltolini Velho, R.; Halben, N.; Chekerov, R.; Keye, J.; Plendl, J.; Sehouli, J.; Mechsner, S. Functional changes of immune cells: Signal of immune tolerance of the ectopic lesions in endometriosis? Reprod. BioMed. Online 2021, 43, 319–328. [Google Scholar] [CrossRef]
- Ramírez-Pavez, T.N.; Martínez-Esparza, M.; Ruiz-Alcaraz, A.J.; Marín-Sánchez, P.; Machado-Linde, F.; García-Peñarrubia, P. The role of peritoneal macrophages in endometriosis. Int. J. Mol. Sci. 2021, 22, 10792. [Google Scholar] [CrossRef]
- Zou, G.; Wang, J.; Xu, X.; Xu, P.; Zhu, L.; Yu, Q.; Peng, Y.; Guo, X.; Li, T.; Zhang, X. Cell subtypes and immune dysfunction in peritoneal fluid of endometriosis revealed by single-cell RNA-sequencing. Cell Biosci. 2021, 11, 98. [Google Scholar] [CrossRef]
- Lampé, R.; Szűcs, S.; Ormos, M.; Ádány, R.; Póka, R. Effect of normal and preeclamptic plasma on superoxide-anion production of neutrophils from healthy non-pregnant women. J. Reprod. Immunol. 2008, 79, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, A.V.; Augusto, K.L.; Portela, M.C.; Sucupira, L.C.; Oliveira, L.A.; Pouchaim, A.J.; Nóbrega, L.R.; Magalhães, T.F.; Sobreira, L.R. Endometriosis and ovarian cancer: An integrative review (endometriosis and ovarian cancer). Asian Pac. J. Cancer Prev. 2017, 18, 11–16. [Google Scholar] [PubMed]
- Lin, W.-C.; Chang, C.Y.-Y.; Hsu, Y.-A.; Chiang, J.-H.; Wan, L. Increased risk of endometriosis in patients with lower genital tract infection. Medicine 2016, 95, e2773. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, J.; Lu, J.; Sun, X. Ovarian endometrioma infiltrating neutrophils orchestrate immunosuppressive microenvironment. J. Ovarian Res. 2020, 13, 44. [Google Scholar] [CrossRef]
- Dmowski, W.P.; Gebel, H.M.; Braun, D.P. The role of cell-mediated immunity in pathogenesis of endometriosis. Acta Obstet. Gynecol. Scand. 1994, 159, 7–14. [Google Scholar]
- Satake, E.; Koga, K.; Takamura, M.; Izumi, G.; Elsherbini, M.; Taguchi, A.; Makabe, T.; Takeuchi, A.; Harada, M.; Hirata, T.; et al. The roles of polymorphonuclear myeloid-derived suppressor cells in endometriosis. J. Reprod. Immunol. 2021, 148, 103371. [Google Scholar] [CrossRef]
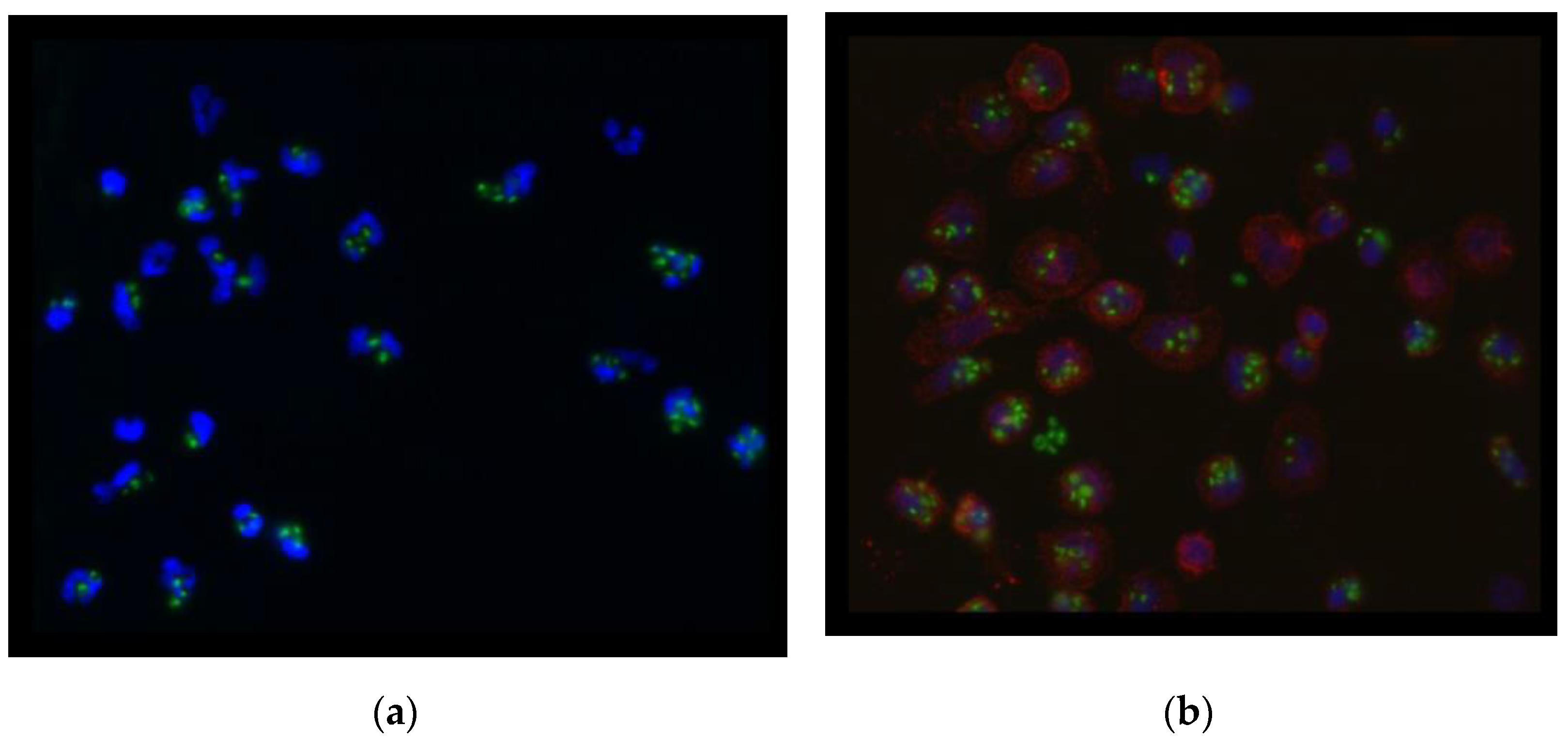
| Endometriosis Patients (n = 8) | Healthy Women (n = 16) | p-Value | |
|---|---|---|---|
| Age (year) | 33.38 (±4.57) | 32.06 (±4.25) | NS |
| BMI a (kg/m2) | 21.64 (±2.64) | 20.78 (±2.27) | NS |
| Gravidity b | 1 (0–2) | 1 (0–3) | NS |
| Parity b | 0 (0–1) | 1 (0–3) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukács, L.; Kovács, A.R.; Pál, L.; Szűcs, S.; Lampé, R. Evaluating the Phagocytic Index of Peripheral Leukocytes in Endometriosis by Plasma Experiments. Medicina 2022, 58, 925. https://doi.org/10.3390/medicina58070925
Lukács L, Kovács AR, Pál L, Szűcs S, Lampé R. Evaluating the Phagocytic Index of Peripheral Leukocytes in Endometriosis by Plasma Experiments. Medicina. 2022; 58(7):925. https://doi.org/10.3390/medicina58070925
Chicago/Turabian StyleLukács, Luca, Anna Rebeka Kovács, László Pál, Sándor Szűcs, and Rudolf Lampé. 2022. "Evaluating the Phagocytic Index of Peripheral Leukocytes in Endometriosis by Plasma Experiments" Medicina 58, no. 7: 925. https://doi.org/10.3390/medicina58070925
APA StyleLukács, L., Kovács, A. R., Pál, L., Szűcs, S., & Lampé, R. (2022). Evaluating the Phagocytic Index of Peripheral Leukocytes in Endometriosis by Plasma Experiments. Medicina, 58(7), 925. https://doi.org/10.3390/medicina58070925







