Gender and Advanced Urothelial Cancer: Outcome, Efficacy and Toxicity following Chemotherapy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Time between Diagnosis and Metastasis Detection
3.3. Overall Survival (OS)
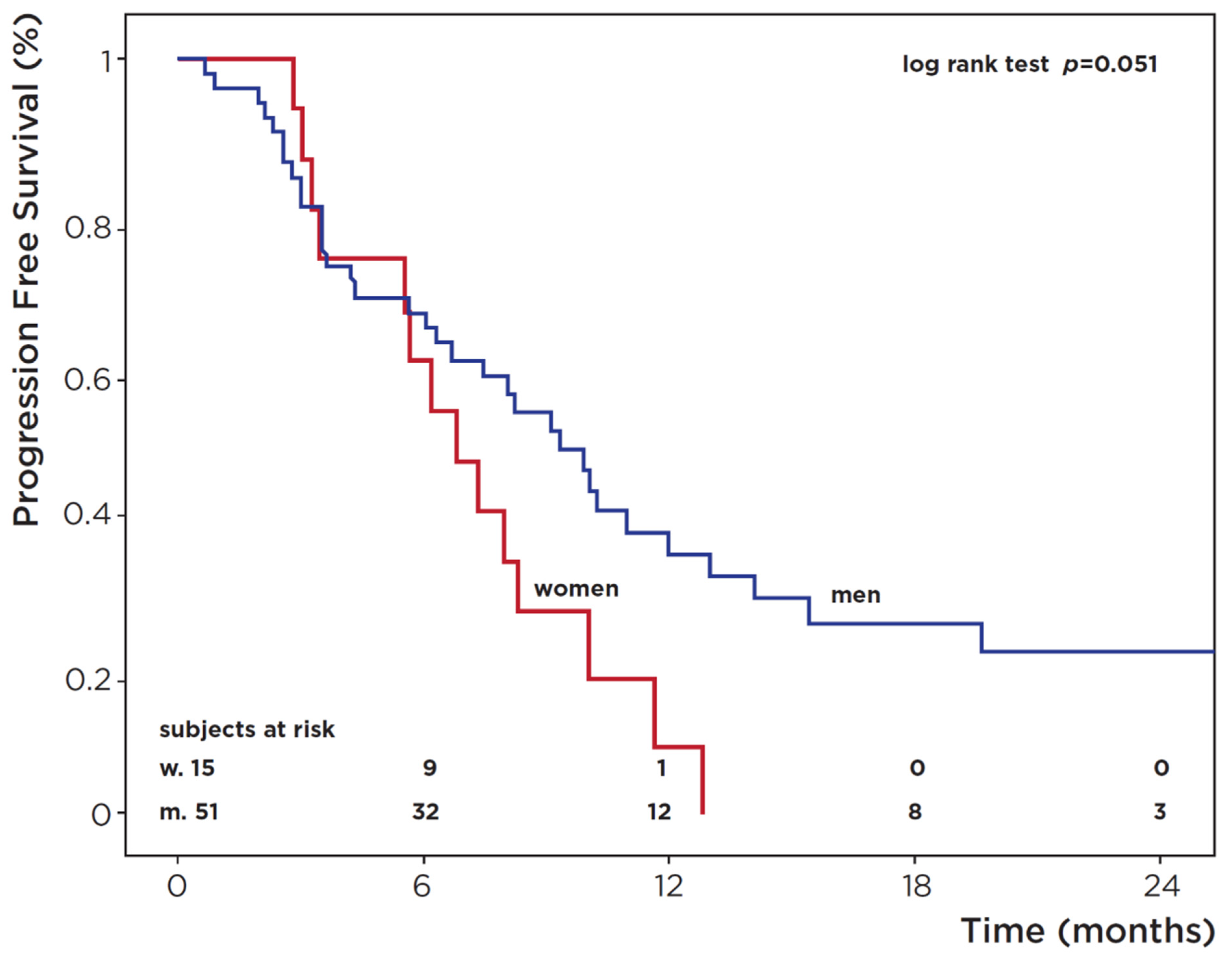
3.4. Radiological Response and Progression-Free Survival (PFS)
3.5. Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hemelt, M.; Yamamoto, H.; Cheng, K.K.; Zeegers, M.P. The effect of smoking on the male excess of bladder cancer: A meta-analysis and geographical analyses. Int. J. Cancer 2009, 124, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO classification of tumours of the urinary system and male genital organs-Part B: Prostate and bladder tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palou, J.; Sylvester, R.J.; Faba, O.R.; Parada, R.; Peña, J.A.; Algaba, F.; Villavicencio, H. Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease-specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette-Guérin. Eur. Urol. 2012, 62, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Fajkovic, H.; Halpern, J.A.; Cha, E.K.; Bahadori, A.; Chromecki, T.F.; Karakiewicz, P.I.; Breinl, E.; Merseburger, A.S.; Shariat, S.F. Impact of gender on bladder cancer incidence, staging, and prognosis. World J. Urol. 2011, 29, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.S.; Smith, C.L.; Hsieh, J.T.; Yu, J.; Kim, I.Y.; Jian, W.; Sonpavde, G.; Ayala, G.E.; Younes, M.; Lerner, S.P. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer 2006, 106, 2610–2616. [Google Scholar] [CrossRef]
- Goto, T.; Miyamoto, H. The role of estrogen receptors in urothelial cancer. Front. Endocrinol. 2021, 12, 643870. [Google Scholar] [CrossRef] [PubMed]
- Weiner, A.B.; Keeter, M.K.; Manjunath, A.; Meeks, J.J. Discrepancies in staging, treatment, and delays to treatment may explain disparities in bladder cancer outcomes: An update from the National Cancer Data Base (2004–2013). Urol. Oncol. 2018, 36, e9–e237. [Google Scholar] [CrossRef]
- Cohn, J.A.; Vekhter, B.; Lyttle, C.; Steinberg, G.D.; Large, M.C. Sex disparities in diagnosis of bladder cancer after initial presentation with hematuria: A nationwide claims-based investigation. Cancer 2014, 120, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.K.; Daignault, S.; Zhang, Y.; Lee, C.T. Patterns of hematuria referral to urologists: Does a gender disparity exist? Urology 2008, 72, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Henning, A.; Wehrberger, M.; Madersbacher, S.; Pycha, A.; Martini, T.; Comploj, E.; Jeschke, K.; Tripolt, C.; Rauchenwald, M. Do differences in clinical symptoms and referral patterns contribute to the gender gap in bladder cancer? BJU Int. 2013, 112, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wolff, I.; Brookman-May, S.; May, M. Sex difference in presentation and outcomes of bladder cancer: Biological reality or statistical fluke? Curr. Opin. Urol. 2015, 25, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Compérat, E.; Cowan, N.C.; De Santis, M.; Gakis, G.; Lebret, T.; Ribal, M.J.; Van der Heijden, A.G.; Sherif, A.; European Association of Urology. EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur. Urol. 2014, 65, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parulekar, W.; Murray, N.; Feld, R.; Evans, W.K.; Tu, D.; Shepherd, F.A. Influence of sex on toxicity and treatment outcome in small-cell lung cancer. J. Clin. Oncol. 2005, 23, 850–856. [Google Scholar] [CrossRef]
- Nicolson, T.J.; Mellor, H.R.; Roberts, R.R. Gender differences in drug toxicity. Trends Pharmacol. Sci. 2010, 31, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.D. Gender differences in pharmacological response. Int. Rev. Neurobiol. 2008, 83, 1–10. [Google Scholar] [CrossRef]
- Haines, L.; Bamias, A.; Krege, S.; Lin, C.C.; Hahn, N.; Ecke, T.H.; Moshier, E.; Sonpavde, G.; Godbold, J.; Oh, W.K.; et al. The impact of gender on outcomes in patients with metastatic urothelial carcinoma. Clin. Genitourin. Cancer 2013, 11, 346–352. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Kunath, F.; Keck, B.; Bertz, S.; Brookman-May, S.; May, M.; Vergho, D.; Hartmann, A.; Riedmiller, H.; Wullich, B.; Burger, M. Is gender becoming relevant in uro-oncological research? A bibliographical analysis. World J. Urol. 2013, 31, 1065–1072. [Google Scholar] [CrossRef]
- Shariat, S.F.; Sfakianos, J.P.; Droller, M.J.; Karakiewicz, P.I.; Meryn, S.; Bochner, B.H. The effect of age and gender on bladder cancer: A critical review of the literature. BJU Int. 2010, 105, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Horstmann, M.; Witthuhn, R.; Falk, M.; Stenzl, A. Gender-specific differences in bladder cancer: A retrospective analysis. Gend. Med. 2008, 5, 385–394. [Google Scholar] [CrossRef]
- Mungan, N.A.; Aben, K.K.; Schoenberg, M.P.; Visser, O.; Coebergh, J.W.; Witjes, J.A.; Kiemeney, L.A. Gender differences in stage-adjusted bladder cancer survival. Urology 2000, 55, 876–880. [Google Scholar] [CrossRef]
- Tracey, E.; Roder, D.; Luke, C.; Bishop, J. Bladder cancer survivals in New South Wales, Australia: Why do women have poorer survival than men? BJU Int. 2009, 104, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, B.K.; Grimsrud, T.K.; Haug, E.S. Bladder cancer survival: Women better off in the long run. Eur. J. Cancer 2018, 95, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlig, A.; Seif Amir Hosseini, A.; Simon, J.; Lotz, J.; Trojan, L.; Schmid, M.; Uhlig, J. Gender specific differences in disease-free, cancer specific and overall survival after radical cystectomy for bladder cancer: A systematic review and meta-analysis. J. Urol. 2018, 200, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, A.; Strauss, A.; Seif Amir Hosseini, A.; Lotz, J.; Trojan, L.; Schmid, M.; Uhlig, J. Gender-specific differences in recurrence of non-muscle-invasive bladder cancer: A systematic review and meta-analysis. Eur. Urol. Focus 2018, 4, 924–936. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Otto, W.; May, M.; Fritsche, H.M.; Dragun, D.; Aziz, A.; Gierth, M.; Trojan, L.; Herrmann, E.; Moritz, R.; Ellinger, J.; et al. Analysis of sex differences in cancer-specific survival and perioperative mortality following radical cystectomy: Results of a large German multicenter study of nearly 2500 patients with urothelial carcinoma of the bladder. Gend. Med. 2012, 9, 481–489. [Google Scholar] [CrossRef]
- Madeb, R.; Messing, E.M. Gender, racial and age differences in bladder cancer incidence and mortality. Urol. Oncol. 2004, 22, 86–92. [Google Scholar] [CrossRef]
- Paciotti, M.; Nguyen, D.D.; Modonutti, D.; Haeuser, L.; Lipsitz, S.; Mossanen, M.; Kibel, A.S.; Lughezzani, G.; Trinh, Q.D.; Cole, A.P. Impact of high-intensity local treatment on overall survival in stage IV upper tract urothelial carcinoma. Urol. Oncol. 2021, 39, e1–e436. [Google Scholar] [CrossRef]
- Shah, R.B.; Montgomery, J.S.; Montie, J.E.; Kunju, L.P. Variant (divergent) histologic differentiation in urothelial carcinoma is under-recognized in community practice: Impact of mandatory central pathology review at a large referral hospital. Urol. Oncol. 2013, 31, 1650–1655. [Google Scholar] [CrossRef]
- Cai, T.; Tiscione, D.; Verze, P.; Pomara, G.; Racioppi, M.; Nesi, G.; Barbareschi, M.; Brausi, M.; Gacci, M.; Luciani, L.G.; et al. Concordance and clinical significance of uncommon variants of bladder urothelial carcinoma in transurethral resection and radical cystectomy specimens. Urology 2014, 84, 1141–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pons, F.; Orsola, A.; Morote, J.; Bellmunt, J. Variant forms of bladder cancer: Basic considerations on treatment approaches. Curr Oncol. Rep. 2011, 13, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Frank, I.; Cheville, J.C.; Thompson, R.H.; Weight, C.J.; Thapa, P.; Boorjian, S.A. The impact of squamous and glandular differentiation on survival after radical cystectomy for urothelial carcinoma. J. Urol. 2012, 188, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B. Histological variants of urothelial carcinoma: Diagnostic, therapeutic and prognostic implications. Mod. Pathol. 2009, 22 (Suppl. 2), S96–S118. [Google Scholar] [CrossRef]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between smoking and risk of bladder cancer among men and women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef]
- van Osch, F.H.; Jochems, S.H.; van Schooten, F.J.; Bryan, R.T.; Zeegers, M.P. Quantified relations between exposure to tobacco smoking and bladder cancer risk: A meta-analysis of 89 observational studies. Int. J. Epidemiol. 2016, 45, 857–870. [Google Scholar] [CrossRef] [Green Version]
- Lammers, R.J.; Witjes, W.P.; Hendricksen, K.; Caris, C.T.; Janzing-Pastors, M.H.; Witjes, J.A. Smoking status is a risk factor for recurrence after transurethral resection of non-muscle-invasive bladder cancer. Eur. Urol. 2011, 60, 713–720. [Google Scholar] [CrossRef]
- Boström, P.J.; Alkhateeb, S.; Trottier, G.; Athanasopoulos, P.Z.; Mirtti, T.; Kortekangas, H.; Laato, M.; van Rhijn, B.; van der Kwast, T.; Fleshner, N.E.; et al. Sex differences in bladder cancer outcomes among smokers with advanced bladder cancer. BJU Int. 2012, 109, 70–76. [Google Scholar] [CrossRef]
- Keck, B.; Ott, O.J.; Häberle, L.; Kunath, F.; Weiss, C.; Rödel, C.; Sauer, R.; Fietkau, R.; Wullich, B.; Krause, F.S. Female sex is an independent risk factor for reduced overall survival in bladder cancer patients treated by transurethral resection and radio- or radiochemotherapy. World J. Urol. 2013, 31, 1023–1028. [Google Scholar] [CrossRef]

| Characteristic | Total (n = 75) n (%) | Males (n = 57) n (%) | Females (n = 18) n (%) | p-Value ° |
|---|---|---|---|---|
| Ages at diagnosis, years median (range) | 67 (40–85) | 67 (40–85) | 67 (51–80) | 0.22 |
| Stage at diagnosis * | ||||
| II | 8 (11.4) | 6 (11.5) | 2 (11.2) | |
| III | 21 (30.0) | 13 (25.0) | 8 (44.4) | 0.29 |
| IV | 41 (58.6) | 33 (63.5) | 8 (44.4) | |
| Site of primary tumour | ||||
| Bladder | 67 (89.3) | 51 (89.5) | 16 (88.9) | 1.0 |
| Upper urinary tract | 8 (10.7) | 6 (10.5) | 2 (11.1) | |
| Histotype | ||||
| Conventional | 69 (92.0) | 53 (93.0) | 16 (88.9) | 0.63 |
| Variants | 6 (8.0) | 4 (7.0) | 2 (11.1) | |
| Site of metastasis | ||||
| Lung | 13 (17.3) | 10 (17.5) | 3 (16.7) | |
| Liver | 2 (2.7) | 2 (3.5) | 0 (0) | 0.95 |
| Bone | 9 (12.0) | 7 (12.3) | 2 (11.1) | |
| Other | 16 (21.3) | 12 (21.1) | 4 (22.2) | |
| Multiple | 35 (46.7) | 26 (45.6) | 9 (50.0) | |
| Smoking * | ||||
| Current | 12 (19.4) | 7 (14.6) | 5 (35.7) | |
| Never | 6 (9.6) | 3 (6.3) | 3 (21.4) | 0.029 |
| Former | 44 (71.0) | 38 (79.1) | 6 (42.9) |
| Overall Survival Analysis | ||||
|---|---|---|---|---|
| Characteristic | Pts at Start | Deaths | %OS | p-Value ° |
| Sex | ||||
| Female | 18 | 7 | 27.5 | |
| Male | 57 | 29 | 17.4 | 0.047 |
| Age diagnosis | ||||
| ≤65 | 28 | 11 | ||
| >65 | 47 | 25 | - | 0.16 |
| Smoking * | ||||
| Current | 12 | 4 | ||
| Never | 6 | 6 | - | 0.055 |
| Former | 44 | 18 | ||
| Stage * | ||||
| II | 8 | 5 | ||
| III | 21 | 9 | - | 0.24 |
| IV | 41 | 20 | ||
| Histology | ||||
| Conventional | 69 | 31 | - | |
| Variants | 6 | 5 | 0.084 | |
| Site | ||||
| Bladder | 67 | 30 | - | 0.64 |
| UUTt | 8 | 6 | ||
| CHT adjuvant | ||||
| No | 66 | 29 | - | 0.17 |
| Yes | 9 | 7 | ||
| CHT I line | ||||
| Platinum | 71 | 35 | - | 0.25 |
| Other | 4 | 1 | ||
| CHT II line | ||||
| Vinflunine | 15 | 7 | ||
| Other | 16 | 11 | - | 0.64 |
| No | 44 | 18 | ||
| BMI | ||||
| 18.5–24.9 | 40 | 21 | ||
| 25–29.9 | 28 | 11 | - | 0.67 |
| >30 | 7 | 4 | ||
| Total | 75 | 36 | 21.3 | |
| Progression-Free Survival (PFS) | Cox Regression Analysis | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Characteristic | Pts at Start | PD | %PFS | p-Value ° | HR (95% CI) p-Value | HR (95% CI) p-Value |
| Sex | ||||||
| Female | 15 | 14 | 0 | 1 | - | |
| Male | 51 | 34 | 21.6 | 0.051 | 0.53 (0.28–1.02) p = 0.06 | |
| Age diagnosis | ||||||
| ≤65 | 22 | 14 | 27.6 | 1 | 1 | |
| >65 | 44 | 34 | 10 | 0.041 | 1.93 (1.02–3.67) p = 0.044 | 1.28 (0.62–2.62) p = 0.50 |
| Smoking * | ||||||
| Current | 10 | 7 | 26.7 | 1 | 1 | |
| Never | 5 | 5 | 0 | 0.009 | 1.51 (0.65–3.52) p = 0.39 | 1.53 (0.64–3.66) p = 0.34 |
| Former | 41 | 27 | 20.7 | 4.35 (1.57–12.1) p = 0.005 | 4.11 (1.43–11.8) p = 0.009 | |
| Stage * | ||||||
| II | 8 | 7 | ||||
| III | 17 | 12 | - | 0.86 | - | - |
| IV | 37 | 27 | ||||
| Histology | ||||||
| Conventional | 62 | 45 | - | 0.94 | - | - |
| Variants | 4 | 3 | ||||
| Site | ||||||
| Bladder | 59 | 42 | - | 0.74 | - | - |
| UUT | 7 | 6 | ||||
| BMI | ||||||
| 18.5–24.9 | 36 | 28 | 12.9 | 1 | 1 | |
| 25–29.9 | 15 | 15 | 23.7 | 0.035 | 0.49 (0.26–0.92) p = 0.027 | 0.47 (0.23–0.95) p = 0.035 |
| >30 | 5 | 5 | 0 | 1.40 (0.54–3.68) p = 0.49 | 0.97 (0.32–2.93) p = 0.96 | |
| Total | 66 | 48 | 16.3 | |||
| Platinum-Based CHT | Vinflunine | |||||
|---|---|---|---|---|---|---|
| Toxicity | Females, n (%) | Males, n (%) | p Value | Females, n (%) | Males, n (%) | p Value |
| Any AEs | 0.23 | 0.44 | ||||
| G1–2 | 9 (52.9) | 40 (74.1) | 3 (60) | 3 (30) | ||
| G3–4 | 7 (41.2) | 13 (24.1) | 1 (20) | 2 (20) | ||
| Haematological | 0.07 | 0.42 | ||||
| G1–2 | 5 (29.4) | 33 (61.1) | 2 (40) | 2 (20) | ||
| G3–4 | 7 (41.2) | 13 (24.1) | 1 (20) | 3 (30) | ||
| Gastrointestinal | 0.13 | 0.19 | ||||
| G1–2 | 5 (29.4) | 24 (44.4) | 1 (20) | 6 (60) | ||
| G3–4 | 0 (0) | 0 (0) | 1 (20) | 0 (0) | ||
| Renal | 0.21 | 1.0 | ||||
| G1–2 | 2 (11.8) | 16 (29.6) | 0 (0) | 1 (10) | ||
| G3–4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Asthenia | 0.78 | 0.61 | ||||
| G1–2 | 8 (47.0) | 22 (40.7) | 3 (60) | 2 (20) | ||
| G3–4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becattini, L.; Saieva, C.; Doni, L.; Roviello, G.; Spatafora, P.; Catalano, M.; Sessa, F.; Galli, I.C.; Bisegna, C.; Conte, F.L.; et al. Gender and Advanced Urothelial Cancer: Outcome, Efficacy and Toxicity following Chemotherapy. Medicina 2022, 58, 886. https://doi.org/10.3390/medicina58070886
Becattini L, Saieva C, Doni L, Roviello G, Spatafora P, Catalano M, Sessa F, Galli IC, Bisegna C, Conte FL, et al. Gender and Advanced Urothelial Cancer: Outcome, Efficacy and Toxicity following Chemotherapy. Medicina. 2022; 58(7):886. https://doi.org/10.3390/medicina58070886
Chicago/Turabian StyleBecattini, Lucrezia, Calogero Saieva, Laura Doni, Giandomenico Roviello, Pietro Spatafora, Martina Catalano, Francesco Sessa, Ilaria Camilla Galli, Claudio Bisegna, Francesco Lupo Conte, and et al. 2022. "Gender and Advanced Urothelial Cancer: Outcome, Efficacy and Toxicity following Chemotherapy" Medicina 58, no. 7: 886. https://doi.org/10.3390/medicina58070886
APA StyleBecattini, L., Saieva, C., Doni, L., Roviello, G., Spatafora, P., Catalano, M., Sessa, F., Galli, I. C., Bisegna, C., Conte, F. L., Zaccaro, C., Santi, R., Serni, S., Nesi, G., & Villari, D. (2022). Gender and Advanced Urothelial Cancer: Outcome, Efficacy and Toxicity following Chemotherapy. Medicina, 58(7), 886. https://doi.org/10.3390/medicina58070886








