Predictive Values of Procalcitonin and Presepsin for Acute Kidney Injury and 30-Day Hospital Mortality in Patients with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. AKI Predictive Value of PCT and PSS at ED Admission
3.2. 30-Day Hospital Mortality Predictive Value of PCT and PSS at ED Admission
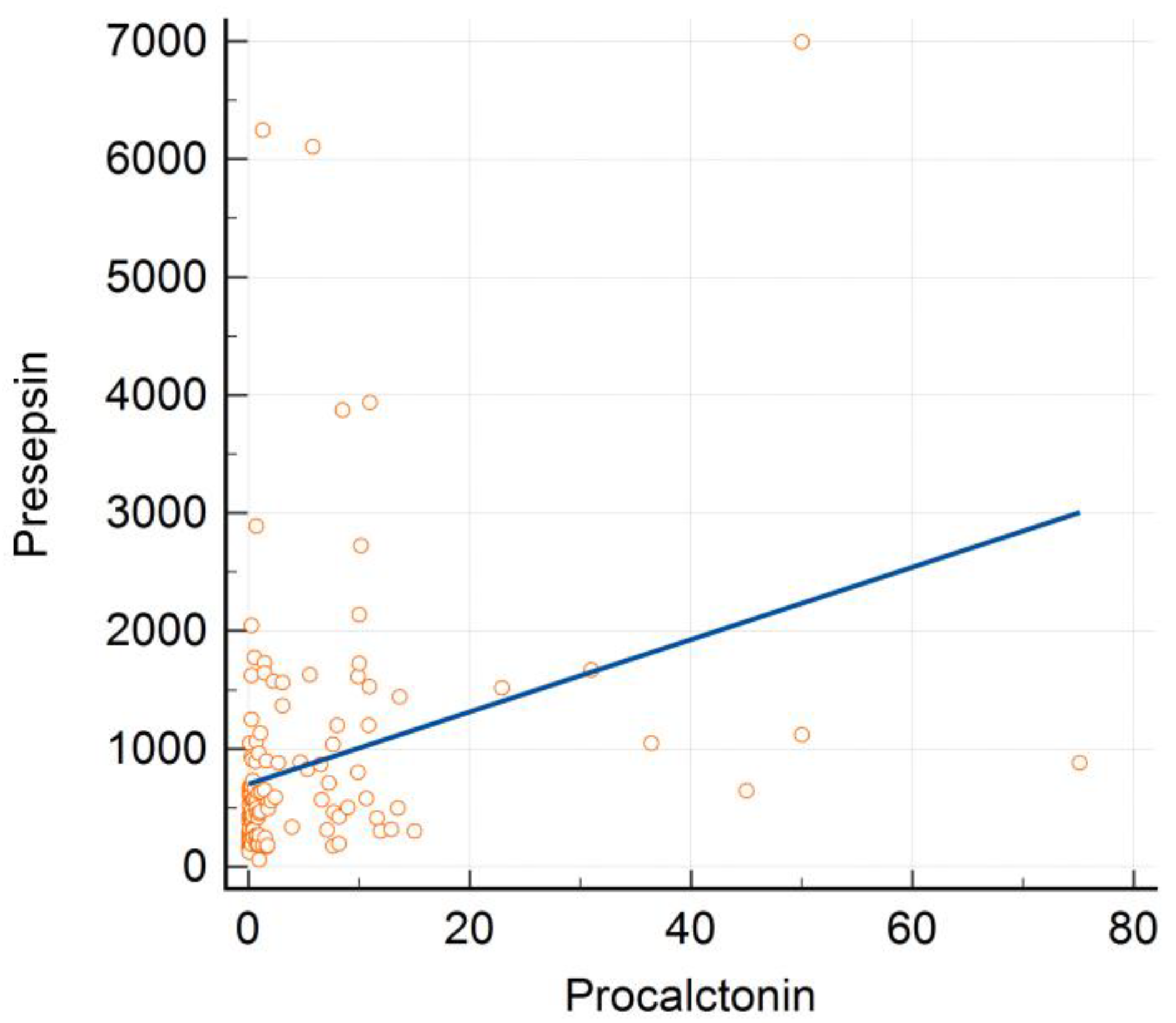
3.3. Correlation between PCT, PSS, and Other Laboratory Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 11 April 2022).
- Central Disaster Management Headquarters; Central Disease Control Headquarters. Coronavirus (COVID-19), Republic of Korea. Available online: http://ncov.mohw.go.kr/en (accessed on 11 April 2022).
- Wang, J.; Wang, Z.; Zhu, Y.; Li, H.; Yuan, X.; Wang, X.; Wang, Y.; Hu, J.; Feng, C.; Liu, C.; et al. Identify the Risk Factors of COVID-19-Related Acute Kidney Injury: A Single-Center, Retrospective Cohort Study. Front. Med. 2020, 7, 436. [Google Scholar] [CrossRef] [PubMed]
- Trifi, A.; Abdellatif, S.; Masseoudi, Y.; Mehdi, A.; Benjima, O.; Seghir, E.; Cherif, F.; Touil, Y.; Jeribi, B.; Daly, F.; et al. COVID-19-induced acute kidney injury in critically ill patients: Epidemiology, risk factors, and outcome. Acute. Crit. Care 2021, 36, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Husain-Syed, F.; Wilhelm, J.; Kassoumeh, S.; Birk, H.-W.; Herold, S.; Vadász, I.; Walmrath, H.-D.; A Kellum, J.; Ronco, C.; Seeger, W. Acute kidney injury and urinary biomarkers in hospitalized patients with coronavirus disease-2019. Nephrol. Dial. Transplant. 2020, 35, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Hardenberg, J.-H.B.; Stockmann, H.; Aigner, A.; Gotthardt, I.; Enghard, P.; Hinze, C.; Balzer, F.; Schmidt, D.; Zickler, D.; Kruse, J.; et al. Critical Illness and Systemic Inflammation Are Key Risk Factors of Severe Acute Kidney Injury in Patients with COVID-19. Kidney Int. Rep. 2021, 6, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.H.; Hirsch, J.S.; Hazzan, A.; Wanchoo, R.; Shah, H.H.; Malieckal, D.A.; Ross, D.W.; Sharma, P.; Sakhiya, V.; Fishbane, S.; et al. Outcomes Among Patients Hospitalized with COVID-19 and Acute Kidney Injury. Am. J. Kidney Dis. 2021, 77, 204–215.e1. [Google Scholar] [CrossRef]
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef]
- Sakran, J.V.; Michetti, C.; Sheridan, M.J.; Richmond, R.; Waked, T.; Aldaghlas, T.; Rizzo, A.; Griffen, M.; Fakhry, S.M. The utility of procalcitonin in critically ill trauma patients. J. Trauma Acute Care Surg. 2012, 73, 413–418. [Google Scholar] [CrossRef]
- Sinha, A.; Sharma, M.K.; Tripathi, K.; Duggal, N.; Tiwari, V.K. Evaluation of Serum Levels of Procalcitonin and C-Reactive Protein as Prognostic Indicators in Burns. Indian J. Plast. Surg. 2021, 54, 308–313. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Liu, Y.-N.; Wang, R.; Xie, L.-X. The accuracy of presepsin (sCD14-ST) for the diagnosis of sepsis in adults: A meta-analysis. Crit. Care 2015, 19, 323. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Li, X.; Guo, P.; Chen, Y.; Li, J.; Tao, T. The accuracy assessment of presepsin (sCD14-ST) for mortality prediction in adult patients with sepsis and a head-to-head comparison to PCT: A meta-analysis. Ther. Clin. Risk Manag. 2019, 15, 741–753. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.R.; He, M.; Kang, Y. A risk score based on procalcitonin for predicting acute kidney injury in COVID-19 patients. J. Clin. Lab. Anal. 2021, 35, e23805. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.; Tuta, L.-A.; David, C.; Bogeanu, C.; Onofrei, S.D.; Stepan, E.; Cuiban, E.; Ciofalca, A.; Feier, L.F.; Pana, C.; et al. Acute kidney injury in moderate and severe COVID-19 patients: Report of two university hospitals. Exp. Ther. Med. 2021, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-B.; Xu, C.; Zhang, R.-B.; Wu, M.; Pan, C.-K.; Li, X.-J.; Wang, Q.; Zeng, F.-F.; Zhu, S. Associations of procalcitonin, C-reaction protein and neutrophil-to-lymphocyte ratio with mortality in hospitalized COVID-19 patients in China. Sci. Rep. 2020, 10, 15058. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Hur, M.; Kim, H.; Lee, C.H.; Lee, J.H.; Kim, H.W.; Nam, M. Prognostic Utility of Procalcitonin, Presepsin, and the VACO Index for Predicting 30-day Mortality in Hospitalized COVID-19 Patients. Ann. Lab. Med. 2022, 42, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Palevsky, P.M.; Liu, K.; Brophy, P.D.; Chawla, L.; Parikh, C.R.; Thakar, C.V.; Tolwani, A.J.; Waikar, S.S.; Weisbord, S.D. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for Acute Kidney Injury. Am. J. Kidney Dis. 2013, 61, 649–672. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cheng, C.; Zheng, X.; Jin, Y.; Duan, G.; Chen, M.; Chen, S. Elevated Procalcitonin Is Positively Associated with the Severity of COVID-19: A Meta-Analysis Based on 10 Cohort Studies. Medicina 2021, 57, 594. [Google Scholar] [CrossRef] [PubMed]
- Sayah, W.; Berkane, I.; Guermache, I.; Sabri, M.; Lakhal, F.Z.; Rahali, S.Y.; Djidjeli, A.; Mahammed, L.L.; Merah, F.; Belaid, B.; et al. Interleukin-6, procalcitonin and neutrophil-to-lymphocyte ratio: Potential immune-inflammatory parameters to identify severe and fatal forms of COVID-19. Cytokine 2021, 141, 155428. [Google Scholar] [CrossRef]
- Hu, R.; Han, C.; Pei, S.; Yin, M.; Chen, X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents 2020, 56, 106051. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Post, A.; Dullaart, R.P.; Bakker, S.J. Sodium status and kidney involvement during COVID-19 infection. Virus Res. 2020, 286, 198034. [Google Scholar] [CrossRef]
- Lim, J.-H.; Park, S.-H.; Jeon, Y.; Cho, J.-H.; Jung, H.-Y.; Choi, J.-Y.; Kim, C.-D.; Lee, Y.-H.; Seo, H.; Lee, J.; et al. Fatal Outcomes of COVID-19 in Patients with Severe Acute Kidney Injury. J. Clin. Med. 2020, 9, 1718. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, Q.; Li, Z.; Shen, L.; Zhang, J.; Wang, P.; Wu, S.; Zhou, T.; Xu, Q.; Chen, X.; et al. Incorporation of Urinary Neutrophil Gelatinase-Associated Lipocalin and Computed Tomography Quantification to Predict Acute Kidney Injury and In-Hospital Death in COVID-19 Patients. Kidney Dis. 2020, 7, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Bas, S.; Gauthier, B.R.; Spenato, U.; Stingelin, S.; Gabay, C. CD14 Is an Acute-Phase Protein. J. Immunol. 2004, 172, 4470–4479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, S.; Suzuki, Y.; Takahashi, G.; Shozushima, T.; Ishikura, H.; Murai, A.; Nishida, T.; Irie, Y.; Miura, M.; Iguchi, H.; et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J. Infect. Chemother. 2012, 18, 891–897. [Google Scholar] [CrossRef]
- Kocyigit, A.; Sogut, O.; Durmus, E.; Kanimdan, E.; Guler, E.M.; Kaplan, O.; Yenigun, V.B.; Eren, C.; Ozman, Z.; Yasar, O. Circulating furin, IL-6, and presepsin levels and disease severity in SARS-CoV-2-infected patients. Sci. Prog. 2021, 104, 368504211026119. [Google Scholar] [CrossRef]
- Assal, H.H.; Abdelrahman, S.M.; Abdelbasset, M.A.; Abdelaziz, M.; Sabry, I.M.; Shaban, M.M. Presepsin as a Novel Biomarker in predicting In-hospital Mortality in Patients with COVID-19 Pneumonia. Int. J. Infect. Dis. 2022, 118, 155–163. [Google Scholar] [CrossRef]
- Zaninotto, M.; Mion, M.M.; Cosma, C.; Rinaldi, D.; Plebani, M. Presepsin in risk stratification of SARS-CoV-2 patients. Clin. Chim. Acta. 2020, 507, 161–163. [Google Scholar] [CrossRef]
- Keskinidou, C.; Vassiliou, A.; Zacharis, A.; Jahaj, E.; Gallos, P.; Dimopoulou, I.; Orfanos, S.; Kotanidou, A. Endothelial, Immunothrombotic, and Inflammatory Biomarkers in the Risk of Mortality in Critically Ill COVID-19 Patients: The Role of Dexamethasone. Diagnostics 2021, 11, 1249. [Google Scholar] [CrossRef]
- Shimoyama, Y.; Umegaki, O.; Kadono, N.; Minami, T. Presepsin and prognostic nutritional index are predictors of septic acute kidney injury, renal replacement therapy initiation in sepsis patients, and prognosis in septic acute kidney injury patients: A pilot study. BMC Nephrol. 2021, 22, 219. [Google Scholar] [CrossRef]
- Mabrey, F.L.; Morrell, E.D.; Bhatraju, P.K.; Sathe, N.A.; Sakr, S.S.; Sahi, S.K.; West, T.E.; Mikacenic, C.; Wurfel, M.M. Plasma Soluble CD14 Subtype Levels Are Associated with Clinical Outcomes in Critically Ill Subjects with Coronavirus Disease 2019. Crit. Care Explor. 2021, 3, e0591. [Google Scholar] [CrossRef]
- Morell-Garcia, D.; Ramos-Chavarino, D.; Bauça, J.M.; Argente Del Castillo, P.; Ballesteros-Vizoso, M.A.; García de Guadia-na-Romualdo, L.; Gómez-Cobo, C.; Pou, J.A.; Amezaga-Menéndez, R.; Alonso-Fernández, A.; et al. Urine biomarkers for the prediction of mortality in COVID-19 hospitalized patients. Sci. Rep. 2021, 11, 11134. [Google Scholar] [CrossRef] [PubMed]
- Hansrivijit, P.; Gadhiya, K.P.; Gangireddy, M.; Goldman, J.D. Risk Factors, Clinical Characteristics, and Prognosis of Acute Kidney Injury in Hospitalized COVID-19 Patients: A Retrospective Cohort Study. Medicines 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
| Variables | Overall (n = 151) | Patients without AKI (n = 95) | Patients with AKI (n = 56) | p-Value |
|---|---|---|---|---|
| Age, years | 77 (67–84) | 77 (67–84) | 79 (65–85) | 0.881 |
| Male | 86 (57.0) | 58 (61.1) | 28 (50.0) | 0.234 |
| Comorbidity | ||||
| Diabetes mellitus | 42 (27.8) | 27 (28.4) | 15 (26.8) | 0.853 |
| Hypertension | 73 (48.3) | 45 (47.4) | 28 (50.0) | 0.866 |
| Chronic liver disease | 8 (5.3) | 3 (3.2) | 5 (8.9) | 0.147 |
| Chronic lung disease | 10 (6.6) | 4 (4.2) | 5 (10.7) | 0.174 |
| Cerebrovascular disease | 49 (32.5) | 30 (31.6) | 19 (33.9) | 0.857 |
| Coronary artery disease | 7 (4.6) | 6 (6.3) | 1 (1.8) | 0.260 |
| Vital sign | ||||
| Systolic blood pressure, mmHg | 115 (97–132) | 116 (100–139) | 110 (88–127) | 0.012 |
| Diastolic blood pressure, mmHg | 66 (56–77) | 67 (57–82) | 61 (53–71) | 0.001 |
| Heart rate, beats/min | 100 (86–116) | 100 (86–114) | 100 (87–118) | 0.318 |
| Respiratory rate, breaths/min | 20 (20–23) | 20 (19–23) | 21 (20–25) | 0.028 |
| Body temperature, °C | 37.2 (36.6–38.2) | 37.4 (36.6–38.1) | 37.2 (36.7–38.3) | 0.774 |
| CURB-65 score | 2 (1–3) | 2 (1–2) | 2 (1–3) | 0.045 |
| Pneumonia severity index | 100 (81–126) | 94 (78–120) | 110 (87–141) | 0.006 |
| Death | 19 (12.6) | 7 (7.4) | 12 (21.4) | 0.020 |
| Variable | Patients without AKI (n = 95) | Patients with AKI (n = 56) | p-Value |
|---|---|---|---|
| WBC count, ×103/μL | 10,920 (7365–15,040) | 11,150 (7465–14,945) | 0.331 |
| BUN, mg/dL | 18.6 (12.1–33.0) | 31.8 (21.0–51.0) | <0.001 |
| Creatinine, mg/dL | 0.72 (0.56–0.84) | 0.73 (0.61–0.92) | 0.392 |
| Albumin, g/dL | 3.6 (3.4–3.9) | 3.3 (2.9–3.7) | 0.004 |
| hsCRP, mg/dL | 9.50 (4.65–20.55) | 13.01 (6.49–23.6) | 0.117 |
| Lactate, mmol/L | 2.12 (1.56–3.38) | 3.30 (2.00–5.74) | 0.003 |
| Procalcitonin, ng/mL | 0.29 (0.06–1.35) | 6.62 (0.89–9.99) | <0.001 |
| Presepsin, pg/mL | 447 (239–658) | 801 (442–1589) | <0.001 |
| Variable | AKI Stage 1 (n = 31) | AKI Stage 2 (n = 11) | AKI Stage 3 (n = 14) | p-Value |
|---|---|---|---|---|
| WBC count, ×103/μL | 11,150 (7090–15,530) | 9750 (6048–20,593) | 11,400 (8235–14,816) | 0.721 |
| BUN, mg/dL | 25.2 (18.6–42.1) | 26.3 (23.0–33.2) c | 56.0 (45.0–71.9) b | 0.002 |
| Albumin, g/dL | 3.4 (3.1–3.7) | 3.5 (2.9–3.7) | 3.1 (2.7–3.5) | 0.554 |
| hsCRP, mg/dL | 11.23 (3.44–21.52) | 14.2 (6.4–23.6) | 21.8 (11.4–29.9) | 0.151 |
| Lactate, mmol/L | 2.45 (1.97–5.37) | 3.70 (2.09–5.90) | 3.67 (2.48–6.26) | 0.588 |
| Procalcitonin, ng/mL | 7.62 (0.93–10.75) | 4.28 (0.56–8.28) | 4.64 (0.79–9.96) | 0.581 |
| Presepsin, pg/mL | 656 (347–1058) b | 1302 (691–1639) a | 1695 (530–2847) | 0.078 |
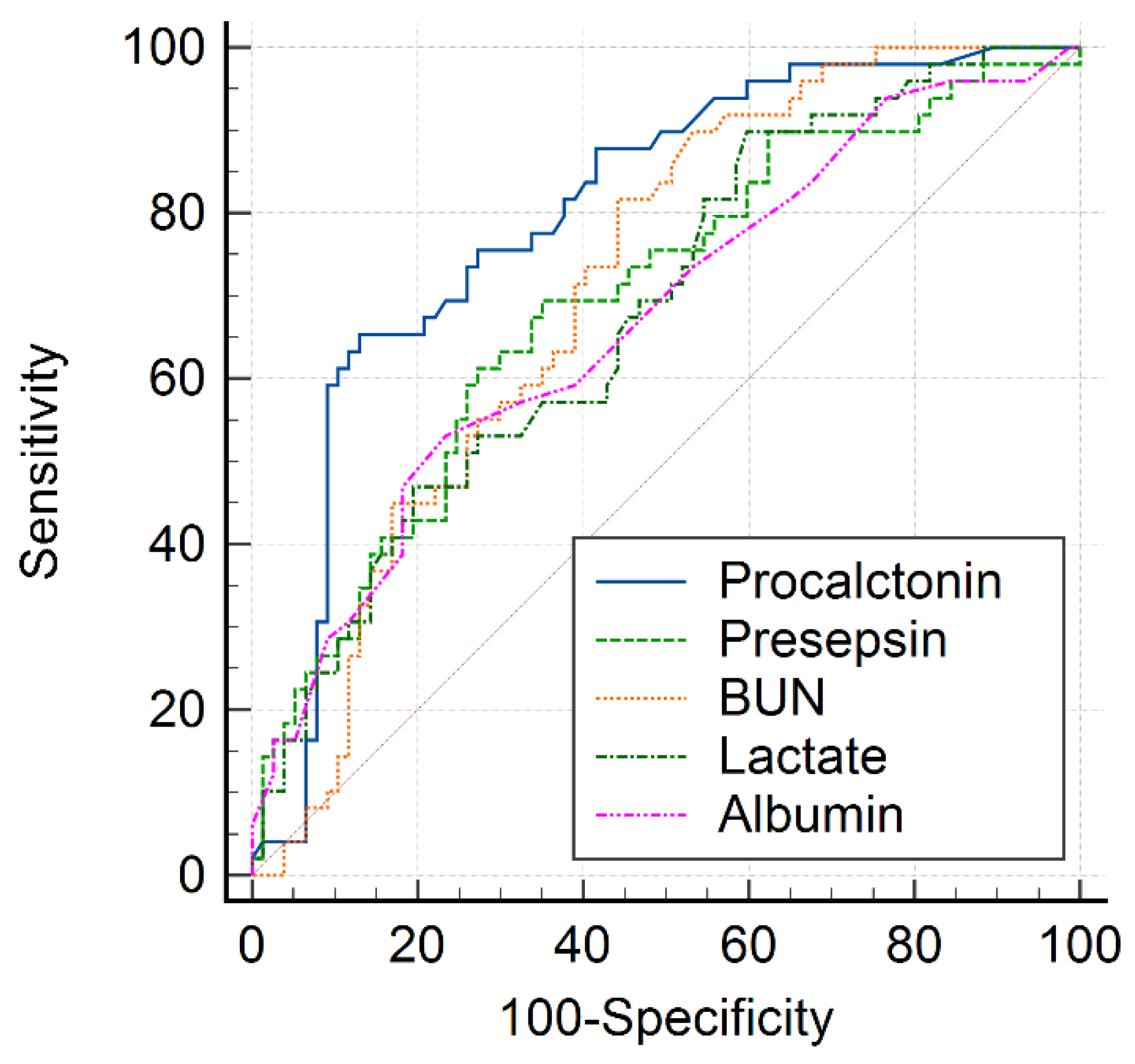
| Variable | AUC | 95% CI | Cutoff Value | Sensitivity (%) | Specificity (%) | p-Value |
|---|---|---|---|---|---|---|
| BUN | 0.659 | 0.582–0.724 | >19.8 | 83.9 | 62.1 | <0.001 |
| Albumin | 0.641 | 0.559–0.717 | <3.2 | 42.9 | 82.1 | 0.003 |
| Lactate | 0.649 | 0.564–0.728 | >1.79 | 87.0 | 37.7 | 0.002 |
| Procalcitonin | 0.811 | 0.739–0.870 | >2.26 | 64.3 | 89.5 | <0.001 |
| Presepsin | 0.700 | 0.615–0.776 | >572 | 66.0 | 69.1 | <0.001 |
| Variable | Survivors (n = 132) | Non-Survivors (n = 19) | p-Value |
|---|---|---|---|
| Age, years | 77 (67–85) | 77 (66–83) | 0.682 |
| Male | 74 (56.1) | 12 (63.2) | 0.627 |
| Comorbidity | |||
| Diabetes mellitus | 38 (28.8) | 4 (21.1) | 0.592 |
| Hypertension | 64 (48.5) | 9 (47.4) | 0.927 |
| Chronic liver disease | 5 (3.8) | 3 (15.8) | 0.063 |
| Chronic lung disease | 9 (6.8) | 1 (5.3) | 0.799 |
| Cerebrovascular disease | 45 (34.1) | 4 (21.1) | 0.305 |
| Coronary artery disease | 7 (5.3) | 0 (0) | 0.597 |
| Vital sign | |||
| Systolic blood pressure, mmHg | 116 (99–133) | 93 (88–119) | 0.243 |
| Diastolic blood pressure, mmHg | 66 (56–79) | 53 (51–65) | 0.023 |
| Heart rate, beats/min | 100 (89–116) | 87 (83–117) | 0.726 |
| Respiratory rate, breaths/min | 20 (20–23) | 20 (19–23) | 0.076 |
| Body temperature, °C | 37.4 (36.7–38.4) | 36.5 (36.0–37.0) | 0.002 |
| CURB-65 score | 2 (1–3) | 3 (2–4) | <0.001 |
| Pneumonia severity index | 97 (80–120) | 138 (125–178) | <0.001 |
| Acute kidney injury | 44 (33.3) | 12 (63.2) | 0.020 |
| Variable | Survivors (n = 132) | Non-Survivors (n = 19) | p-Value |
|---|---|---|---|
| WBC count, × 103/μL | 10,700 (7390–14,930) | 12,100 (6705–17,890) | 0.365 |
| BUN, mg/dL | 21.8 (15.7–39.7) | 29.8 (24.4–57.8) | 0.016 |
| Albumin, g/dL | 3.6 (3.2–3.9) | 2.9 (2.7–3.6) | 0.004 |
| hsCRP, mg/dL | 11.65 (4.80–21.81) | 19.07 (5.03–30.88) | 0.194 |
| Lactate, mmol/L | 2.32 (1.72–3.99) | 3.33 (2.31–6.82) | 0.034 |
| Procalcitonin, ng/mL | 0.70 (0.09–3.51) | 9.98 (4.42–12.32) | < 0.001 |
| Presepsin, pg/mL | 495 (258–845) | 1441 (960–1935) | < 0.001 |
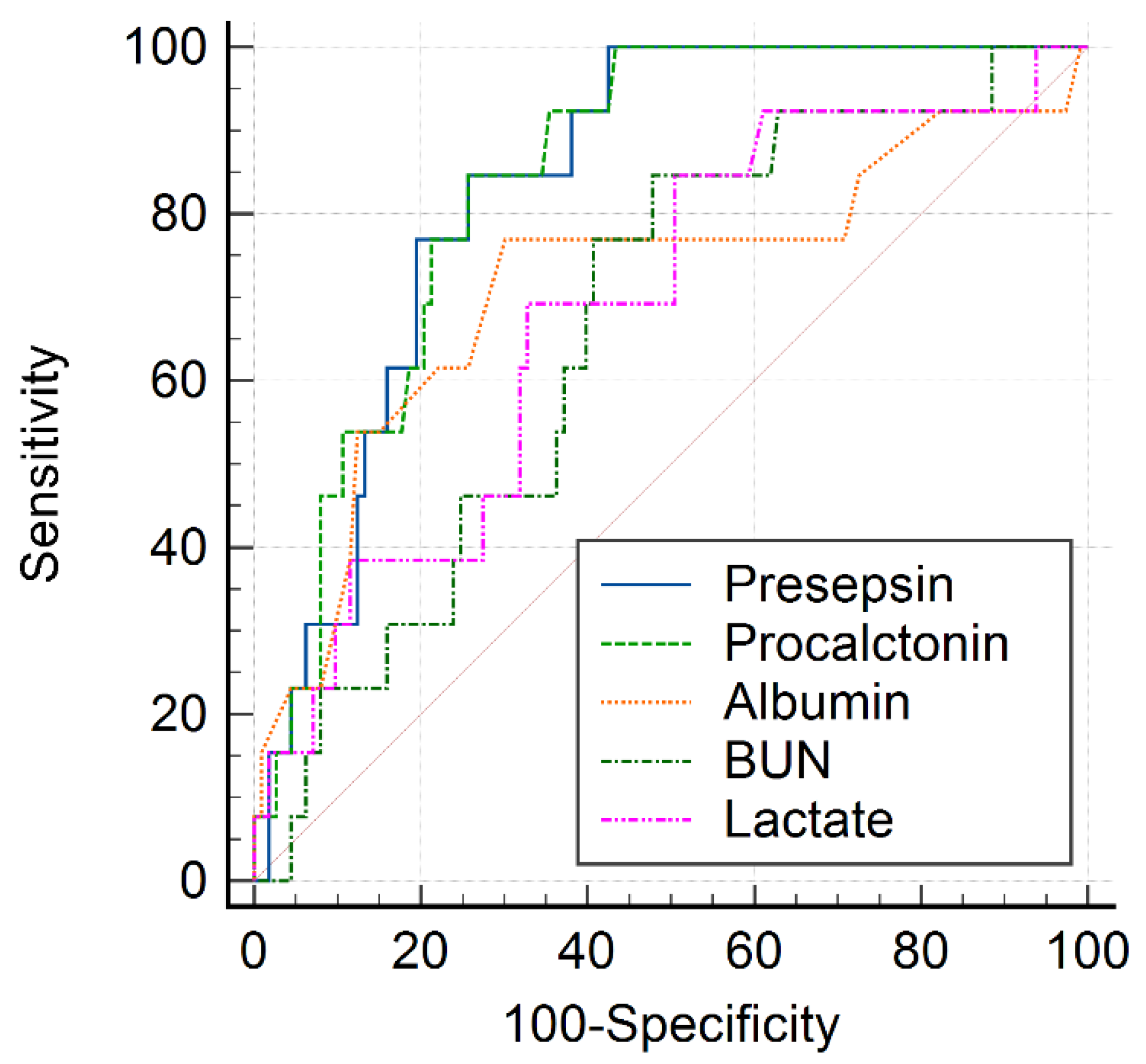
| Variable | AUC | 95% CI | Cutoff Value | Sensitivity (%) | Specificity (%) | p-Value |
|---|---|---|---|---|---|---|
| BUN | 0.671 | 0.590–0.746 | >17.7 | 94.7 | 40.2 | 0.004 |
| Albumin | 0.702 | 0.622–0.773 | <3.3 | 68.4 | 71.2 | 0.009 |
| Lactate | 0.652 | 0.567–0.731 | >2.24 | 84.2 | 48.3 | 0.021 |
| Procalcitonin | 0.769 | 0.694–0.834 | >2.67 | 68.4 | 77.3 | <0.001 |
| Presepsin | 0.846 | 0.774–0.903 | >865 | 84.6 | 76.0 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Hong, D.-Y.; Kim, J.-W.; Park, S.-O.; Lee, K.-R.; Baek, K.-J. Predictive Values of Procalcitonin and Presepsin for Acute Kidney Injury and 30-Day Hospital Mortality in Patients with COVID-19. Medicina 2022, 58, 727. https://doi.org/10.3390/medicina58060727
Kim S-Y, Hong D-Y, Kim J-W, Park S-O, Lee K-R, Baek K-J. Predictive Values of Procalcitonin and Presepsin for Acute Kidney Injury and 30-Day Hospital Mortality in Patients with COVID-19. Medicina. 2022; 58(6):727. https://doi.org/10.3390/medicina58060727
Chicago/Turabian StyleKim, Sin-Young, Dae-Young Hong, Jong-Won Kim, Sang-O Park, Kyeong-Ryong Lee, and Kwang-Je Baek. 2022. "Predictive Values of Procalcitonin and Presepsin for Acute Kidney Injury and 30-Day Hospital Mortality in Patients with COVID-19" Medicina 58, no. 6: 727. https://doi.org/10.3390/medicina58060727
APA StyleKim, S.-Y., Hong, D.-Y., Kim, J.-W., Park, S.-O., Lee, K.-R., & Baek, K.-J. (2022). Predictive Values of Procalcitonin and Presepsin for Acute Kidney Injury and 30-Day Hospital Mortality in Patients with COVID-19. Medicina, 58(6), 727. https://doi.org/10.3390/medicina58060727






